Abstract
The ubiquitous mammalian signaling molecule bisdiphosphoinositol tetrakisphosphate (1,5-(PP)2-myo-InsP4, or InsP8) displays the most congested three-dimensional array of phosphate groups found in nature. The high charge density, the accumulation of unstable P-anhydrides and P-esters, the lack of UV absorbance, and low levels of optical rotation constitute severe obstacles to its synthesis, characterization, and purification. Herein, we describe the first procedure for the synthesis of enantiopure 1,5-(PP)2-myo-InsP4 and 3,5-(PP)2-myo-InsP4 utilizing a C2-symmetric P-amidite for desymmetrization and concomitant phosphitylation followed by a one-pot bidirectional P-anhydride-forming reaction that combines sixteen chemical transformations with high efficiency. The configuration of these materials is unambiguously shown by subsequent X-ray analyses of both enantiomers after being individually soaked into crystals of the kinase domain of human diphosphoinositol pentakisphosphate kinase 2.
Keywords: chiral auxiliaries, inositol, kinases, phosphorylation, second messengers
Cells propagate and integrate information by using phosphorylated second messengers based on the myo-inositol scaffold.[1] Their polar character and relatively low abundance often necessitate tedious isolation procedures, which is especially true for the family of inositol pyrophosphates.[2] The need to have ready access to inositol pyrophosphates for biological studies motivated the development of a facile, scalable, and stereoselective synthesis of these molecules.
Inositol pyrophosphates, such as 5-PP-myo-InsP5 (1), 1-PP-myo-InsP5 (2, myo-InsP7), and 1,5-(PP)2-myo-InsP4 (3, myo-InsP8; Figure 1), can signal by two distinctly different processes, that is, by either binding to a specific receptor[3] or by non-enzymatic transfer of the β-phosphate to phosphorylated serine residues in proteins (transphosphorylation).[4] In vivo, both processes contribute to complex signaling networks that are far from being understood.[5]
Figure 1.
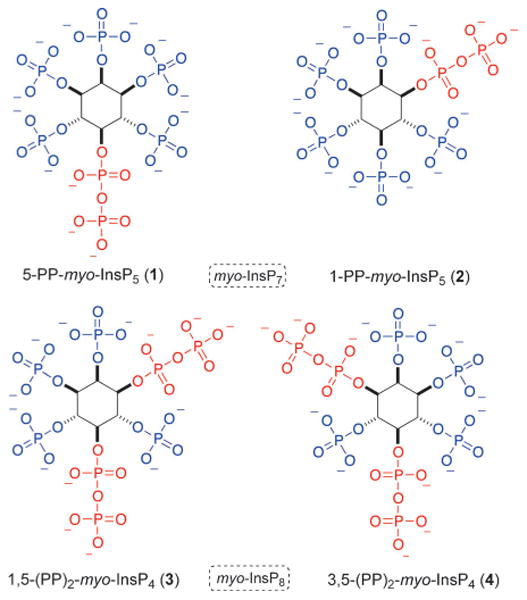
Mammalian inositol pyrophosphates 1–3 and an unnatural enantiomer 4. The dashed box indicates the generic description, with the more precise names given underneath the compounds.
Inositol pyrophosphates and their dedicated kinases (IP6Ks: inositolhexakisphosphate kinases; PPIP5Ks: diphosphoinositol pentakisphosphate kinases)[6] and phosphatases (DIPPs: diphosphoinositol polyphosphate phosphohydrolases)[7] have been linked to a range of biological phenomena, such as insulin signaling,[8] telomere length regulation,[9] pleckstrin homology (PH) domain regulation,[10] cellular energy homeostasis,[11] apoptosis,[12 and regulation of the innate immune system.[13] Some of these studies have been facilitated by the use of chemically synthesized myo-InsP7 and derivatives thereof.[4b,8b,12,13b] In contrast, the specific biological functions of myo-InsP8 remain largely unexplored, mainly because a method to chemically synthesize these molecules in sufficient purity has not been reported. Nonetheless, it has been established that concentrations of myo-InsP8 in mammalian cells are regulated in a stimulus-dependent fashion increasing severalfold following osmotic stress or thermal challenge, whereas bioenergetic stress significantly decreases levels of myo-InsP8.[14]
In mammals, the structure of the myo-InsP8 isomer has been identified as the 1,5 isomer 3.[15] Chemical synthesis of this isomer would greatly aid studies into its function. Having access to both enantiomers 3 and 4 would be even more useful for functional studies, in which biological specificity could be tested.
Different isomers and analogues of myo-InsP7 have been accessed by chemical synthesis, but it is still a challenge to obtain reasonably pure material.[16] The method must be mild enough to protect the delicate P-anhydride, while enforcing the generation of a highly congested structure. The more intense molecular crowding in 1,5-myo-InsP8 presents even more complex synthetic challenges. In fact, the chemical synthesis of only one myo-InsP8 isomer is reported, namely the unnatural and symmetric meso 2,5-isomer; its published purity of approximately 10% underscores the technical demands in synthesizing these molecules.[17]
Synthetic access to enantiopure nonsymmetric myo-InsP7 can be achieved by using a C2-symmetric P-amidite through the desymmetrization of a protected analogue of the meso compound myo-inositol.[18] The efficiency of this approach is based on the fact that the chiral auxiliary also serves as an orthogonal protecting group that can be cleaved under very mild conditions. As all positions in myo-InsP8 are phosphorylated, desymmetrization by phosphorylation[18a, 19] significantly decreases the required number of steps. Consequently, extension of this approach to synthesize myo-InsP8 derivatives 3 and 4 could potentially provide efficient access to these molecules.
The synthesis commenced with myo-inositol (5) that was converted[18a] (Scheme 1) into benzylidene-protected intermediate 6 with a free 5-OH group by the method of Holmes et al.[20] The 5-axial hydroxy group in alcohol 6 was phosphitylated with a bis-fluorenyl-protected P-amidite 7,[21] oxidized, and the benzylidene acetal was selectively cleaved yielding meso-diol 8.
Scheme 1.
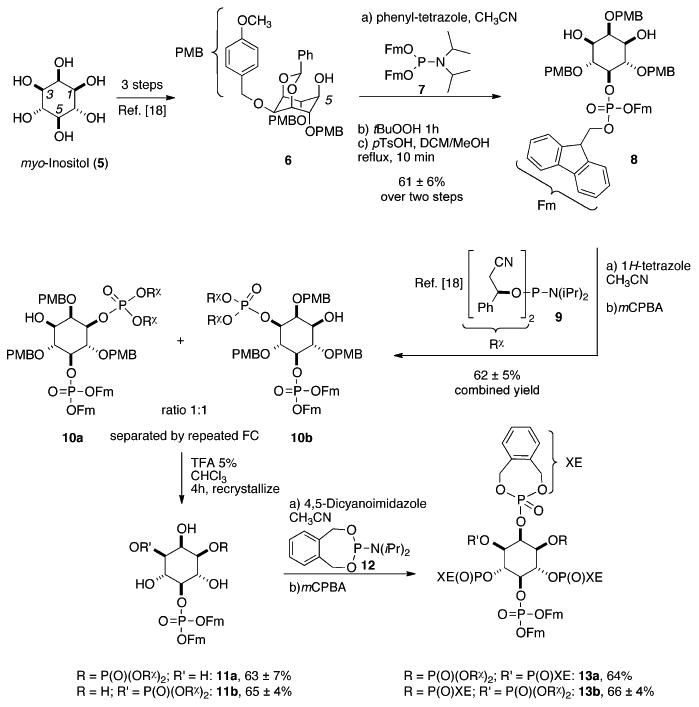
Synthesis of the unsymmetric hexakisphosphates 13a and 13b by phosphitylation with C2-symmetric P-amidite 9, followed by the gram-scale separation of the diastereomers 10a,b. The chiral auxiliary (Rχ) serves as an orthogonal protecting group throughout the synthesis. DCM = dichloromethane, PMB = para-methoxybenzyl, Fm = fluorenylmethyl, tBuOOH = tert-butylhydroperoxide, pTsOH = para-toluenesulfonic acid, mCPBA = meta-chloroperbenzoic acid, TFA = trifluoroacetic acid, FC = flash chromatography, XE = o-xylylene.
Diol 8 was subjected to phosphitylation with C2-symmetric P-amidite 9, followed by oxidation of the PIII intermediate. The obtained mixture of diastereomers 10a/b was then separated by repeated flash chromatography (FC) until an enrichment of either diastereomer, 10a or 10b, to a purity of 90% to 95%, was achieved. Note that with this approach, a total of 2.7 g of the diastereomeric mixture was separated, providing access to more than 1 g of each of the purified diastereomers (diastereomeric ratio, d.r. >9:1). After cleavage of the para-methoxybenzyl (PMB) protecting groups under acidic conditions and crystallization, both diastereomers 11a and 11b were obtained in highly enriched form (d.r. ≥ 98:2), as evidenced by mixing of the separated compounds and 31PNMR spectroscopic analysis (see the Supporting Information). Consequently, enrichment of the diastereomers 10a or 10b prior to cleavage of the PMB groups is sufficient to obtain essentially diastereopure material in the next step. The diastereopure tetraols 11a and 11b were then phosphitylated with an o-xylylene-derived phosphoramidite 12[16g] and oxidized in situ. The obtained unsymmetric hexakisphosphates 13a and 13b were again analyzed for diastereomeric purity by mixing experiments (d.r. ≥ 98:2; Supporting Information).
In the key step, the hexakisphosphates 13a and 13b were subjected to a bidirectional “telescoping” P-anhydride-forming reaction (Scheme 2). This step involved the introduction of both protected pyrophosphates in the 1 or 3 and 5 positions in a single, one-pot reaction without workup of the reaction product between steps. As this process combined sixteen discrete chemical reactions, precise control over every single step was required. Overall, four protecting groups (Fm and chiral auxiliaries Rχ) were removed by replacement with more labile trimethylsilyl groups (TMS) under mild, basic conditions in the presence of the TMS donor N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA). Subsequently, all four TMS groups were cleaved by methanolysis and the nucleophilic dibasic phosphates formed were stabilized by mono-protonation with trifluoroacetic acid (TFA). The monobasic phosphates obtained were then phosphitylated with bis-benzyl P-amidite 14, oxidized in situ, and the generated bis-P-anhydrides 15a and 15b were isolated in almost pure form by simple precipitation. It was mandatory to obtain the bis-P-anhydrides 15a and 15b in high quality, as they were not stable enough for further purification.
Scheme 2.
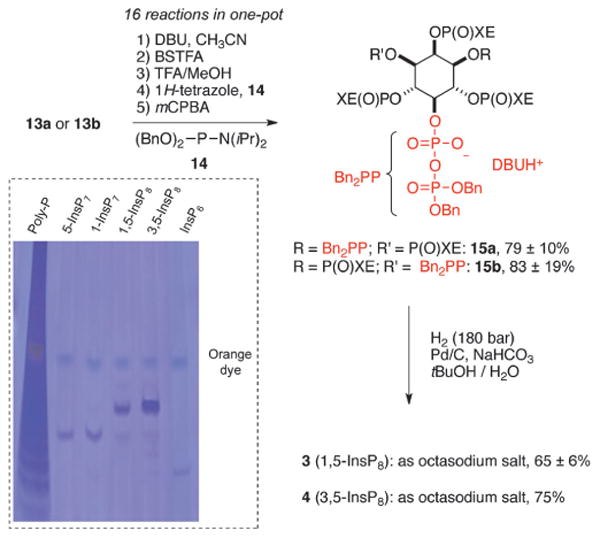
One-pot synthesis of protected 1,5- or 3,5-(PP)2-myo-InsP4 (15a/15b) followed by high-pressure hydrogenation to yield enantiopure myo-InsP8 (3 or 4) as octasodium salts. Dotted Box: PAGE analysis of synthetic myo-InsP8 with synthetic standards (5-myo-InsP7, 1-myo-InsP7) and commercial standards (polyphosphate and myo-InsP6). Stained with toluidine blue. BSTFA = N,O-bis(trimethylsilyl) trifluoroacetamide, DBU = 1,8-diazabicyclo[5.4.0]undecene, TFA = trifluoroacetic acid, mCPBA = meta-chloroperbenzoic acid, Bn = benzyl.
Rapid deprotection by high-pressure hydrogenation of 15a or 15b led to 3 or 4, respectively (Scheme 2), and precipitation from solution gave material of suitable purity for biological studies. The presence of trace impurities (10%) of myo-InsP7 (e.g. 1 and 2) was detected by 31P NMR spectroscopy and PAGE analysis[22] (Scheme 2, dotted box; Supporting Information). Even though α-unprotected P-anhydrides[23] are less prone to hydrolysis compared to P-anhydrides that are fully protected,[16g, 17] complete preservation of the moiety could not be achieved.
It is difficult to assign the absolute configuration of inositolphosphates exclusively by measurement and comparison of optical rotations, as these values are usually very small and strongly depend on the pH value.[19] Optical rotation values of myo-InsP8 have not been reported. However, the naturally occurring isomer of myo-InsP8, 3, has previously been identified in crystal complexes of the kinase domain of human PPIP5K2 (PPIP5K2KD).[15] New crystal complexes of PPIP5K2KD were now prepared, into which either myo-InsP8 enantiomer 3 or 4 were individually soaked (Figure 2). The electron-density maps allowed for an unequivocal assignment of the absolute configuration of each of the myo-InsP8 enantiomers. It is interesting to note that the unnatural enantiomer 4 is also accommodated in the catalytically active site of the enzyme. In addition to the active site of PPIP5K2KD accommodating the natural (3) and unnatural (4) enantiomers, both were also substrates for the phosphatase enzyme DIPP1 (3: Vmax = 124.9 ± 5.5 nmolmg−1 min−1; 4: Vmax = 239.9 ± 9.3 nmolmg−1 min−1, n = 3; measured by inorganic phosphate (Pi) release as described previously (see Ref. [24] and the Supporting Information).
Figure 2.
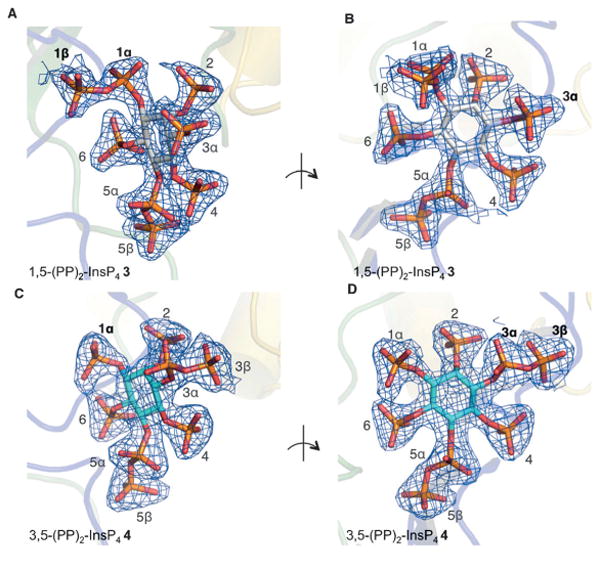
Refined 2 Fo– Fc maps contoured at 2.0σ (panels A, B) or 1.0σ (panels C, D) and shown in blue mesh. Myo-InsP8 molecules are shown in a stick model. The kinase domain of PPIP5K2 is shown in a transparent representation. The phosphate groups around the inositol ring are numbered. Panels A, B: PPIP5K2KD/ADP complexed with myo-InsP8 (1,5-(PP)2-InsP4 3), showing two orientations. Panels C, D: PPIP5K2KD/AMP-PNP complexed with the enantiomer 3,5-(PP)2-InsP4 (4). (PDB codes: 4Q4C and 4Q4D).
Overall, this study unveils a new strategy to synthesize 1,5- and 3,5-myo-InsP8 3 and 4 in scalable quantities, based on desymmetrization by phosphitylation on a gram-scale, followed by a bidirectional P-anhydride synthesis in a one-pot telescoping procedure. The absolute configuration was deduced by cocrystallization with PPIP5K2KD, allowing for the subsequent unambiguous assignment of each enantiomer. This study provides novel strategies to construct the most congested and highly charged three-dimensional assemblies of phosphate groups occurring in nature. These strategies can be applied to target other regioisomers and functionalized analogues and will help to unravel the enigmas associated with the inositol pyrophosphate signaling network.
Footnotes
We are grateful to Prof. Jay Siegel and Prof. John Robinson for continuous support. This work was supported by the Swiss National Science Foundation (SNF, Ambizione Grant PZ00P2_136816 to H.J.J.), U.Z.H., and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Supporting Information (Experimental Details) for this article is available on the WWW under http://dx.doi.org/10.1002/anie.
Contributor Information
Samanta Capolicchio, Department of Chemistry, University of Zürich (UZH), Winterthurerstrasse 190, 8057 Zürich (Switzerland).
Dr. Huanchen Wang, Inositol Signaling Group, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC (USA)
Divyeshsinh T. Thakor, Department of Chemistry University of Zürich (UZH) Winterthurerstrasse 190, 8057 Zürich (Switzerland)
Dr. Stephen B. Shears, Inositol Signaling Group, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC (USA)
Dr. Henning J. Jessen, Email: Henningjacob.jessen@chem.uzh.ch, Department of Chemistry University of Zürich (UZH) Winterthurerstrasse 190, 8057 Zürich (Switzerland).
References
- 1.a) Kutateladze TG. Nat Chem Biol. 2010;6:507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Streb H, Irvine RF, Berridge MJ, Schulz I. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 2.a) Laussmann T, Eujen R, Weisshuhn CM, Thiel U, Vogel G. Biochem J. 1996;315:715–720. doi: 10.1042/bj3150715. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Laussmann T, Hansen A, Reddy KM, Reddy KK, Falck JR, Vogel G. FEBS Lett. 1998;426:145–150. doi: 10.1016/s0014-5793(98)00329-9. [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Huang KX, Quiocho FA, O'Shea EK. Nat Chem Biol. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]; b) Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK, Juluri KR, Xu Y, Prestwich GD, Parang K, Snyder SH. Proc Natl Acad Sci USA. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Bennett M, Onnebo SMN, Azevedo C, Saiardi A. Cell Mol Life Sci. 2006;63:552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kilari RS, Weaver JD, Shears SB, Safrany ST. FEBS Lett. 2013;587:3464–3470. doi: 10.1016/j.febslet.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shears SB. Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Shears SB, Weaver JD, Wang H. Adv Biol Regul. 2013;53:19–27. doi: 10.1016/j.jbior.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Thomas MP, Potter BVL. FEBS J. 2014;281:14–33. doi: 10.1111/febs.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Fridy PC, Otto JC, Dollins DE, York JD. J Biol Chem. 2007;282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]; b) Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]; c) Choi JH, Williams J, Cho J, Falck R, Shears SB. J Biol Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]; e) Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. J Biol Chem. 2001;276:39179–39185. doi: 10.1074/jbc.M106842200. [DOI] [PubMed] [Google Scholar]
- 7.Safrany ST, Ingram SW, Cartwright JL, Falck JR, McLennan AG, Barnes LD, Shears SB. J Biol Chem. 1999;274:21735–21740. doi: 10.1074/jbc.274.31.21735. [DOI] [PubMed] [Google Scholar]
- 8.a) Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, Snowman AM, Moran TH, Mezey E, Snyder SH. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, Yang SN, Barma DK, Falck JR, Saiardi A, Barker CJ, Berggren PO. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 9.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Proc Natl Acad Sci USA. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 11.Szijgyarto Z, Garedew A, Azevedo C, Saiardi A. Science. 2011;334:802–805. doi: 10.1126/science.1211908. [DOI] [PubMed] [Google Scholar]
- 12.Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, Tokhunts R, Koldobskiy MA, Fu C, Barrow R, Wu M, Fiedler D, Barrow JC, Snyder SH. Mol Cell. 2014;54:119–132. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Prasad A, Jia Y, Chakraborty A, Li Y, Jain SK, Zhong J, Roy SG, Loison F, Mondal S, Sakai J, Blanchard C, Snyder SH, Luo HR. Nat Immunol. 2011;12:752–760. doi: 10.1038/ni.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pulloor NK, Nair S, Kostic AD, Bist P, Weaver JD, Riley AM, Tyagi R, Uchil PD, York JD, Snyder SH, Garcia-Sastre A, Potter BVL, Lin R, Shears SB, Xavier RJ, Krishnan MN. PLoS Pathog. 2014;10:e1003981. doi: 10.1371/journal.ppat.1003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Choi K, Mollapour E, Choi JH, Shears SB. Mol Pharmacol. 2008;74:527–536. doi: 10.1124/mol.107.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Choi K, Mollapour E, Shears SB. Cell Signalling. 2005;17:1533–1541. doi: 10.1016/j.cellsig.2005.03.021. [DOI] [PubMed] [Google Scholar]; c) Pesesse X, Choi K, Zhang T, Shears SB. J Biol Chem. 2004;279:43378–43381. doi: 10.1074/jbc.C400286200. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Falck JR, Hall TMT, Shears SB. Nat Chem Biol. 2012;8:111–116. doi: 10.1038/nchembio.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Albert C, Safrany ST, Bembenek ME, Reddy KM, Reddy K, Falck J, Brocker M, Shears SB, Mayr GW. Biochem J. 1997;327:553–560. doi: 10.1042/bj3270553. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Falck JR, Reddy KK, Ye JH, Saady M, Mioskowski C, Shears SB, Tan Z, Safrany S. J Am Chem Soc. 1995;117:12172–12175. [Google Scholar]; c) Laussmann T, Reddy KM, Reddy KK, Falck JR, Vogel G. Biochem J. 1997;322:31–33. doi: 10.1042/bj3220031. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Reddy KM, Reddy KK, Falck JR. Tetrahedron Lett. 1997;38:4951–4952. [Google Scholar]; e) Riley AM, Wang H, Weaver JD, Shears SB, Potter BVL. Chem Commun. 2012;48:11292–11294. doi: 10.1039/c2cc36044f. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Wu M, Dul BE, Trevisan AJ, Fiedler D. Chem Sci. 2013;4:405–410. doi: 10.1039/C2SC21553E. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhang H, Thompson J, Prestwich GD. Org Lett. 2009;11:1551–1554. doi: 10.1021/ol900149x. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Wu M, Chong LS, Capolicchio S, Jessen HJ, Resnick AC, Fiedler D. Angew Chem. 2014;126:7320–7325. doi: 10.1002/anie.201402905. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2014;53:7192–7197. doi: 10.1002/anie.201402905. [DOI] [PubMed] [Google Scholar]
- 17.Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, Falck JR, Shears SB, York JD, Mayr GW. J Biol Chem. 2008;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Capolicchio S, Thakor DT, Linden A, Jessen HJ. Angew Chem. 2013;125:7050–7054. doi: 10.1002/anie.201301092. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:6912–6916. doi: 10.1002/anie.201301092. [DOI] [PubMed] [Google Scholar]; b) Jessen HJ, Capolicchio S, Pavlovic I, Thakor DT. Synlett. 2014;25:1494–1498. [Google Scholar]
- 19.Jordan PA, Kayser-Bricker KJ, Miller SJ. Proc Natl Acad Sci USA. 2010;107:20620–20624. doi: 10.1073/pnas.1001111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway SJ, Gardiner J, Grove SJA, Johns MK, Lim ZY, Painter GF, Robinson DEJE, Schieber C, Thuring JW, Wong LSM, Yin MX, Burgess AW, Catimel B, Hawkins PT, Ktistakis NT, Stephens LR, Holmes AB. Org Biomol Chem. 2010;8:66–76. doi: 10.1039/b913399b. [DOI] [PubMed] [Google Scholar]
- 21.a) Cremosnik GS, Hofer A, Jessen HJ. Angew Chem. 2014;126:290–294. doi: 10.1002/anie.201306265. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2014;53:286–289. [Google Scholar]; b) Watanabe Y, Nakamura T, Mitsumoto H. Tetrahedron Lett. 1997;38:7407–7410. [Google Scholar]; c) Jessen HJ, Ahmed N, Hofer A. Org Biomol Chem. 2014;12:3526–3530. doi: 10.1039/c4ob00478g. [DOI] [PubMed] [Google Scholar]
- 22.Pisani F, Livermore T, Rose G, Chubb JR, Gaspari M, Saiardi A. PLoS One. 2014;9:e85533. doi: 10.1371/journal.pone.0085533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessen HJ, Schulz T, Balzarini J, Meier C. Angew Chem. 2008;120:8847–8850. doi: 10.1002/anie.200803100. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8719–8722. doi: 10.1002/anie.200803100. [DOI] [PubMed] [Google Scholar]
- 24.Weaver JD, Wang H, Shears SB. Biosci Rep. 2013;33:229–241. doi: 10.1042/BSR20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]


