Abstract
Collaborative efforts from the fields of biology, materials science, and engineering are leading to exciting progress in the development of nanomedicines. Since the targets of many therapeutic agents are localized in subcellular compartments, modulation of nanoparticle-cell interactions for an efficient cellular uptake through the plasma membrane, and the development of nanomedicines for precise delivery to subcellular compartments remain formidable challenges. The cellular internalization routes have a determining effect on the post-internalization fate and intracellular localization of nanoparticles. This review highlights the cellular uptake routes most relevant to the field of non-targeted nanomedicine, and presents an account of ligand targeted nanoparticles for receptor mediated cellular internalization as a strategy for modulating the cellular uptake of nanoparticles. Ligand targeted nanoparticles have been the main impetus behind the progress of nanomedicines towards the clinic. This strategy has even resulted in a remarkable development towards effective oral delivery of nanomedicines that can overcome the intestinal epithelial cellular barrier. A detailed overview of the recent developments towards subcellular targeting that is emerging as a platform for the next generation organelle specific nanomedicines is also provided. Each section of the review includes prospect, potential, and concrete expectations from the field of targeted nanomedicines and strategies to meet those expectations.
Keywords: Nanomedicine, nanoparticle cellular uptake, non-targeted and targeted nanoparticles, intracellular distribution, subcellular targeting
1. Introduction
The multidisciplinary and integrative research efforts in the field of nanomedicine have led to the development of a variety of nanoparticle-based carrier systems potentially suitable for site specific delivery of diagnostic and therapeutic agents [1]. The original foundation for the recent revolutionary leaps taken by nanomaterials for biomedical application is considered to be laid in 1960 through the famous lecture of R. Feyman “There is plenty of room at the bottom” [2]. However; the work of Paul Ehrlich, who coined the visionary idea of “magic bullets”, related to the cell-specific diagnostics and cell-targeted therapies is also of seminal importance [3,4]. The field of nanomedicine has established its ability to overcome the poor solubility, non-specific cytotoxicity, poor bioavailability, and less than optimum pharmacokinetic and pharmacodynamics associated with the cytotoxic agents employed in cancer chemotherapy. With some nanomedicines already making their way into the clinic, liposomes, polymeric nanoparticles, dendrimers, and gold nanoparticles have demonstrated remarkable potential as carrier systems in the development of nanomedicines [5–8]. The whole idea of nanomedicines, on one hand, has been augmented by the development of a wide range of nanomaterials with a high degree of control over their physical (e.g., size, surface charge, shape, mechanical strength) and chemical attributes. While, on the other hand, a better understanding of the physiopathological nature of different diseases and insight into the interaction of nanomaterials with biological systems at various levels (i.e., systemic, organ, tissue, and cell) are of paramount importance for further development and to realize bench-to-bedside translation. The recent success of nanomedicines stems from some key developments of multidisciplinary nature. The non-fouling nature of hydrophilic materials such as polyethylene glycol (PEG) and polycarboxybetaine (PCB) [9,10] against biological materials, and observation of the enhanced permeation and retention (EPR) effect are two such examples [11]. The development of hydrophilic polymers functionalization at the surface of nanoparticles imparts a stealth character against the immune system and enhances their systemic circulation [12]. The groundbreaking discovery of the EPR effect [13,14] associated with the tumor because of the abnormal and leaky microvasculature has emerged as the foundation for the passively targeted first generation of nanomedicines preferentially accumulating in tumor tissue [15, 16]. The combination of hydrophilic polymers induced long systemic circulation and the EPR effect results in the accumulation of nanoparticle based carrier systems in the tumor tissue followed by the release of therapeutic agent either in the proximity to a diseased tissue or inside the cells after internalization. The EPR effect is a scientific observation that results from many complex biological processes reviewed in ref 11, however; the therapeutic outcome based on exploiting the EPR effect can be inconsistent due mainly to the heterogeneity associated with the tumor tissues. Recently, the specific affinity of receptors to certain ligand molecules has led to the second generation of nanomedicines that are preferentially targeted to particular organs, tissues, or cells. The ligands, with specific affinity towards a particular receptor or molecule differentially expressed at the target site, are displayed at the surface of nanocarriers that results in the preferential accumulation and uptake at the site of action [1,17]. Although some concerns have been raised about poor systemic circulation, enhanced clearance by the mononuclear phagocyte system and limited tissue penetration, the new paradigm of ligand conjugated actively targeted nanocarriers have shown to improve the cellular uptake and efficacy of their payload when compared to their passively targeted counterparts [18,19]. The enhanced cellular uptake of nanoparticles at the disease site is of paramount importance because targets for many theranostic agents, against several disorders including cancer, are localized in the subcellular compartments [20]. This fact on one hand highlights the importance of a better understanding of cellular uptake mechanisms while on the other hand it has fueled recent research activities focused on the development of nanocarriers capable of subcellular and organelle level targeting, referred to as third generation of nanomedicines [21]. After giving an account of endocytic pathways relevant for the non-targeted and ligand conjugated targeted nanoparticles, we herein provide a comprehensive review on the recent developments and outline future strategies in designing nanomedicines capable of efficient intracellular trafficking and subcellular targeting.
2. Endocytic routes and non-ligand targeted nanomedicines
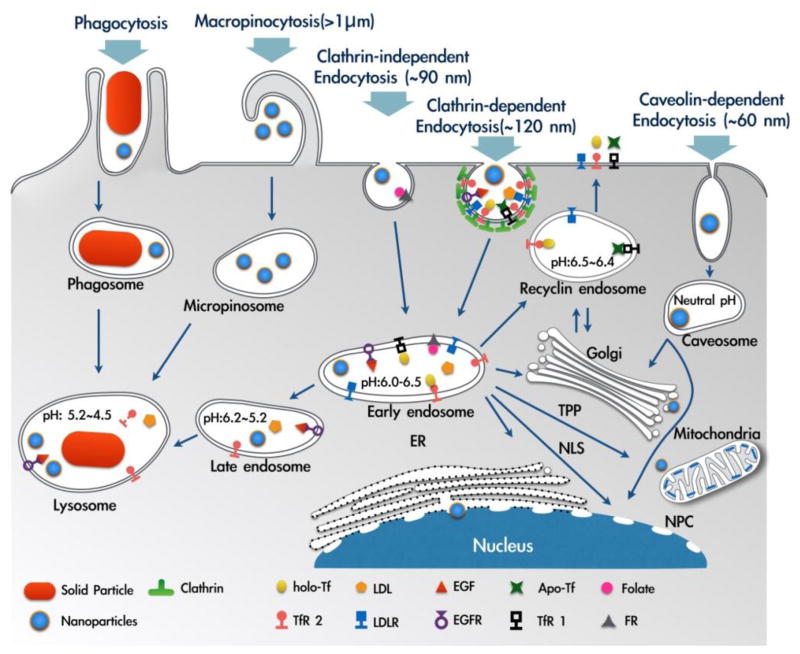
Precise release of drugs in specific organ, tissue and cells [22] has been the primary focus of nanoparticles based therapeutic strategies. The drug loaded nanoparticles have to overcome a number of transport barriers to reach the specific cells [23]. Particularly for intracellular targeting, an efficient translocation of nanoparticles across the plasma membrane barrier is a prerequisite. Plasma membrane provides an independent environment to develop the normal function of the different types of cells and presents high complexity. Plasma membrane also has a critical function in the cellular adhesion, communication, and division, and endocytosis plays a crucial role in the regulation of these critical functions of plasma membrane. The process of endocytosis involves the generation of new intracellular membrane enclosed vesicles from the plasma membrane with a concomitant internalization of lipids, proteins and extracellular fluid (Fig. 1). The phenomenon opposite to endocytosis, named as exocytosis, is the fusion of inner vesicles with the plasma membrane as a means to transporting molecules either to plasma membrane or to extracellular space [24]. The endocytic and exocytic trafficking are highly dynamic and well regulated and it has been estimated that cells can internalize up to five times their volume and membrane area in one hour [25]. Phagocytosis and pinocytosis are the two main endocytosis pathways employed by the cells. The phagocytosis route is mainly used by dendritic cells, neutrophils and macrophages [26]. The pinocytosis route is present in all types of cells and can be further subdivided as clathrin-mediated endocytosis, caveolae-mediated endocytosis, clathrin/caveolae-independent endocytosis, and macropinocytosis. An efficient uptake of nanoparticles is important for an effective intracellular drug delivery; we believe that an understanding of the biological pathways for cellular internalization of nutrients and solutes can facilitate the development of nanoparticles that can perform precise intracellular targeting with enhanced therapeutic outcomes.
Fig. 1.
Illustration of internalization pathways discussed in this article (phagocytosis, macropinocytosis, clathrin-dependent endocytosis, clathrin-independent endocytosis, and caveolin-dependent endocytosis). The fate of internalized cargo and localization to subcellular compartments are also depicted. ER: endoplasmic reticulum, NLS: nuclear localization signal, NPC: nuclear pore complex, TPP: triphenylphosphonium cation. Adapted and reproduced with permission from [90,92].
2.1 Phagocytosis
The phagocytosis is an endocytosis process exhibited by several types of cells, including epithelial cells, fibroblast, immune cells, specific phagocytic cells (monocyte, macrophages, and neutrophils), cells that generate inflammatory mediators (basophils, eosinophils, and mast cells), and natural killer cells [26]. In mammalian organisms, the phagocytosis is used to engulf the disabled particles, senescent cells, and infectious microorganisms (bacteria and virus) as a response of innate and adaptive immunity [27]. One of the main characteristics of this unique form of endocytosis is the large size of the endocytosed vesicles >250 nm, known as phagosomes [28]. The process of phagocytosis can be triggered either through the interaction of cell surface receptors with particular ligands presented by the foreign agent or through the interaction of specific cell surface receptors with soluble factors that recognize the foreign agent and facilitate phagocytosis (opsonization). The soluble factors involved in the opsonization process include proteins of the complement system, antibodies, acetylcholine, laminin, fibronectin, C-reactive protein, and type-I collagen [29]. The most important receptors that participate in phagocytosis are the Fc receptor family for IgG (FcγRI, FcγRIIA and FcγRIIA), the complement receptors (CR1, CR3 and CR4), and the α5β1 integrin [30]. A great deal of scientific efforts has been focused on controlling the nanoparticles internalization via phagocytosis. The cellular internalization of nanoparticles via phagocytosis in macrophages involves attractive forces (i.e., van der Waals, electrostatic, ionic, hydrophobic/hydrophilic) between the cells and nanoparticles surfaces. In addition, the phagocytosis of nanoparticles can also be triggered by the receptor mediated recognition of opsonins adsorbed on the surface of nanoparticles. Mitragotri and coworkers [31,32] have revealed that the particle geometry can help in modulating their cellular internalization via phagocytosis. Different local particle shapes at the point of cell attachment generates different angles between the membrane and particle. This contact angle has a significant effect on the ability of macrophages to internalize particles via actin-driven movement of the macrophage membrane. Six distinct shapes of nanoparticles investigated the Mitragotri and coworkers include spheres (radius 1.0–12.5 μm), oblate ellipsoids (major axis 4 μm, aspect ratio 4), prolate ellipsoids (major axis 2–6 μm, aspect ratio 1.3–3), elliptical disks (major axis 3–14 μm, aspect ratio 2–4, thickness 400–1000 nm), rectangular disks (major axis 4–8 μm, aspect ratio 1.5–4.5), and UFOs (sphere radius 1.5 μm, ring radius 4 μm). The authors demonstrated that elongated particles with higher aspect ratio are less prone to the phagocytosis. Geng et al. [33] have also reported a similar finding. Interestingly, a higher aspect ratio has also recently been implicated with a preferential localization into endosomes and lysosomes [34], hence, care should be taken while exploiting the shape of particles for modulating the phagocytosis and intracellular targeting simultaneously.
2.2. Pinocytosis
2.2.1. Clathrin-mediated endocytosis
Clathrin-mediated endocytosis (CME) is the most studied process for trafficking of materials into the eukaryotic cells. CME is a complex pathway that involves intercellular signaling, membrane recycling, and uptake of nutrients [35]. The vesicle formation starts with the participation of extensive protein machinery to induce a curvature in the membrane (e.g., epsin [36], amphiphysin [BAR domain, Bin/amphiphysin/Rvs] [37], endophilin [38], and the FCHo2 F-BAR domain [39]), adaptor protein complexes (e.g., the AP-2 heterotetrameric complex [a-b2-m2-s2] [40], AP180 [41]), and clathrin assembly lymphoid myeloid leukemia [CALM] protein [42]), which are essential for the formation of the spherical clathrin-coated pit [43]. The release of a vesicle from the plasma membrane occurs by the activity of GTPase dynamin, a protein that is assembled as a ring around the neck of a newly formed invagination [44,45]. When the coated-vesicles are released, the clathrin pit is disassembled by the action of auxilin and heat shock cognate 70 (HSC70)-dependent proteins [46]. After internalization through CME, the uncoated-vesicles are either guided to early endosomes, or recycled to the plasma membrane surface. The vesicles can also be targeted to more mature endosomes and later to compartments such as lysosomes and multivesicular bodies. Majority of the receptor mediated cellular uptake of nanoparticles occur through CME. Particular examples are provided in the later sections dealing with the ligand targeted nanoparticles. In case of non-targeted nanoparticles, the uptake route depends on their physical attributes including particle size, shape and surface charge, and also on the type of cell line. The cationic nanoparticles of ~100 nm size derived from the polylactide-co-polyethylene glycol (PLA-PEG) have been found to internalize exclusively via CME [47]. The poly(L-lysine), which is a cationic polymer, functionalized at the surface of poly(lactide-co-glycolide) (PLGA) nanoparticles have also been found to significantly enhance the cellular uptake via CME [48]. Another study reported mesoporous silica nanoparticles (~110 nm) labeled with fluorescein isothiocyanate showing efficient internalization into human mesenchymal stem cells (hMSCs) and adipocytes (3T3-L1) predominantly via CME [49]. Although the underlying mechanisms that mediate the internalization of nontargeted nanoparticles are not fully understood, it seems that the high rate of cellular internalization via CME under normal cell activity has resulted in many studies concluding the CME as a main route of internalization for nanoparticles of size ~100 nm. The positively charged nanoparticles of ~100 nm diameter have been shown to internalize predominantly through CME, which can be a result of increased interaction between the positively charged nanoparticles and negatively charged cell surface that enhances the nanoparticles internalization via CME simply because the CME is the most probable internalization route for nanoparticles of ~100 nm diameter. To the best of our knowledge, there is no systematic study on ~100 nm size nanoparticles investigating the effect of nanoparticles shape on the cellular internalization route. All the reported literature in this size context deals with the nanoparticles of spherical shape.
2.2.2. Clathrin-independent endocytosis
Clathrin-independent endocytosis (CIE) pathway was initially described as a mode of entry for a number of bacterial toxins and cell surface proteins, and recently was proposed in the plasma membrane repair, cellular polarization, cellular spreading, and modulation of intercellular signaling [50]. CIE does not require the presence of coat proteins for the vesicle formation and internalization; however, the actin and actin-associated proteins are important players for the vesicle formation during CIE [51]. The CIE involves different subtypes of pathways that include the participation of proteins such as Arf-6, RhoA, and Cdc42 [52]. Studies have shown that Arf-6-dependent CIE participates in the endocytosis of the major histocompatibility complex (MHC) class I [53], the β-integrins [54], the glucose transporter GLUT1, and other proteins that are involved in amino acid uptake and cell-extracellular matrix interactions [55]. In addition, RhoA and Cdc42 endocytosis are dependent on the lipid rafts for vesicle formation. RhoA is a dynamin-dependent pathway that has been described in the internalization of the β-chain of the interleukin-2 receptor (IL-2R-β) and other proteins in both immune cells and fibroblasts [56]. In contrast, the Cdc42 is a dynamin-independent pathway described as a principal route for the uptake of cholera toxin B (CtxB) and the Helicobacter pylori vacuolating toxin (VacA) [57,58]. The cargos entering the cell through CIE are usually delivered to the early endosomes, followed by the transfer to late endosomes and lysosomes. In addition, the cargo can be routed to the trans-Golgi network or recycled back to the plasma membrane [59]. CIE is the internalization route described preferentially for polyplexes of self-branched and trisaccharide-substituted chitosan oligomers nanoparticles (SBTCO) for the delivery of DNA [60], and for cowpea mosaic virus (CPMV), which has been extensively studied in the last years as a strategy for vaccine development, in vivo vascular imaging, and tissue-targeted delivery [61]. Recent studies suggest that CIE is involved in a new mechanism for the uptake of nanoparticles that was described as a type of macropinocytosis. This new mechanism was found to be dependent on the actin filaments and dynamin, and was designated as excavator shovel like mechanism [62]. In another study, Garaiova et al. [63] showed that the nanoparticles derived from trisaccharide-substituted chitosan oligomers (SBTCO) generated a higher uptake and better transfection efficacy than the nanoparticles prepared from a linear chitosan (LCO). SBTCO were primarily taken up by the cells via CIE, and successfully escaped from the endocytic vesicles. In contrast, LCO suspension in the cell culture medium resulted in the nanoparticles aggregation and a relatively lower extent of cellular internalization was observed when compared to the SBTCO nanoparticles.
2.2.3. Caveolae
Caveolae are the flask-shaped invaginations (60–80 nm) of plasma membrane that participate in different cellular processes including cholesterol homeostasis, endocytosis of proteins, and signal transduction [64]. Caveolae are abundant in several types of cells, such as fibroblasts, smooth muscle, adipocytes and endothelial cells, and are absent in neurons and leukocytes. Interestingly, in case of adipocytes caveolae can occupy as much as ~50% of plasma membrane [65], and the percentage of caveolae can be as high as ~70% of plasma membrane as in case of the endothelial cells in blood capillaries [66]. Initial studies have revealed the caveolin (CAV1, CAV2 and CAV3) as the main protein constituent of caveolae with an estimate of about 140–150 of CAV1 protein molecules per caveolae [67]. Cavins, the coat proteins (cavin 1–4), are known to work together with caveolins to regulate the formation of caveolae, and also potentially participate in the signals that regulate the caveolae fate [68]. The intracellular destinations of caveolae have been a subject of controversy for many years. Nevertheless, it has emerged that in endothelial cells caveolae are able to perform transendothelial transport, which may be utilized for the release of nanoparticles in the subendothelial tissues [69]. The material that is endocytosed via caveolin-mediated pathway is initially localized into caveosomes. The neutral pH of caveosomes can be considered as a means to avoid the hydrolytic environment of lysosomes. The sorting of caveosomes cargo to the Golgi apparatus and endoplasmic reticulum may also be exploited for the targeted delivery of theranostic agents to these subcellular compartments. The negative surface charge has been found to trigger the cellular internalization predominantly via caveolae [91]. Liu et al. have employed the rabies virus glycoprotein RVG29 (29-amino-acid peptide) as a targeting moiety for DNA conjugated poly(amido amine) (PAMAM) dendrimer, exhibiting significant accumulation of carrier in the brain of mice after intravenously administration. The cellular internalization mechanism of PAMAM–RVG29 in the brain capillary endothelial cells was found to be through a combination of clathrin and caveolae mediated energy-dependent endocytosis that involved an interaction with GABAB receptor [70]. Interestingly, previous studies about the mechanism of rabies virus glycoprotein crossing the blood brain barrier (BBB) and cellular internalization have revealed a specific interaction with the nicotinic acetylcholine receptor (AchR) [71]. This study suggests that RVG29 conjugated nanoparticles may be employed for crossing the BBB and delivery of drugs to brain.
2.2.4. Macropinocytosis
Macropinocytosis is an actin-driven endocytic process by which cells internalize considerable volumes of extracellular fluid through large vesicles (diameter of 0.5–10 μm) known as macropinosomes [72]. Macropinocytosis is a typical route for the uptake of apoptotic cell fragments [73], viruses [74] and bacteria [75], and contributes substantially to the antigen presentation in major histocompatibility complex II (MHCII) [76, 77]. Unlike the receptor-mediated endocytosis and phagocytosis, the activation of macropinocytosis is not regulated by the direct action of a receptor or the cargo molecules. In this case, the activation of tyrosine kinase receptor such as the epidermal growth factor and the platelet-derived growth factor receptor leads to an increment of the actin polymerization, actin-mediated ruffling, and macropinosome formation [78]. Interestingly, macropinosomes share some proteins (Cdc42, Arf6 and Rab5) with others endocytosis processes, suggesting a relationship between the mechanisms of macropinosomes biogenesis and other endocytosis routes [79, 80]. The macropinosomes are sensitive to cytoplasmic pH and undergo acidification and fusion events [81]. In macrophages, the macropinosomes present a fate similar to endosomes, and during their maturation gain and loss markers that are typical for early and late endosome before their fusion with lysosomes [82]. Micron size particles are generally known to internalize the cells via macropinocytosis [83], however; most of the literature reports highlight that the nanoparticles undergo cellular internalization via more than one endocytic pathway. Recently, Zhang et al. [84] reported lapatinib loaded nanoparticles formulated with a core of albumin and a lipid corona formed by the egg yolk lecithin. The nanoparticles exhibited ~62 nm and a zeta potential of 22.80 mV and were demonstrated to internalize the BT-474 cells through energy-dependent endocytosis involving clathrin-dependent pinocytosis and macropinocytosis.
2.3. Differential uptake of nutrients in cancer cells-A novel gateway for nanomedicines
The essential nutrients, such as lipids, free forms of fat-soluble vitamins and carotenoids, transport across the plama membrane by simple diffusion [85]. The transport of some nutrients such as glucose or lipoproteins is actively controlled by the endocytosis pathways described earlier in this section [86,87]. The uptake of nutrients in normal cells is a well regulated process, however; abnormally high cell proliferation rate for example in case of cancer progression concominantly triggers a higher rate of nutrients uptake. Several studies support that the actively proliferating cells could adapt their endocytosis mechanisms to meet the enhanced nutrients requirement. Recently, a study on human melanoma cells revealed a significantly increased expression of the aminoacid transporter for leucine (LAT1) and glutamine (ASCT2) [88]. Another study on pancreatic tumor xenograft model suggests that the macropinocytosis is an important route for nutrient uptake in tumor cells [89]. This study reveals the enhanced rate of albumin uptake for meeting a higher glutamine demand of cancer cells (MIA PaCa-2 cells) as a functional contribution of the macropinocytosis stimulated by the oncogenic Ras proteins. These hyperactive endocytic mechanisms have not been explored in context of nanomedicine and present a potential strategy for enhancing the uptake of therapeutic nanoparticles in cancer cells. For any future development in this context, an indepth understanding of the differential physiopathological processes associated with the diseased and normal cells is a prerequisite to enhance the relevance of differential nutrient uptake phenomena as a gateway for cellular internalization of nanomedicines.
Keeping in view the fact that size, geometry, surface charge and mechanical properties of nanoparticles can influence the cellular uptake efficiency, several studies have been focused on understanding the optimal properties of nanoparticles to accomplish an effective cellular uptake and consequently an enhanced pharmacological effect. These details are comprehensively covered in the following excellent review articles [11,90,91]. In addition to tuning the physical characteristics, receptor mediated cellular internalization has also been extensively employed for achieving an enhanced cellular uptake of nanomaterials, and examples relevant to the field of nanomedicine are reviewed in the following section.
3. Receptor-mediated cellular internalization of ligand targeted nanomedicines
The receptors overexpressed on the surface of target disease cells have been widely explored to improve the cellular uptake of nanoparticles as well as to minimize the off-site toxicity concerns. Currently, several receptors are known for active disease cell targeting; the representative examples include folate receptor (FR), transferrin receptor (TfR), epidermal growth factor receptor (EGFR), G-protein coupled receptor (GPCR), low-density lipoprotein receptor (LDLR), and lectins [92]. The receptor mediated cellular internalization of nanoparticles has been studied for the intracellular delivery of various cargos such as small molecule drugs, DNA, siRNA, and miRNA. Different types of high affinity ligands, the moieties that specifically bind to a particular receptor, have been functionalized at the surface of nanoparticles in order to augment their interaction with cells, cellular uptake, and subsequent internalization. The major types of targeting ligands include high affinity small molecules, peptides, aptamers and antibodies. In this section, the recent developments related to the targeting strategies using ligand-conjugated nanoparticles will be addressed highlighting the receptors (FR, TfR, EGFR, PMSA, Integrin, FcRn) that have demonstrated potential for augmenting the field of nanomedicine.
3.1. Folate receptor (FR) targeting
The folate receptor (FR) is overexpressed in a variety of frequent tumor types (e.g., ovarian, lung, brain, and colorectal cancer) [93]. FR is a glycoprotein with molecular weights of 38–44 kDa and exists in isoforms FR-α and FR-β. The FR-α is expressed in various epithelial cancer types (e.g., ovarian 90%, endometrial 90%, brain 90%, and renal carcinomas 75%) as well as on some normal epithelial membranes such as kidneys [94]. In contrast, the FR-β is found on activated macrophages [95] and on the surface of hematopoietic malignancies such as chronic myelogenous leukemia [96]. For targeting of chronic inflammatory diseases such as type 2 diabetes, atherosclerosis, and rheumatoid arthritis, the FR-β can prove to be an important target. Folic acid (441 Da) shows a high affinity (KD = 10−9 M) to the FR, which allows the selective delivery of folate-conjugated nanocarriers to the FR-expressing disease cells [97]. The use of small molecules like folic acid ligand compared with the peptides and antibodies as targeting ligands can provide several benefits. The main advantages include an easy scale-up for clinical applications, facile chemical modification, no toxicity and immune reactions due to its function as a vitamin, and the high stability in acidic or basic media and at high temperature.
The concept and efficacy of folate targeting systems have been extensively studied in vitro and in vivo using folate-conjugated nanoparticles, chemotherapeutics, liposomes, and oligonucleotides [98]. The PEGylated liposomal doxorubicin (DOX) containing folate-PEG-distearoyl-phophatidyl-ethanolamine showed higher therapeutic activity than that of non-targeted one in the KB (human nasopharyngeal epidermoid carcinoma cell line) and KB-V (vincristine-resistant derivative) xenograft model and in the J6456 intra-cavitary therapy model, both of which overexpress the FR [99]. Jiang et al. developed folate-conjugated human serum albumin nanoparticle loaded with docetaxel and investigated the antitumor activity of the nanoparticles in human hepatoma cell line and in an in vivo model [100]. The folate-conjugated nanoparticles were found to show superior antitumor activity based on in vivo inhibition ratios. Chemotherapeutic drugs loaded folate functionalized polymer derived nanoparticles have been reported to exhibit significantly higher in vivo efficacy as compared to the non-targeted nanoparticle therapeutics as evaluated in an ovarian peritoneal metastasis model [101]. High affinity of folate moieties is also of interest for use in the fields of immunotherapy as well as leukemia cells. Lu et al. were able to demonstrate that a treatment with folate-conjugated hapten resulted in more immunogenic tumor cells and finally the enhanced anticancer immune reaction against hapten-treated tumor cells [102]. For targeting the acute myelogenous leukemia (AML) blast cells with overexpressed FR-β (70%), the folate conjugated liposomal DOX resulted in a stronger inhibition of colony formation than that of the non-targeted analog in MV4-11 (human acute myelocytic leukemia) and K562 (human erythromyeloblastoid leukemia) cells and AML patient cells as well [103]. These studies suggest the efficacy and the potency of folate targeting in pre-clinical as well as clinical application.
3.2. Transferrin receptor (TfR) targeting
The transferrin receptor (TfR), which is a cell membrane glycoprotein with a homodimer of two identical transmembrane subunits, can mediate cellular uptake of iron from a plasma glycoprotein (i.e., transferrin) [104]. The two subunits of TfR are each of 84,910 Da and have 760 amino acid residues, especially three N-linked glycosylation sites and an O-linked glycosylation site, which are necessary for normal function of the receptor [105,106]. The main function of TfR is to regulate the cellular uptake of iron from transferrin (~80 kDa) and cell growth [107], and TfR is probably expressed on all cells and the level of TfR expression varies among different cells. A high expression of TfR is known for immature erythroid, placental tissue, rapidly dividing cells, and its expression is about 100-fold higher in cancer cells than the regular expression of normal cells [108], which makes TfR as one of the most attractive targets for cancer therapy by receptor-mediated endocytosis of drug nanoparticles. Transferrin (Tf) and TfR binding single chain antibody fragment (TfRscFv) have been used as ligands for transferrin receptor-mediated intracellular delivery of nanotherapeutics. The examples of TfR targeted nanomedicines that are at various stages of clinical trials include CALAA-01 (a four component nanomedicine in phase I clinical trial constituted by Tf-functionalized PEG, Tf and adamentane functionalized PEG, SiRNA and a cyclodextrin bearing polymer), MBP-426 (liposome based oxaliplatin loaded Tf conjugated nanomedicine in Phase II clinical trial), SGT-53 (intracellular delivery of p53 plasmid DNA using TfRscFv conjugated liposome based nanomedicine in Phase Ib clinical trial), and SGT-94 (RB94 plasmid DNA delivery using TfRscFv conjugated liposome in Phase I clinical trial) [109]. These successful examples have triggered substantial interest in the further development of TfR targeted nanomedincine. Choi et al. reported that PEGylated gold nanoparticles with different amounts of human transferrin for active targeting leading to an efficient nanoparticle internalization into TfR over expressing cancer cells (Neuro2A cells) as well as in the tumor site in Neuro2A tumor-bearing mice, suggesting greater intracellular delivery of therapeutic agents to the target cancer cells [110]. For effective treatment of brain glioma cells, the transferrin-conjugated magnetic silica PLGA nanoparticles containing anticancer drugs (DOX and/or paclitaxel) were evaluated in intracranial U-87 MG-luc 2 xenograft mice. The targeted nanoparticles resulted in an enhanced anti-glioma activity compared to the non-targeted nanoparticles [111]. The transferrin receptor mediated internalization of the anticancer drugs into the brain tumor has been associated with the highly overexpressed TfR in brain capillary endothelium and glioma cells. In addition, Hong et al. showed that the endocytosis process of Tf-PEG-niosomes in KB cells could be inhibited by low temperature and by the free Tf, indicating the high specificity towards TfR [112]. As expected, the hydroxycamptothecin (HCPT)-loaded Tf-PEG-niosomes resulted in the strongest cytotoxicity to three carcinomatous cell lines (KB, K562, and S180 cells) and significantly stronger inhibition (71%) of tumor growth in S180 tumor-bearing mice compared to the non-targeted PEG-niosomes. Overall, these studies represent the efficacy of Tf targeting using various nanoparticles (e.g., metal, polymer, and liposome-based carriers) in vitro and in vivo. A recent report from Salvati et al. [113] is of worth mentioning that reports disappearance of targeting ability of Tf functionalized nanoparticles in the biological media. The protein constituents of the biological media were implicated for neutralization of TfR targeting of Tf ligands that were covalently localized at the surface of nanoparticles through a PEG linker. This finding is of paramount importance necessitating the evaluation of any potential targeting nanomedicine in the biological medium rather than in buffer saline. It further highlights the importance of evaluation of optimum ligand density to achieve a balance between desired systemic circulation and targeted cellular uptake.
3.3. Epidermal growth factor receptor (EGFR) targeting
The epidermal growth factor receptor (EGFR, also known as HER1) is a member of the ErbB tyrosine kinase family including HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4) [114,115]. The EGFR, which is overexpressed in various solid tumors including colorectal, brain, breast, ovarian, pancreatic, and prostate cancers, can stimulate tumor growth, invasion, and metastasis [116]. The small molecules and monoclonal antibodies have been used as EGFR-targeting ligands; for examples, epidermal growth factor (EGF), transforming growth factor-α (TGF-α), heparin binding EGF-like growth factor, epigen, betacelluin, and epiregulin [117]. Upon binding of ligands to EGFRs, the homodimerization or heterodimerization of the monomeric EGFR occurs with other members of the ErbB family receptors and other cell surface tyrosine kinases for the cellular signaling.
Anti-EGFR ILs-DOX (Fab′ fragments of anti-EGFR antibody ceuximab conjugated DOX loaded immunoliposomes targeted to EGFR in Phase I clinical trial) represents a notable example of EGFR targeted nanomedicines [109]. In a recent attempt of targeting HER1, Shevtsov et al. developed the superparamagnetic iron oxide nanoparticles conjugated with recombinant human epidermal growth factor (SPION-EGF) for the enhanced magnetic resonance imaging of malignant brain tumors and demonstrated more efficient tumor imaging than that of non-targeted SPION in an orthotopic model of C6 gliomas [118]. In addition, the use of cisplatin-encapsulated gelatin nanoparticles with EGF improved in vitro and in vivo targeting ability and anticancer effect in A549 cells and tumor-bearing mice model (high EGFR expression) compared to HFL1 cells (low EGFR expression) [119]. For HER2 (14–91% of patients with breast cancer) targeting, the monoclonal antibody trastuzumab (also known as Herceptin) is now widely used for patients with HER2-positive breast cancers [120]. In pre-clinical studies, Lee et al. demonstrated that the DOX-loaded PEG-PLGA-Au half-shell nanoparticles with Herceptin resulted in higher accumulation in tumor sites of SK-BR-3 (breast cancer cell line with high HER2 expression) bearing mice by receptor-mediated endocytosis and finally stronger therapeutic efficacy based on chemotherapy and hyperthermia compared to non-targeted nanoparticles [121]. MM302 (HER2 targeted scFv antibody fragment conjugated DOX loaded liposome) represents an example of HER2 targeted nanomedicine in Phase I clinical trial [109]. Currently, HER3, which is the only member of EGFR family lacking intrinsic tyrosine kinase activity, has gained significant interest for targeting of EGFR [122]. The fully humanized HER3 antibody U3-1287 (AMG 888) showed a reduced growth of cancer cells and improved tumor suppression in xenografted human HNSCC FaDu model. Therefore, based on these research results, the cellular targeting strategies by receptor-mediated endocytosis will potentially impact in clinical application to treat various diseases, specifically cancers.
3.4. Prostate-specific membrane antigen (PSMA) targeting
A type 2 integral membrane glycoporotein PSMA is a valid cancer target overexpressed on the surface of prostate carcinomas and the neovasculature of majority of the solid tumors [123,124]. In this regard, a notable example is represented by the BIND-014, docetaxel leaded polymer (PLA-PEG and PLGA-PEG) based nanomedicine in Phase II clinical trial, designed for targeted delivery by employing a small molecule ligand (S,S-2-[3-[5-amino-1-carboxypentyl]-ureido]-pentanedioic acid, ACUPA) against PSMA [18]. A combinatorial approach, screening about 100 formations, was employed to achieve the effective nanomedicine attributes including particle size, targeting ligand density, surface hydrophilicity, drug loading, systemic circulation and drug release properties (Fig. 2). This thorough optimization resulted in a highly effective PSMA-positive prostate cancer targeted nanomedicine with remarkable results of tumor shrinkage in human at doses below the typical clinical doses of solvent based docetaxel formulation (Fig. 2).
Fig. 2.
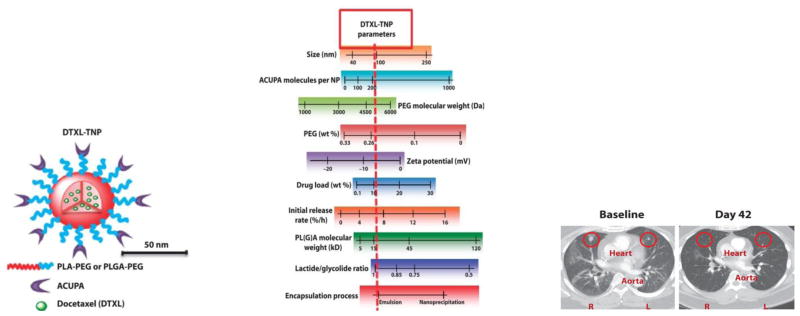
(A) Schematic illustration of PSMA targeted BIND-014 docetaxel loaded polymeric nanoparticles. (B) Depiction of achievable nanomedicine attribtutes, red line represents the optimized properties. (C) CT scan evidencing a remarkable regression of lung metastases in 51 year male patient treated with two cycles of BIND-014. Reproduced with permission from [18].
3.5. Integrins targeting
Integrins represent a family of heterodimers transmembrane receptors that are involved in a number of vital cellular functions (adhesion, migration, invasion, stress responses, proliferation, differentiation, survival, and apoptosis) through modulating the endothelial cells-extracellular matrix interactions and regulation of intracellular signaling [92]. Among >24 surface receptors, derived from the dimerization of about 18α and 8β subunits, αvβ3 receptors are differentially overexpressed in tumor related endothelial cells during angiogenesis compared to the endothelial cells in normal tissues [125–128]. Various strategies have been employed to develop high affinity ligands for αvβ3 receptors targeted nanomedicines where cyclic-RGD peptides have emerged as the most promising targeting ligand [92,129–131]. A recent report from Graf et al. [132] employed a Pt(IV) prodrug loaded PLGA-PEG nanoparticles functionalized with cyclic-RGD for αvβ3 receptors targeted delivery. The encapsulated drug was found to be more efficacious with higher tolerance when compared to cisplatin in in vivo orthotopic human breast cancer xenograft model. There are no examples of liposome or polymer based integrins targeted nanomedicines in a clinical trial phase.
3.6. Neonatal Fc-receptor (FcRn) targeting-An avenue to oral delivery of nanomedicine
Nanomedicine administration is currently limited to the parenteral routes, however; for convenience and patient compliance oral route of administration is of particular interest, especially against the diseases that require frequent administration [133]. Oral route of administration suffers from a poor intestinal absorption of nanomaterials because of their inability to cross the intestinal epithelium cellular barrier [134]. It has been reported that FcRn in neonatal intestine is responsible for safe transport of breast milk immunoglobulin (IgG) to offspring, highlighting a gateway to overcome intestinal epithelium cellular barrier [135]. In adults the FcRn receptor expression level in apical region of epithelial cells of small intestine is equivalent to the neonatal expression. Interestingly, the affinity between Fc region of IgG and FcRn is pH dependent, high binding affinity at acidic pH (<6.5) as compared to the physiological pH (~7.4). Capitalizing on these facts, Pridgen et al. [136] have reported a ground breaking proof of concept study demonstrating unprecedented potential of FcRn targeting as an avenue to oral delivery of nanomedicine. PLA-PEG polymer nanoparticles surface functionalized with polyclonal IgG Fc fragment (NP-Fc) employed in this study exhibited transepithelial transport both in vitro and in vivo. Oral administration of fluorescently labeled NP-Fc in fasted wild-type mice resulted in observation of fluorescence revealing the ability of Fc functionalized nanoparticles to overcome the intestinal epithelial barrier and entering into the lamina propria (Fig. 3). The efficiency of absorption for targeted nanoparticles was 11.5 times higher than the nontargeted particles. The particles were also observed to localize in other organs, which shows that they were able to enter systemic circulation (Fig. 3). Furthermore, oral administration of insulin loaded NP-Fc in fasted wild-type mice resulted in a hypoglycemic response. A control experiment with the FcRn knockout mice did not result in a hypoglycemic response, which established that the superior outcome is because of FcRn targeting. Although this novel avenue is still at its infancy but the initial results are promising enough to expect an impact on future development of nanomedicines that can be administered orally.
Fig. 3.
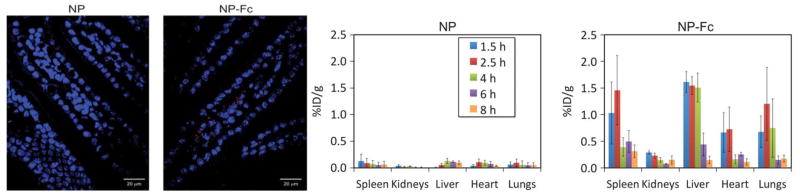
FcRn targeted nanoparticles can be seen as red puncta in the confocal fluorescence images of sections of mouse duodenum (left panel). Comparison of organ localization of FcRn targeted and non-targeted PLA-PEG nanoparticles (right panel). Reproduced with permission from [136].
4. Intracellular trafficking and subcellular targeting
4.1 From endosomes/lysosomes to cytoplasm
Ligand conjugated nanoparticles with an ability of specific tissue or cellular level targeting has already been successfully demonstrated, and this novel paradigm goes in parallel with the EPR effect for achieving an increased intratumoral concentration of the cytotoxic anticancer drugs. Many interesting therapeutic targets are localized in the intracellular compartments. This has triggered increasing efforts focused on developing sophisticated nanoparticles designs with an ability to precisely navigate across the physiological barriers and selectively deliver the therapeutic and diagnostic agents to the intracellular targets [137]. Despite different cellular entry pathways, endosomes are the first intracellular compartments encountered by the internalized nanoparticles. Early endosomes are characterized by a lower luminal pH (~6–6.5, necessary for the activity of endosome-specific enzymes) that has been exploited to trigger the release of pH responsive nanoparticles into the cytosol. Polyamines based carrier systems can efficiently impart neutralization effect after being protonated under the acidic conditions of endosome. This neutralization leads to an increase in endosomal pH that triggers the ionic transport into the lumen of endosome resulting in swelling and possible release of endocytosed nanoparticles (proton sponge effect) [138]. The choice of material to induce proton sponge effect has been limited to polyamines, however; these materials suffer from the poor biocompatibility and high toxicity inherent with the high pKa (~9) of amine groups that can induce membrane lysis at acidic as well as physiological pH. The protection of amine groups by employing acid sensitive protecting groups that cleave and expose the amino groups only under the endosomal pH to trigger the endosomal release, and incorporation of low pKa heterocycles as pendant groups have been reported to reduce the toxicity of the polymer system [139]. A timely response to the pH is very crucial as failing to respond efficiently can delay the release resulting in either transfer of endocytosed nanoparticles to the hydrolytic enzymes containing lysosomes for degradation or directed to the plasma membrane via recycling route.
The intracellular spatiotemporally controlled trafficking and the fate of endocytosed cargo are regulated by RAB proteins (one of the five major subfamilies of the superfamily G-proteins) in combination with a number of effectors or regulatory molecules. For cancer progression, an increased activity of the RAB proteins has been implicated with an increased rate of growth factor receptors recycling to the plasma membrane. The therapeutic intervention to modulate RAB activity provides interesting and rather less explored potential targets [140,141].
A well regulated endocytosis and endosomal trafficking is of paramount importance as an improper functioning of endosomal trafficking has been linked to the neurodegenerative diseases (e.g., Alzheimer’s disease, Huntington’s disease, autism) [142–145]. Na-H exchangers (NHEs) is a family of nine Na+/H+ exchanger isoforms found in mammals. NHE6 has been found in endosomes from a number of different cell types and is associated with the regulation of lumen pH in early, late, recycling endosomes, and lysosomes, where pH decreases to 5 [146,147]. With the maturity of endosome a spatiotemporal control of the lumen pH is essential to control the degradation rate and the recycling time of the internalized material (dissociation of ligand-receptor conjugates and recycling of the receptors to the plasma membrane). Some NHEs (NHE1-NHE5) are localized in plasma membrane and play a crucial role in initiating and development of cancer [148]. Interestingly, all the malignant tumors have a reversed hydrogen gradient as compared to the normal tissues. The intracellular pH of the tumor cells is alkaline (7.12–7.7) whereas the pH in the extracellular environment is acidic (6.2–6.9) [149, 150]. Maintenance of such a distinct hydrogen ion dynamic has been attributed to the abnormally high activity of NHE1, which is essential for the survival of cancer cells under hostile extracellular environment. The dysfunctional pH control system has also been associated with the development of multidrug resistance. While maintaining an alkaline cytoplasmic pH, NHEs play an important role in maintaining a highly acidic pH in the lumens of intracellular organelles. The NHE1 inhibitors are being employed as selective tumor drugs [34, 151, 152]. The field of nanomedicine is yet to contribute in this context; it is of paramount importance that these attributes should be considered while developing nanoparticles for cell membrane, endosome and lysosome targeting.
Lysosomes are considered as digesting or recycling components of the cell machinery because of the presence of capethesins proteases. The resulting degradation products from the enzymatic activity on the cargo materials are released in the cytoplasm to meet the nutritional need of the cell. The tumor cells exhibit higher activity of lysosomal capethesin and its release in the extracellular environment has been implicated with the promotion of tumor growth. The concept of lysosomotropic was introduced by Christian de Duve who defined the lysosomes with their hydrolytic enzymes as suicide bags [153]. Since then the destabilization of lysosomes via lysosomal membrane permeabilization (LMP) and release of its hydrolytic contents in the cytoplasm as a means of triggering cell death has been highlighted as potential targets for therapy. Multidrug resistance acquired by the cancer even at the early stage of its development makes the common LMP triggering stimuli irrelevant. Consequently, more potent lipophilic amine based cationic amphiphiles are being employed as lysosomotropic detergents. Recently, inhibiting the role of acid sphingomyelinase (ASM) in supporting the lysosomal membrane integrity was exploited to induce the LMP using cationic amphiphilic drugs (CADs). For the several cancer cell types tested, CADs killed cells at concentrations much lower than the concentrations that induce cytotoxicity in the normal cells [154–156].
In case the endosome or lysosome is not the final therapeutic target, it is necessary to devise a strategy for endosomal release and for the protection of administered drug from degradation under hostile environment of lysosomes. This has been achieved by encapsulating drugs into a variety of nanoparticle based carrier systems. These nanoparticles are not only required to protect the sensitive drug molecules from degradation but also needed to release the drug in a time dependent manner [157]. A delayed release may expose the drug to the aggressive endosomal or lysosomal environment (low pH and hydrolytic enzymes) leading to the loss of any therapeutic impact. Polymer nanoparticles and liposomes are being widely explored as biocompatible, bioresponsive and biodegradable nanocarrier systems. Various strategies have been explored to affect the release of a cargo from endosome into the cytoplasm. These include the use of cell penetrating peptides, stimuli responsive polymers (endoosmolytic mechanism to induce the endosomal disruption and release by exploiting differential redox or pH environment) or use of fusogenic liposomes (fusogenic mechanism: emptying the particle in cytoplasm during the fusion of liposomal particles with the endosomal membrane) [158]. Zheng et al. have reported pH responsive nanoparticles derived from PEGylated polyphosphazene laterally functionalized with N,N-diisopropylethylenediamine (DPA) [159]. The DPA units imparted pH triggered release property to the resulting nanoparticles as demonstrated by loading and release of DND-26, a fluorescent dye that preferentially accumulates in acidic compartments in a cell. DND-26 was found to be distributed in the whole cell in contrast to its accumulation in the endosome and lysosome when cells were exposed to the free DND-26. DPA functional group is of particular interest because of its distinct pKa value (6.3) as compared to the pKa (7.4) of N,N-dimehtylethyleneamine. A judicial choice of such functionalities can offer modulation of payload release from nanoparticles at a particular pH [160]. Another pH responsive nanoparticle platform, derived from a block copolymer of PEG and polyamide where the polyamide block is laterally functionalized with citraconic amide and succinic amide, was reported by Lee et al. [161]. The interesting feature of this platform is the degradation of the citraconic amide at the endosomal pH leading to the side chain degradation resulting in the polymer charge reversal and consequently destabilization of nanoparticles and release of loaded lysozyme. Despite of several reports on stimuli responsive materials, there is hardly any nanoparticles platform at the clinical stage, which highlights a need to further bridge the gap between different disciplines actively working on the development of materials for biomedical applications. Binauld and Stenzel [162] have comprehensively reviewed the polymeric materials that can degrade under acidic pH, which should be of help for designing clinically relevant polymers.
Cytoplasm is home to a wide range of biological processes (signaling, metabolic and pathogenic) that have been demonstrated to be the therapeutic targets for many diseases. Access for nanomedicine to the cytoplasm has mainly been through the endosomes; however, some cell penetrating peptides (CPP) can enter into the cytoplasm by directly traversing the plasma membrane. The ability of CPP to directly translocate across the plasma membrane is greatly lost when conjugated with a cargo [163,137]. Some CPPs have, in fact, been employed as anticancer agent per se (e.g., Azurin against solid tumors under Phase I clinical, trial and XG-102 targeting c-Jun-N-terminal kinases under Phase II clinical trial). Co-administration of a CPP (iRGD) via systemic injection has resulted in improved therapeutic index of a variety of codelivered drug entities (DOX, nab-paclitaxel, DOX liposomes, and trastuzumab) [164]. The underlying mechanism of this effect stems from the ability of the iRGD to bind to αv integrins, which are exclusively expressed on the tumor vessels endothelium. After preferentially homing to the tumors, iRGD is proteolytically transformed into the CRDGK/R that loses its affinity with the αv integrins but acquires affinity to the neuropilin 1 (NRP-1) that triggers the tissue penetration by enhancing the vascular permeability. The whole process is tumor specific because of the αv integrins binding specificity of iRGD. The iRGD assisted improved therapeutic index has been demonstrated only in the mouse tumor models, the efficacy of this platform is yet to be shown in human patients.
4.2 Endoplasmic reticulum and Golgi apparatus
An alternate route to cellular entry that avoids the acid pH and hydrolytic lysosomal environment is represented by the retrograde trafficking pathway, which leads the endosomal cargo to the Golgi apparatus (GA) and endoplasmic reticulum (ER) [165]. ER and GA are responsible for calcium homeostasis, folding of membrane and secretory protein, and lipid biosynthesis. Retrograde trafficking pathway is involved in the recycling of certain receptors (e.g., mannose-6 phosphate receptor) and has been exploited by certain toxins (e.g., ricin toxin, shiga toxin, anthrax toxin lethal factor, and cholera toxin) for localizing in and interfering with the function of ER [166,167]. An irregular ER function recognized as ER stress results in unfolded protein response (UPR) that leads to protein synthesis inhibition and refolding of proteins, and clearance of misfolded proteins. ER stress can lead to apoptosis that has been a cause of heart diseases (cardiac hypertrophy, degeneration of cardiomyocytes), liver diseases, neurodegenerative diseases, and diabetes [168,169]. UPR has also been proposed as anticancer target because of its critical role in tumor cell resistance to hypoxia and tumor progression [170]. A nanoparticle platform capable of targeting ER stress or UPR is still awaited, however; there are some notable examples available in the literature that may lay a foundation for such an endeavor in future. In an in vitro experiment PLGA (95 nm ±20) based nanocarriers are observed to accumulate predominantly in the Golgi apparatus in case of human bronchial epithelial (HBE) and opossum kidney (OK) renal tubule cells as revealed by the compartments labeling using immunofluorescence [171]. Remarkably more particles were localized in the GA as compared to the lysosomes. The authors proposed that the PLGA particles were able to avoid localization in late endosome or lysosomes and were apparently transferred from the early endosome to the GA via ER. The presence of albumin in the culture medium was suggested to have coated the nanoparticles resulting in a receptor mediate cellular internalization and subsequent associated with the GA via a retrograde route. The resolution of the analytical method used in this study was not able to conclude if the particles are within the GA or are localized in the Golgi associated vesicles of late endosomes. In a later study employing Raman spectroscopy in combination with optical microscopy, it was revealed that the PEO functionalized PCL and PLGA nanoparticles were incorporated into the Golgi-associated vesicles of late endosomes in Human HeLa cells (cell line CCL-2) [172]. The cellular internalization pathways can determine the intercellular destination of nanoparticles and nanoparticles approaching the cells can be internalized via more than one pathway. The physiochemical nature and cell type used can influence the preference for a particular internalization pathway. PLGA nanoparticles internalized by the MDCK epithelial cells were predominantly transported to the lysosomes with some particles found localizing in the ER and Golgi complex emphasizing the critical importance of the internalization pathways [173]. The extent of nanoparticles uptake, mechanism of cellular uptake and subsequent localization in subcellular compartments can vary with cell type for the same type of nanomaterial.
4.3 Mitochondria
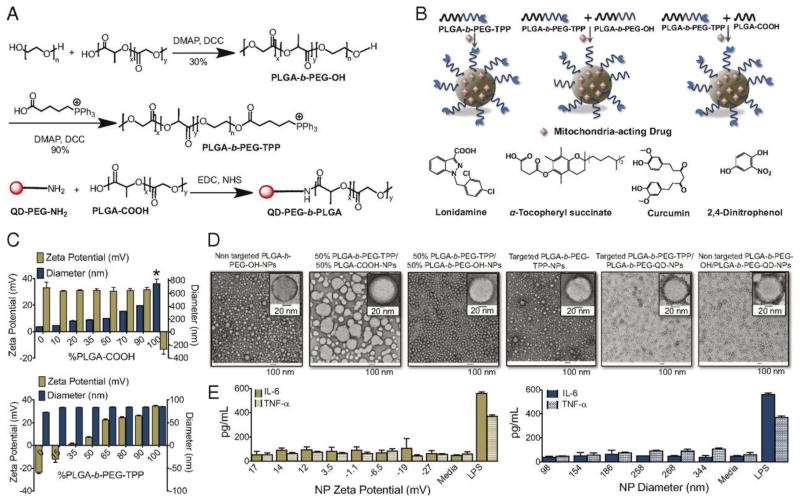
Mitochondria are unique among the cellular organelles comprising of two-membrane structure (inner and outer mitochondrial membranes) with the mitochondrial DNA enclosed in the inner membrane. The protein constituents of the mitochondria are comprised of mitochondrial genome coded proteins or of nucleus origin. The mitochondrial diseases, therefore, originate from defective nuclear and mitochondrial genome. Mitochondrial dysfunction has been implicated for a number diseases, including cancer, neurodegenerative and neuromuscular diseases, obesity, and diabetes, and has been recognized for hosting vital therapeutic targets [174–178]. A synergy of highly dense inner membrane (abundant in saturated phospholipids) and high membrane potential (negative inside) compared to the plasma membrane results in a highly controlled transportation across the mitochondrial membranes [179,180]. Because of the highly selective and impermeable nature of the mitochondrial membrane, targeting and delivering therapeutic agents to mitochondria has been a formidable challenge. Cations are generally known to target the mitochondria; primarily because of the high membrane potential [181–183]. Among different cations, triphenylphosphonium (TPP) cation [184], fulfilling the prerequisite of a balance between the delocalized positive charge and the lipophilicity, has been shown to successfully cross this barrier and reach to the inner leaflet of the inner mitochondrial membrane. Consequently, TPP has been exploited as a vector for delivering covalently conjugated small molecule based drugs to mitochondria [185–188]. The targeted delivery of therapeutic agents to mitochondria using liposomes based nanocarrier platform has been demonstrated. In a series of attempts, TPP has been incorporated in the lipid bilayer membrane of liposomes by covalently conjugating with stearyl moieties. The resulting nanocarrier exhibited efficient delivery of anticancer drugs (sclaeol and ceramide) to mitochondria [189–191]. In addition to TPP, the octaarginine functionalized liposome has also been found to deliver cargo to the mitochondria [192]. Depending on the surface density of octaarginine, the liposomes exhibited different cellular internalization pathways. For high octaarginine surface density the liposomes efficiently escaped through macropinocytosis into the cytosol [193], whereas for a low octaarginine surface density liposomes were internalized via a clatherin mediated endocytosis followed by the transfer to lysosome for degradation [194]. Despite of the recent efforts showing some improvement, immune response and in vivo toxicity is a general concern associated with these targeting platforms [195,192]. Compared to the liposomes, polymer based nanocarriers for mitochondrial targeted delivery are rather less frequent. In a recent attempt Marrache et al. [196] reported a TPP end functionalized PLGA-PEG (PLGA-PEG-TPP) nanoparticles based delivery system for targeted delivery of various mitochondria-acting drugs (Fig. 4). In order to optimize the particle size and surface charge for an optimum mitochondrial uptake, they prepared various formulations by blending different ratios of PLGA-b-PEG-TPP with –COOH end functionalized PLGA (PLGA-COOH) or with hydroxy end capped PLGA-PEG (PLGA-b-PEG-OH). By varying the ratios between these polymers, authors could prepare nanoparticles of different sizes (ranging from ~80 nm to ~400 nm) with a constant surface charge (~ +30 mV) and nanoparticles of approximately same particle size but with different surface charge (ranging from ~ −25 mV to +30 mV). Compared to the control nanoparticles with negative surface charge, the positively charged nanoparticles exhibited a high accumulation in the mitochondria of human cervical cancer (HeLa) cell. Interestingly, the positively charged targeted nanoparticles were able to escape from early endosome and get localized in the mitochondria, whereas the negative charged non-targeted nanoparticles were found localized in the endosomes even after 4 h incubation. Authors attributed this observation to the buffering effect of positively charged nanoparticles that can prevent acidification of endosomal vesicles, which may increase the ATPase-mediated influx of protons and counter ions. The resulting osmotic swelling ruptures the endosomal membrane leading to the cargo release in the cytosol. In an attempt to demonstrate the non-aggregating behavior of positively charged nanoparticles that is generally induced by the negatively charged serum proteins, authors further reported stability of the nanoparticles by monitoring the change in the nanoparticles size in the cell culture media 10% (vol/vol) FBS in DMEM or 10% (vol/vol) FBS in H2O for 7 days. The charged nanoparticles below 200 nm were found not to induce an immune response when tested for the production of proinflammatory cytokines (IL-6 and TNF-α) in RAW 264.7 macrophages by ELISA. The formulations prepared by encapsulation of mitochondria-acting drugs, including lonidamine and α-tocopheryl succinate for cancer (HeLa cells), the mitochondrial antioxidant curcumin for Alzheimer’s disease (human neuroblastoma IMR-32 cells), and the mitochondrial uncoupler 2,4-dinitrophenol for obesity (3T3-L1 cells), the PLGA-b-PEG-TPP nanoparticles exhibited marked improvement in the therapeutic index of all the employed drugs when compared to the non-targeted nanoparticles or the drugs alone. In a similar attempt Wang et al. have demonstrated a preferential mitochondrial localization of TPP functionalized nanoparticles derived from poly-L-lysine (PLL) [197]. Although TPP functionalized nanocarriers have shown promising in vitro results, the in vivo performance of these materials is needed to be evaluated for potential clinical applications.
Fig. 4.
(A) Synthesis of TPP end capped and QD functionalized PLGA-PEG block copolymers. (B) Schematic illustration of drug loaded targeted nanoparticles. (C) Modulation of size and zeta potential. (D) TEM images of the fabricated nanoparticles. (E) IL-6 and TNF-α secretion profiles of nanoparticles with different zeta potentials (left) and diameters (right). Reproduced with permission from [196].
4.4 Nucleus
Nucleus, a double lipid bilayer wrapped organelle hosting therapeutic targets (proteins, nuclear receptors, and DNA) of a variety of diseases, has been a focus of targeted drug and DNA (DNA as a drug for gene therapy) delivery. For most instances, the nanoparticles deliver the drugs into the cell and the drug molecules diffuse through the cytosol to reach the nuclear target. Some reports have suggested an improved accumulation of the therapeutic agents in the nucleus as a result of tuning the molecular design of polymeric material employed for the fabrication of nanoparticles-cargo complex. Polyplexes derived from copolymers of N-(2-hydroxypropyl)methacrylamide (HPMA) and methacrylamide monomers bearing pendant L-lysine based peptide groups with different numbers of L-lysine repeat units were reported to exhibit an improved nuclear accumulation (plasmid DNA delivery) when the number of L-lysine repeat units was ten (pHK10 and pHK15 stands for polymers with 10 and 15 L-lysine repeat units, Fig. 5) [198]. pHK10 copolymer interacted with the plasmid DNA resulting in the nanoparticles with lower aspect ratio (width ~25 nm and length ~74 nm) when compared to the nanoparticles (width ~18 nm and length ~102 nm) formed by the copolymer pHK15. In this study, a higher aspect ratio was implicated with the poor internalization and higher localization into the endosomes and lysosomes thus delaying the nuclear delivery. Interestingly, the particles were reported to be internalized via caveolin-mediated endocytosis.
Fig. 5.
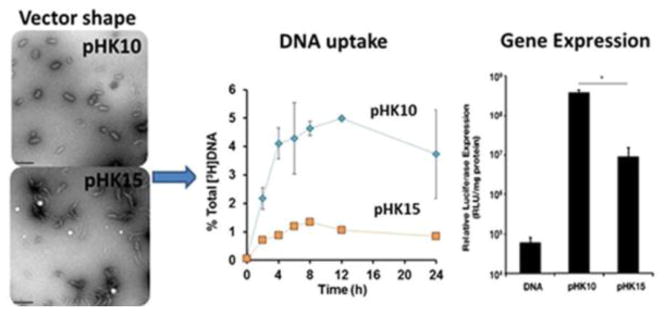
TEM images revealing the effect of the number of L-lysine units on the vector shape (left). A comparison of DNA uptake capacity of pHK10 and pHK15 (middle) and gene expression (right). Reprinted with permission from [198]. Copyright (2013) American Chemical Society.
Caveolin-mediated endocytosis is especially of interest as this cell internalization pathway results in delivering the endocytosed material to caveosomes/caveolae that have neutral pH, can bypass the acidic and enzymatic degradation in lysosomes, is known for sorting the cargo to GA and ER that resides in close proximity to nucleus, and have been proposed to provide nanomedicines with a safe route to nucleus [199–201]. Certain functionalities such as L-arginin [202] and saccharide [203] moieties as constituents of carrier polymer systems have been reported to sort the polyplexes to GA and ER. Particularly the saccharide functionalization strategy mimics the natural process of transporting the glycosylated proteins from the ER to the nucleus [204, 205]. Further studies focusing on exploiting this avenue by developing new polymers with glycosylated or arginine may provide a convenient way of caveolae-mediated nucleus targeting. In the similar vein, β-cholanic acid based hydrophobically modified glycol chitosan polymer nanoparticles (~359 nm) were found within the cytoplasm of the HeLa cells just after two minutes of incubation with almost no localization in the lysosomes. About 20% of the particles were found localized in the lysosomes after 60 minutes of incubation with most of the particles accumulating in the perinuclear region [206]. This approach has an inherent limitation of low efficacy considering the amount of drug able to reach the nucleus. In case, the drug resistance machinery of the cell has been activated the drug molecules have an even meager chance to reach to the target. However, establishing a strategy to navigating through the cytosol and reaching precisely at the nuclear target is a nontrivial task. A more precise understanding of the transport mechanisms active in the cytosol would be helpful for designing intelligent carrier systems for delivery of cargo to the specific targets.
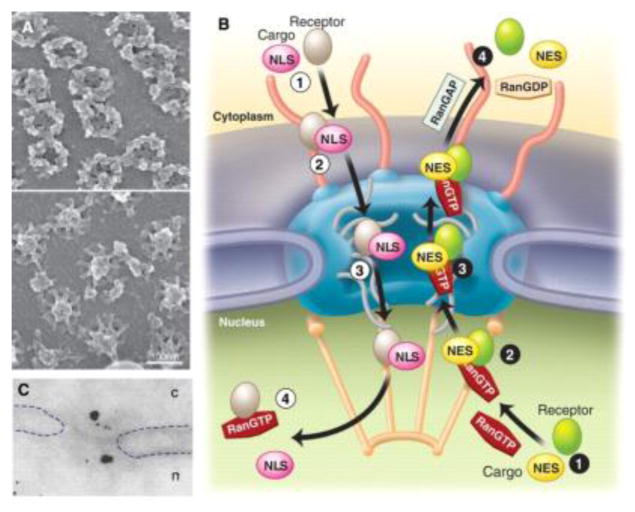
After surmounting the plasma membrane barrier, nuclear envelope presents another barrier for the nuclear delivery. The transportation across the nuclear envelope occurs through the nuclear pore complexes (NPCs, Fig. 6), the perforations in the nuclear envelope. The NPC constituent proteins, nucleoporins (Nup), determine their assembly, structural, and functional aspects. Nature employs two modes of transportation for trafficking across the NPCs, passive and active. The small ions and macromolecules (~9 nm) are transported across the nuclear envelop via passive diffusion through the NPCs while macromolecules larger than 40kDa (39 nm) are sorted via nuclear transport receptor mediated active transport that is facilitated by the oligopeptide sequences specifically binding to the receptors, known as nuclear localization signals (NLSs). FG-Nups constituent of NPCs, contain phenylalanine-glycine (FG) domains, are reported to line the inner NPC channel while extending on both sides of the nuclear envelope. Multiple, stochastic, low-affinity interactions between the transport receptors and FG-Nups play an important role in creating a barrier for translocation of cargo across the NPC [207].
Fig. 6.
(A) Scanning electron microscopic images of cytoplasmic (upper) and nucleoplasmic sides of nuclear envelope revealing the NPCs. (B) Schematic depiction of receptor mediated transportation across the NPCs. (C) Immunoelectron microscopic image of gold nanoparticles translocation between nucleus (n) and cytoplasm (c). Reproduced with permission from [207].
The conjugation of nuclear localization signal (NLS) to the nanoparticle based carrier systems has been demonstrated to direct the cargo to the nuclear target. Cheng et al. [208] demonstrated that nanoparticles derived from NLS (CGGGPKKKRKVGG) functionalized PLGA nanoparticles (~72 nm) and NLS functionalized quantum dots conjugated PLGA nanoparticles (~168 nm) were observed to be localized in the nucleus of HeLa cells. In the similar vein, NLS functionalized DOX loaded PLGA nanoparticles (~226 nm) were shown to deliver great amount of the drug to the nucleus (MCF7 cells) when compared to the free drug of PLGA nanoparticles lacking an NLS. The conjugation with NLS further enhanced the cell cycle (G2/M phase) blocking capacity and induced higher extent of apoptosis [209].
Recently, engineering dual (cellular and nucleus) targeted nanoparticles with a goal of enhancing cellular uptake and localization to the nuclear compartment has emerged as an interesting challenge. For instance, Hoang et al. [210] developed PEG-PLA block copolymer micelles (~30 nm) labeled with Indium-111 (Auger electron emitterm, 111In), conjugated with trastuzumab fab (HER2 specific antibody) and NLS (CGYGPKKKRKVGG) with a further option of loading with the antimetabolite methotrexate. Based on the in vitro subcellular fractionation using cells expressing high levels of HER2 (MDA-MB-231, MDA-MB-361 and SK-BR-3 cells lines), the NLS block copolymer micelles showed higher accumulation in the nuclei and the duel targeting platform exhibited an improved antiproliferative effect. Yu et al. [211] also employed the dual targeted strategy and fabricated DOX loaded folic acid and NLS (Ac-CGYGPKKKRKVGG) functionalized chitosan (modified with cholesterol) micelles (~250 nm). The dual targeting led to a higher cellular uptake (KB cells) because of the folic acid and increased nucleus localization due to the NLS, and highest tumor suppression (KB tumor xenograft models, BALB/c nude mice) when compared to the nontargeted or singly targeted DOX loaded micelles.
As presumed in some of the studies above, the nuclear access to these large nanoparticles can only be possible if they undergo a degradation or micellar disassembly process after delivery in the cytoplasm. Keeping in view the NPC channel size (~39 nm), a comprehensive evaluation of the size range of nanoparticles with different physiochemical properties that can translocate across the NPC channels is necessary for designing an optimum nuclear targeting nanoparticles.
5. Outlook
The success of nanoparticles based carrier systems in human trials for the targeted delivery of therapeutic agents reflects an active progress of nanomedicines towards the clinic. For the development of more potent nanomedicines, an in depth understanding of cellular uptake mechanisms is of paramount importance. Nanoparticles enter the cells via a combination different internalization routes. Depending on the size, shape and surface charge of the nanoparticles, a particular cellular internationalization route may be preferred on the others. The fact that the cellular internalization routes determine the fate and intracellular localization of nanoparticles emphasizes that the development of reliable strategies to control or at least influence the nanoparticle cellular internalization route can hugely augment the therapeutic outcome. In the same vein, a systematic investigation of abnormally high rate of nutrient uptake during the tumor progression as a route for delivery of drugs loaded nanoparticles may offer a unique opportunity for nanoparticles based cancer therapy. With the new goals set for the subcellular organelle level targeting, the field of nanomedicine is now moving to the next level of complexity. The task is challenging, however; there are notable reports showing promising results highlighting the potential improvements that can be expected from organelle level targeting. Endosomses are the organelles with slightly acidic pH that are encountered by all the nanoparticles. Polyamines have been generally employed to trigger the release of nanoparticles from the endosomes via proton sponge effect, however; questions have been raised regarding the poor biocompatibility of toxicity of polyamines because of their high pKa (~9). The development of low pKa materials capable of triggering the endosomal release may result in the clinical advantage of proton sponge effect. The concept of subcellular targeted nanoparticles is at its infancy and only a limited number of strategies have so far been reported for ER, mitochondria, and nucleus targeting. More detailed investigations are needed to assess the impact and relevance of subcellular targeting for their future clinical applications. For subcellular targeting, it is desirable that the design of the nanoparticles still present the non-immunogenic stealth character with high systemic circulation combined with the ability of overcoming biological barriers and target site specificity. It is a formidable challenge, however; nanoparticles capable of exhibiting a sequential multistage stage targeting can be an interesting strategy and coming years should witness the success of this new paradigm of nanomedicines.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) (grant U54-CA151884), the National Heart, Lung, and Blood Institute (NHLBI) Program of Excellence in Nanotechnology (PEN) (contract #HHSN268201000045C), the National Institute of Biomedical Imaging and Bioengineering (NIBIB) R01 grant (EB015419-01) and the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics. Dr. Farokhzad declares financial interests in BIND Therapeutics, Selecta Biosciences and Blend Therapeutics; three biotechnology companies developing nanoparticle technologies for medical applications. C.V. acknowledges the support from Center for the Development of Nanoscience and Nanotechnology (Grant FB0807) and the Postdoctoral Program of Becas-Chile/CONICYT.
Footnotes
The rest of the authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feynman RP. There’s plenty of room at the bottom. Eng Sci (CalTech) 1960;23:22–36. [Google Scholar]
- 3.Ehrlich P. Address in Pathology, On chemiotherapy. 17th Intl Congress Med Br Med J. 1913;16:353–359. doi: 10.1136/bmj.2.2746.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 5.Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandare J, Calderón M, Dagia NM, Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chem Soc Rev. 2012;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Chen S, Jiang S. Dual-Functional Biomimetic Materials: Nonfouling Poly(carboxybetaine) with Active Functional Groups for Protein Immobilization. Biomacromolecules. 2006;7:3311–3315. doi: 10.1021/bm060750m. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotech. 2013;31:553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced Drug Delivery Reviews. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987;223:42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 14.Prabhakar U, Blakey DC, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P. Challenges and key considerations of the enhanced permeability and retention effect (EPR) for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds AR, Moein Moghimi S, Hodivala-Dilke K. Nanoparticle-mediated gene delivery to tumour neovasculature. Trends in molecular medicine. 2003;9:2–4. doi: 10.1016/s1471-4914(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis. Annual review of medicine. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 18.Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, DeWitt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnajaran B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128–139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajendran L, Knölker H-J, Simons K. Subcellular targeting strategies for drug design and delivery. Nature Reviews Drug Discovery. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 21.Huang JG, Leshuk T, Gu FX. Emerging nanomaterials for targeting subcellular organelles. Nano Today. 2011;6:478–492. [Google Scholar]
- 22.Kim BY, Rutka JT, Chan WC. Nanomedicine. The New England journal of medicine. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nature Materials. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annual review of biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. The Journal of cell biology. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein SC. Phagocytosis of microbes: insights and prospects. Trends in cell biology. 1995;5:141–142. doi: 10.1016/s0962-8924(00)88967-9. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends in cell biology. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 29.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nature reviews Immunology. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 2009;29:2127–2136. [PubMed] [Google Scholar]
- 35.Kirchhausen T. Clathrin. Annual review of biochemistry. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 36.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 37.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 38.Capraro BR, Shi Z, Wu T, Chen Z, Dunn JM, Rhoades E, Baumgart T. Kinetics of endophilin N-BAR domain dimerization and membrane interactions. The Journal of biological chemistry. 2013;288:12533–12543. doi: 10.1074/jbc.M112.435511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henne WM, Kent HM, Ford MG, Hegde BG, Daumke O, Butler PJ, Mittal R, Langen R, Evans PR, McMahon HT. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure. 2007;15:839–852. doi: 10.1016/j.str.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Cocucci E, Aguet F, Boulant S, Kirchhausen T. The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150:495–507. doi: 10.1016/j.cell.2012.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanlandingham PA, Barmchi MP, Royer S, Green R, Bao H, Reist N, Zhang B. AP180 couples protein retrieval to clathrin-mediated endocytosis of synaptic vesicles. Traffic. 2014;15:433–450. doi: 10.1111/tra.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Molecular biology of the cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 44.Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 45.Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nature cell biology. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- 46.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 47.Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochemical and Biophysical Research Communications. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 48.Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cellmembrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29:4244–4252. doi: 10.1016/j.biomaterials.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang D-M, Hung Y, Ko B-S, Hsu S-C, Chen W-H, Chien C-L, Tsai C-P, Kuo C-T, Kang J-C, Yang C-S, Mou C-Y, Chen Y-C. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. The FASEB Journal. 2005;19:2014–2016. doi: 10.1096/fj.05-4288fje. [DOI] [PubMed] [Google Scholar]
- 50.Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochemistry and cell biology. 2008;129:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson AS, Smythe E, Ayscough KR. Functions of actin in endocytosis. Cellular and molecular life sciences: CMLS. 2009;66:2049–2065. doi: 10.1007/s00018-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Current opinion in cell biology. 2011;23:413–420. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. The Journal of cell biology. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 55.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Molecular cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 57.Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. The Journal of cell biology. 1998;140:553–563. doi: 10.1083/jcb.140.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauthier NC, Monzo P, Kaddai V, Doye A, Ricci V, Boquet P. Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol Biol Cell. 2005;16:4852–4866. doi: 10.1091/mbc.E05-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nature reviews. Molecular cell biology. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garaiova Z, Strand SP, Reitan NK, Lélu S, Størset SØ, Berg K, Malmo J, Folasire O, Bjørkøy A, de Davies CL. Cellular uptake of DNA–chitosan nanoparticles: The role of clathrin- and caveolae-mediated pathways. Int J Biol Macromol. 2012;51:1043–1051. doi: 10.1016/j.ijbiomac.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Plummer EM, Manchester M. Endocytic Uptake Pathways Utilized by CPMV Nanoparticles. Mol Pharm. 2013;10:26–32. doi: 10.1021/mp300238w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lerch S, Dass M, Musyanovycha A, Landfester K, Mailänder V. Polymeric nanoparticles of different sizes overcome the cell membrane barrier. Eur J Pharm Biopharm. 2013;84:265–274. doi: 10.1016/j.ejpb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 63.Garaiova Z, Strand SP, Reitan NK, Lélu S, Størset SØ, Berg K, Malmo J, Folasire O, Bjørkøy A, de Davies CL. Cellular uptake of DNA–chitosan nanoparticles: The role of clathrin- and caveolae-mediated pathways. International Journal of Biological Macromolecules. 2012;51:1043–1051. doi: 10.1016/j.ijbiomac.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Parton RG, Simons K. The multiple faces of caveolae, Nature reviews. Molecular cell biology. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 65.Thorn H, Stenkula KG, Karlsson M, Ortegren U, Nystrom FH, Gustavsson J, Stralfors P. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Molecular biology of the cell. 2003;14:3967–3976. doi: 10.1091/mbc.E03-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB. Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae. IUBMB life. 2011;63:659–667. doi: 10.1002/iub.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 68.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nature reviews. Molecular cell biology. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 69.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nature biotechnology. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Huang R, Han L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 71.Lafon M. Rabies virus receptors. J Neurovirol. 2005;11:82–87. doi: 10.1080/13550280590900427. [DOI] [PubMed] [Google Scholar]
- 72.Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. Journal of cell science. 2006;119:4758–4769. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- 73.Fiorentini C, Falzano L, Fabbri A, Stringaro A, Logozzi M, Travaglione S, Contamin S, Arancia G, Malorni W, Fais S. Activation of rho GTPases by cytotoxic necrotizing factor 1 induces macropinocytosis and scavenging activity in epithelial cells. Molecular biology of the cell. 2001;12:2061–2073. doi: 10.1091/mbc.12.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mercer J, Helenius A. Virus entry by macropinocytosis. Nature cell biology. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 75.Kolb-Maurer A, Wilhelm M, Weissinger F, Brocker EB, Goebel W. Interaction of human hematopoietic stem cells with bacterial pathogens. Blood. 2002;100:3703–3709. doi: 10.1182/blood-2002-03-0898. [DOI] [PubMed] [Google Scholar]
- 76.Steinman RM, Swanson J. The endocytic activity of dendritic cells. The Journal of experimental medicine. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. The Journal of experimental medicine. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 79.Schafer DA, D’Souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1:892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- 80.Nobes C, Marsh M. Dendritic cells: new roles for Cdc42 and Rac in antigen uptake? Current biology: CB. 2000;10:R739–741. doi: 10.1016/s0960-9822(00)00736-3. [DOI] [PubMed] [Google Scholar]
- 81.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. The Journal of cell biology. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. The Journal of cell biology. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Zhang S, Ruan S-b, Zhang Q-y, He Q, Gao H-l. Lapatinib-incorporated lipoprotein-like nanoparticles: preparation and a proposed breast cancer-targeting mechanism. Acta Pharmacologica Sinica. 2014;35:846–852. doi: 10.1038/aps.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients. 2013;5:3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan T, Hong S, Yao Y, Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts. but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell research. 2007;17:772–782. doi: 10.1038/cr.2007.73. [DOI] [PubMed] [Google Scholar]
- 87.Hussain MM. Intestinal lipid absorption and lipoprotein formation. Current opinion in lipidology. 2014 doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M, Haass NK, Rasko JEJ, Holst J. Targeting glutamine transport to suppress melanoma cell growth. International Journal of Cancer. 2014 doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
- 89.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 91.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv Drug Deliv Rev. 2013;65:121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Müller C, Schibli R. Prospects in Folate Receptor-Targeted Radionuclide Therapy. Front Oncol. 2013;3:249. doi: 10.3389/fonc.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 95.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 96.Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, Ratnam M. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 97.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 98.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 99.Gabizon A, Tzemach D, Gorin J, Mak L, Amitay Y, Shmeeda H, Zalipsky S. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor expressing tumor models. Cancer Chemother Pharmacol. 2010;66:43–52. doi: 10.1007/s00280-009-1132-4. [DOI] [PubMed] [Google Scholar]
- 100.Jiang S, Gong X, Zhao X, Zu Y. Preparation, characterization, and antitumor activities of folate-decorated docetaxel-loaded human serum albumin nanoparticles. Drug Deliv. 2014 doi: 10.3109/10717544.2013.879964. [DOI] [PubMed] [Google Scholar]
- 101.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, Zhang T, Wang AZ. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32:8548–8554. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Sega E, Leamon CP, Low PS. Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Deliv Rev. 2004;56:1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Li H, Lu Y, Piao L, Wu J, Liu S, Marcucci G, Ratnam M, Lee RJ. Targeting human clonogenic acute myelogenous leukemia cells via folate conjugated liposomes combined with receptor modulation by all-trans retinoic acid. Int J Pharm. 2010;402:57–63. doi: 10.1016/j.ijpharm.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 105.Schneider C, Owen MJ, Banville D, Williams JG. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984;311:675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- 106.Omary MB, Trowbridge IS. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981;256:12888–12892. [PubMed] [Google Scholar]
- 107.Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, Joo SW. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac J Cancer Prev. 2014;15:517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 108.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 109.van der Meel R, Vehmeijer LJC, Kok RJ, Storm G, van Gaal EVB. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv Drug Del. 2013;65:1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 110.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 112.Hong M, Zhu S, Jiang Y, Tang G, Pei Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J Control Release. 2009;133:96–102. doi: 10.1016/j.jconrel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 113.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature Nanotechnology. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 114.Mirghani H, Amen F, Moreau F, Guigay J, Hartl DM, Lacau St Guily J. Oropharyngeal cancers: Relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer. 2014;50:1100–1111. doi: 10.1016/j.ejca.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 115.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 116.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 117.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 118.Shevtsov MA, Nikolaev BP, Yakovleva LY, Marchenko YY, Dobrodumov AV, Mikhrina AL, Martynova MG, Bystrova OA, Yakovenko IV, Ischenko AM. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int J Nanomedicine. 2014;9:273–287. doi: 10.2147/IJN.S55118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30:5737–5750. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 121.Lee SM, Park H, Choi JW, Park YN, Yun CO, Yoo KH. Multifunctional nanoparticles for targeted chemophotothermal treatment of cancer cells. Angew Chem Int Ed Engl. 2011;50:7581–7586. doi: 10.1002/anie.201101783. [DOI] [PubMed] [Google Scholar]
- 122.Kol A, Terwisscha van Scheltinga AG, Timmer-Bosscha H, Lamberts LE, Bensch F, de Vries EG, Schröder CP. HER3, serious partner in crime: Therapeutic approaches and potential biomarkers for effect of HER3-targeting. Pharmacol Ther. 2014 doi: 10.1016/j.pharmthera.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 124.Schülke N, Varlamova OA, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, Maddon PJ, Heston WDW, Olson WC. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci U S A. 2003;100:12590–12595. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tucker GC. Integrins: Molecular Targets in Cancer Therapy. Curr Oncol Rep. 2006;8:96–103. doi: 10.1007/s11912-006-0043-3. [DOI] [PubMed] [Google Scholar]
- 126.Gottschalk KE, Kessler H. The structures of Integrins and integrin-ligand complexes: implications for drug design and signal transduction. Angew Chem Int Ed. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 127.Eliceiri BP, Cheresh DA. The role of alpha v integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 129.Arap W, Pasqualini R, Ruoslahti E. Chemotherapy targeted to tumor vasculature. Curr Opin Oncol. 1998;10:560–565. doi: 10.1097/00001622-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 130.Liu S. Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjugate Chem. 2009;20(12):2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Auzzas L, Zanardi F, Battistini L, Burreddu P, Carta P, Rassu G, Curti C, Casiraghi G. Targeting v3 Integrin: Design and Applications of Mono- and Multifunctional RGD-Based Peptides and Semipeptides. Current Medicinal Chemistry. 2010;17:1255–1299. doi: 10.2174/092986710790936301. [DOI] [PubMed] [Google Scholar]
- 132.Graf Prodrug N, Bielenberg DR, Kolishetti N, Muus C, Banyard J, Farokhzad OC, Lippard SJ. αVβ3 Integrin-Targeted PLGA-PEG Nanoparticles for Enhanced Anti-tumor Efficacy of a Pt(IV) ACS Nano. 2012;6:4530–4539. doi: 10.1021/nn301148e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, van der Born K, Wanders J, de Boer RF, Martin C, Fumoleau P. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38:349–358. doi: 10.1016/s0959-8049(01)00371-9. [DOI] [PubMed] [Google Scholar]
- 134.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 135.Brambell FW. The transmission of immune globulins from the mother to the foetal and newborn young. Proc Nutr Soc. 1969;28:35–41. [PubMed] [Google Scholar]
- 136.Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC. Transepithelial Transport of Fc-Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rajendran L, Knölker H-J, Simons K. Subcellular targeting strategies for drug design and delivery. Nature Reviews Drug Discovery. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 138.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A Versatile Vector for Gene and Oligonuceotide Transfer into Cells in Culture and in Vivo: Polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guidry EN, Farand J, Soheili A, Parish CA, Kevin NJ, Pipik B, Calati KB, Ikemoto N, Waldman JH, Latham AH, Howell BJ, Leone A, Garbaccio RM, Barrett SE, Parmar RG, Truong QT, Mao B, Davies IW, Colletti SLL. Sepp-Lorenzino, improving the in vivo therapeutic index of siRNA polymer conjugates through increasing pH responsiveness. Bioconjugate Chem. 2014;25:296–307. doi: 10.1021/bc400442p. [DOI] [PubMed] [Google Scholar]
- 140.Agola JO, Jim PA, Ward HH, Basuray S, Wandinger-Ness A. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet. 2011;80:305–318. doi: 10.1111/j.1399-0004.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stein M-P, Dong J, Wandinger-Ness A. Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Advanced Drug Delivery Reviews. 2003;55:1421–1437. doi: 10.1016/j.addr.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 142.Li X, DiFiglia M. The recycling endosome and its role in neurological disorders. Prog Neurobiol. 2012;97:127–141. doi: 10.1016/j.pneurobio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Nixon RA, Mathews PM, Cataldo AM. The neuronal endosomal-lysosomal system in Alzheimer’s disease. J Alzheimers Dis. 2001;3:97–107. doi: 10.3233/jad-2001-3114. [DOI] [PubMed] [Google Scholar]
- 144.Walsh CA, Morrow EM, Rubenstein JLR. Autism and brain development. Cell. 2008;135:396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ouyang Q, Lizarraga SB, Schmidt M, Yan U, Gong J, Elisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yap CC, Winckler B. Acid indigestion in the endosome: linking signaling dysregulation to neurodevelopmental disorders. Neuron. 2013;80:4–6. doi: 10.1016/j.neuron.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 148.Ohgaki R, van IJzendoorn SCD, Matsushita M, Hoekstra D, Kanazawa H. Organellar Na+/H+ Exchangers: Novel Players in Organelle pH Regulation and Their Emerging Functions. Biochemistry. 2011;50:443–450. doi: 10.1021/bi101082e. [DOI] [PubMed] [Google Scholar]
- 149.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 150.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 151.Harguindey S, Arranz JL, Orozco JDP, Rauch C, Fais S, Cardone RA, Reshkin SJ. Cariporide and other new and powerful NHE1inhibitors as potentially selective anticancer drugs-an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. Journal of Translational Medicine. 2013;11:282–298. doi: 10.1186/1479-5876-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 153.de Duve C. Lysosomes revisited. Eur J Biochem. 1983;137:391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]
- 154.Petersen NHT, Olsen OD, Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S, Ostenfeld MS, Ulanet D, Dovmark TH, Lønborg A, Vindeløv SD, Hanahan D, Arenz C, Ejsing CS, Kirkegaard T, Rohde M, Nylandsted J, Jäättelä M. Transformation-Associated Changes in Sphingolipid Metabolism Sensitize Cells to Lysosomal Cell Death Induced by Inhibitors of Acid Sphingomyelinase. Cancer Cell. 2013;24:379–93. doi: 10.1016/j.ccr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 155.Gulbins Erich, Kolesnick Richard N. It Takes a CAD to Kill a Tumor Cell with a LMP. Cancer Cell. 2013;24:279–81. doi: 10.1016/j.ccr.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 156.Saftig P, Sandhoff K. Cancer: Killing from the inside. Nature. 502:312–313. doi: 10.1038/nature12692. [DOI] [PubMed] [Google Scholar]
- 157.Kong M, Park H, Cheng X, Chen X. Spatial-temporal event adaptive characteristics of nanocarrier drug delivery in cancer therapy. Journal of Controlled Release. 2013;172:281–291. doi: 10.1016/j.jconrel.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 158.Duncan R, Richardson SCW. Endocytosis and Intracellular Trafficking as Gateways for Nanomedicine Delivery: Opportunities and Challenges. Mol Pharmaceutics. 2012;9:2380–2402. doi: 10.1021/mp300293n. [DOI] [PubMed] [Google Scholar]
- 159.Zheng C, Xu J, Yao X, Xu J, Qiu L. Polyphosphazene nanoparticles for cytoplasmic release of doxorubicin with improved cytotoxicity against Dox-resistant tumor cells. Journal of Colloid and Interface Science. 2011;355:374–382. doi: 10.1016/j.jcis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 160.Yu H, Xu Z, Chen X, Xu L, Yin Q, Zhang Z, Li Y. Reversal of Lung Cancer Multidrug Resistance by pH-Responsive Micelleplexes Mediating Co-Delivery of siRNA and Paclitaxel. Macromol Biosci. 2014;14:100–109. doi: 10.1002/mabi.201300282. [DOI] [PubMed] [Google Scholar]
- 161.Lee Yan, Fukushima Shigeto, Bae Younsoo, Hiki Shigehiro, Ishii Takehiko, Kataoka Kazunori. A Protein Nanocarrier from Charge-Conversion Polymer in Response to Endosomal pH. J Am Chem Soc. 2007;129:5362–5363. doi: 10.1021/ja071090b. [DOI] [PubMed] [Google Scholar]
- 162.Binauld S, Stenzel MH. Acid-degradable polymers for drug delivery: a decade of innovation. Chem Commun. 2013;49:2082–2102. doi: 10.1039/c2cc36589h. [DOI] [PubMed] [Google Scholar]
- 163.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci USA. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tarragó-Trani MT, Storrie B. Alternate routes for drug delivery to the cell interior: Pathways to the Golgi apparatus and endoplasmic reticulum. Advanced Drug Delivery Reviews. 2007;59:782–797. doi: 10.1016/j.addr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 167.Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoshida H. ER stress and diseases. FEBS Journal. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 169.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 170.Leleu X, Xu L, Jia X, Sacco A, Farag M, Hunter ZR, Moreau AS, Ngo HT, Hatjiharissi E, Ho AW, Santos DD, Adamia S, O’Connor K, Ciccarelli B, Soumerai J, Manning RJ, Patterson CJ, Roccaro AM, Ghobrial IM, Treon SP. Endoplasmic reticulum stress is a target for therapy in Waldenstrom macroglobulinemia. Blood. 2009;113:626–634. doi: 10.1182/blood-2007-10-116848. [DOI] [PubMed] [Google Scholar]
- 171.Cartieraa MS, Johnsona KM, Rajendranb V, Caplanb MJ, Saltzmana WM. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30:2790–2798. doi: 10.1016/j.biomaterials.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chernenko T, Matthäus C, Milane L, Quintero L, Amiji M, Diem M. Label-free raman spectral imaging of intracellular delivery and degradation of polymeric nanoparticle systems. ACS Nano. 2009;3:3552–3559. doi: 10.1021/nn9010973. [DOI] [PubMed] [Google Scholar]
- 173.He B, Jia Z, Du W, Yu C, Fan Y, Dai W, Yuan L, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. The transport pathways of polymer nanoparticles in MDCK epithelial cells. Biomaterials. 2013;34:4309–4326. doi: 10.1016/j.biomaterials.2013.01.100. [DOI] [PubMed] [Google Scholar]
- 174.Galluzzi L, Kroemer G, Fulda S. Targeting mitochondria for cancer therapy. Nature Reviews Drug Discovery. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 175.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 176.Yamada Y, Akita H, Kogure K, Kamiya H, Harashima H. Mitochondrial drug delivery and mitochondrial disease therapy-an approach to liposome-based delivery targeted to mitochondria. Mitochondrion. 2007;7:63–71. doi: 10.1016/j.mito.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 177.Schon EA, DiMauro S. Medicinal and genetic approaches to the treatment of mitochondrial disease. Curr Med Chem. 2003;10:2523–2533. doi: 10.2174/0929867033456503. [DOI] [PubMed] [Google Scholar]
- 178.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 179.Yamada Y, Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Adv Drug Delivery Rev. 2008;60:1439–1462. doi: 10.1016/j.addr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 180.Jean SR, Tulumello DV, Wisnovsky SP, Lei EK, Pereira MP, Kelley SO. Molecular Vehicles for Mitochondrial Chemical Biology and Drug Delivery. ACS Chem Biol. 2014;9:323–333. doi: 10.1021/cb400821p. [DOI] [PubMed] [Google Scholar]
- 181.Smith RAJ, Hartley RC, Murphy MP. Mitochondria-Targeted Small Molecule Therapeutics and Probes. Antioxidants & Redox Signaling. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 182.Murphy MP. Targeting lipophilic cations to mitochondria. Biochimica et Biophysica Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 183.Reily C, Mitchell T, Chacko BK, Benavides GA, Murphy MP, Darley-Usmar VM. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biology. 2013;1:86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 185.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, Scroggins B, Neckers L, Altieri DC. Combinatorial drug design targeting multiple cancer signaling networks controlled. J Clin Invest. 2009;119:454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 187.Chalmers S, Caldwell ST, Quin C, Prime TA, James AM, Cairns AG, Murphy MP, McCarron JG, Hartley RC. Selective uncoupling of individual mitochondria within a cell using a mitochondria-targeted photoactivated protonophore. J Am Chem Soc. 2012;134:758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boddapati SV, Tongcharoensirikul P, Hanson RN, D’Souza GG, Torchilin VP, Weissig V. Mitochondriotropic liposomes. J Liposome Res. 2005;15:49–58. doi: 10.1081/lpr-64958. [DOI] [PubMed] [Google Scholar]
- 190.Patel NR, Hatziantoniou S, Georgopoulos A, Demetzos C, Torchilin VP, Weissig V, D’Souza GG. Mitochondria-targeted liposomes improve the apoptotic and cytotoxic action of sclareol. J Liposome Res. 2010;20:244–249. doi: 10.3109/08982100903347931. [DOI] [PubMed] [Google Scholar]
- 191.Boddapati SV, D’Souza GG, Erdogan S, Torchilin VP, Weissig V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008;8:2559–2563. doi: 10.1021/nl801908y. [DOI] [PubMed] [Google Scholar]
- 192.Yamada Y, Harashima H. Delivery of bioactive molecules to the mitochondrial genome using a membrane-fusing, liposome-based carrier, DF-MITO-Porter. Biomaterials. 2012;33:1589–1595. doi: 10.1016/j.biomaterials.2011.10.082. [DOI] [PubMed] [Google Scholar]
- 193.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 194.Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochimica et Biophysica Acta. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 195.Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. Journal of Controlled Release. 2012;159:393–402. doi: 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. PNAS. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang X-H, Peng H-S, Yang L, You F-T, Teng F, Tang A-W, Zhang F-J, Li X-H. Poly-L-lysine assisted synthesis of core-shell nanoparticles and conjugation with triphenylphosphonium to target mitochondria. J Mater Chem B. 2013;1:5143–5152. doi: 10.1039/c3tb20884b. [DOI] [PubMed] [Google Scholar]
- 198.Shi J, Choi JL, Chou B, Johnson RN, Schellinger JG, Pun SH. Effect of polyplex morphology on cellular uptake, intracellular trafficking, and transgene expression. ACS Nano. 2013;7:10612–10620. doi: 10.1021/nn403069n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, Beltram Fabio. A caveolae-mediated internalization of extracellular HIV-1 Tat fusion proteins visualized in real time. Mol Ther. 2003;8:284–294. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 200.Carver LA, Schnitzer JE. Caveolae: Mining little caves for new cancer targets. Nature Reviews Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 201.Reilly MJ, Larsen JD, Sullivan MO. Polyplexes Traffic through Caveolae to the Golgi and Endoplasmic Reticulum en Route to the Nucleus. Mol Pharm. 2012;9:1280–1290. doi: 10.1021/mp200583d. [DOI] [PubMed] [Google Scholar]
- 202.Li H, Luo T, Sheng R, Sun J, Wang Z, Cao A. Endoplasmic reticulum localization of poly(ω-aminohexyl methacrylamide)s conjugated with (L-)-arginines in plasmid DNA delivery. Biomaterials. 2013;34:7923–7938. doi: 10.1016/j.biomaterials.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 203.Fichter KM, Ingle NP, McLendon PM, Reineke TM. Polymeric nucleic acid vehicles exploit active interorganelle trafficking mechanisms. ACS Nano. 2013;7:347–364. doi: 10.1021/nn304218q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liao H-J, Carpenter G. Role of the Sec61 Translocon in EGF Receptor Trafficking to the Nucleus and Gene Expression. Mol Biol Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen T-LL, Wang PY, Luo W, Gwon SS, Flay NW, Zheng J, Guo C, Tanzer ML, Vertel BM. Aggrecan Domains Expected to Traffic Through the Exocytic Pathway are Misdirected to the Nucleus. Exp Cell Res. 2001;263:224–235. doi: 10.1006/excr.2000.5093. [DOI] [PubMed] [Google Scholar]
- 206.Nam HY, Kwon SM, Chung H, Lee S-Y, Kwon S-H, Jeon H, Kim Y, Park JH, Kim J, Her S, Oh Y-K, Kwon IC, Kim K, Jeong SY. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. Journal of Controlled Release. 2009;135:259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 207.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 208.Cheng F-Y, Wang SP-H, Su C-H, Tsai T-L, Wu P-C, Shieh D-B, Chen J-H, Hsieh PC-H, Yeh Chen-Sheng. Stabilizer-free poly(lactide-co-glycolide) nanoparticles for multimodal biomedical probes. Biomaterials. 2008;29:2104–2112. doi: 10.1016/j.biomaterials.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 209.Misra R, Sahoo SK. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. European Journal of Pharmaceutical Sciences. 2010;39:152–163. doi: 10.1016/j.ejps.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 210.Hoang B, Reilly RM, Allen C. Block copolymer micelles target auger electron radiotherapy to the nucleus of HER2-positive breast cancer cells. Biomacromolecules. 2012;13:455–465. doi: 10.1021/bm201479t. [DOI] [PubMed] [Google Scholar]
- 211.Yu J, Xie X, Xu X, Zhang L, Zhou X, Yu H, Wu P, Wang T, Che X, Hu Z. Development of dual ligand-targeted polymeric micelles as drug carriers for cancer therapy in vitro and in vivo. J Mater Chem B. 2014;2:2114–2126. doi: 10.1039/c3tb21539c. [DOI] [PubMed] [Google Scholar]