Abstract
The ER stress-inducible transcription factor, x-box binding protein 1 (XBP1), which enhances protein glycosylation in the endoplasmic reticulum (ER), was shown to also enhance protein glycosylation outside the ER, via a process called O-GlcNAcylation, which protected the heart from ischemia/reperfusion damage.
Keywords: heart, cardiac, ischemia/reperfusion, ER stress, unfolded protein response, XBP1, O-GlcNacylation
O-GlcNAcylation is a reversible post-translational modification (PTM) that takes place outside of the ER and affects the functions of target proteins. In cardiac myocytes, O-GlcNAcylation increases acutely in response to a variety of conditions, including hypoxia, ischemia, ischemia/reperfusion, and oxidative stress, during which O-GlcNAcylation is generally protective. However, chronic increases in O-GlcNAcylation during diseases, such as diabetes exacerbate cardiac dysfunction and damage. In contrast to O-GlcNAcylation, N-linked glycosylation (N-glycosylation) of proteins occurs in the ER, is relatively permanent, and is required for folding and trafficking of proteins in the ER and Golgi. N-glycosylation can be impaired by many of the same stresses that affect O-GlcNAcylation in the heart. Impaired N-glycosylation causes ER stress, subsequent activation of the unfolded protein response (UPR), and activation of the transcription factor, X-box binding protein 1 (XBP1), which induces genes that restore N-glycosylation in the ER and promote adaptation to ER stress. A recent study, published in the journal Cell, showed that XBP1 also enhances O-GlcNAcylation, which protects the heart from ischemia/reperfusion damage1. Thus, in response to potentially damaging stress, XBP1 coordinates glycosylation inside and outside of the ER to confer protection.
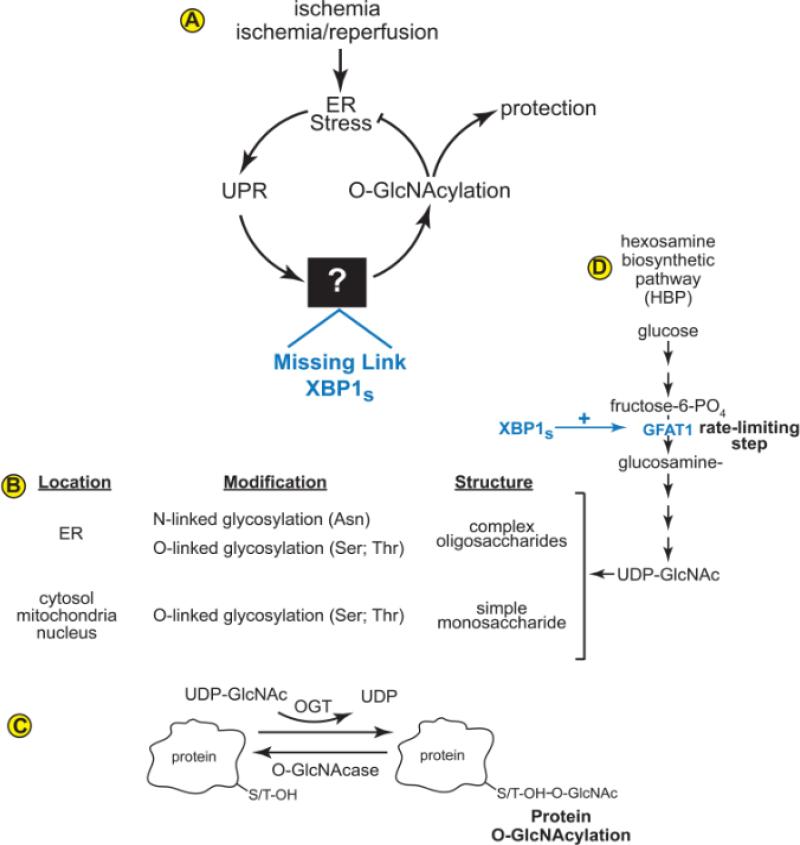
The study by Wang et al showed that XBP1 is the missing link between protein O-GlcNAcylation and the UPR1 (Fig. A). In the heart, ischemia/reperfusion leads to ER stress, activation of the UPR and XBP1, which, Wang et al determined to be a direct transcriptional activator of the gene encoding the rate-limiting step in the hexosamine biosynthetic pathway, which supplies the substrate required for O-GlcNAcylation, i.e. uridine diphosphate N-acetyl glucosamine (UDP-GlcNAc). These findings reveal previously unappreciated links between the UPR, hexosamine biosynthesis, and O-GlcNAcylation that may serve protective roles in several tissues other than the heart.
Figure.
(A) XBP1 is the Missing Link between the UPR and O-GlcNAcylation. The contributions made by Wang et al are shown in blue. (B) Proteins are glycosylated in different cell compartments. (C) A key substrate for O-GlcNAcylation, which is used by the enzyme OGT (O-GlcNAc transferase), is UDP-GlcNAc (uridine diphosphate N-acetyl glucosamine). (D) UDP-GlcNAc is generated by the hexose biosynthetic pathway (HBP). O-GlcNAc groups can be removed from proteins by OGA (O-GlcNAcase). The contributions made by Wang et al are shown in blue.
The UPR is a conserved intracellular signaling system that is activated in response to an imbalance in ER protein homeostasis, or ER proteostasis2. Conditions that impair protein folding in the ER, such as the lack of oxygen and nutrients in the ischemic heart, result in ER stress, which can lead to an imbalance in proteostasis due to the accumulation of potentially toxic misfolded proteins. One of the major ER stress signaling pathways is mediated by the ER-transmembrane protein, inositol-requiring protein-1 (IRE1). By way of mRNA splicing, IRE1 converts the XBP1 mRNA from a transcript that encodes a protein that does not have transcriptional activity, XBP1 unspliced (XBP1u), to a transcript that encodes a form of XBP1 that is a potent transcription factor, XBP1 spliced (XBP1s). XBP1s-responsive genes encode proteins that, among other things, augment ER protein-folding in various ways, including restoration and fortification of protein glycosylation in the ER lumen.
Protein glycosylation is a widespread PTM that has substantial impact on the function of the heart. It can take place in the ER lumen, as well as in the cytosol, mitochondria, and nucleus (Fig. B)3. The O-linked protein glycosylation that takes place outside the ER is responsible for the post-translational modification of over 1,000 different proteins. O-linked glycosylation involves the post-translational addition of the monosaccharide, N-acetylglucosamine (GlcNAc), to a serine or threonine in target proteins. In contrast to N-linked glycosylation, there is no known consensus sequence on target proteins that are modified by O-linked glycosylation outside the ER. O-linked glycosylation in the cytosol, nucleus, and mitochondria, also called O-GlcNAcylation, is mediated by O-GlcNAc transferase (OGT), which uses uridine diphospho-β-N-acetylglucoseamine (UDP-GlcNAc) as the glycosyl donor (Fig. C). N-glycosylation and O-GlcNAcylation share the intermediate, UDP-GlcNAc, which is generated from glucose by the hexosamine biosynthetic pathway (Fig. D). The rate-limiting step in UDP-GlcNAc formation is the conversion of fructose-6-phosphate to glucosamine-6-phosphate by the enzyme, glutamine:fructose amidotransferase (GFAT).
Before the study by Wang et al, it had been shown that ischemia could activate the UPR and XBP1 in cultured cardiac myocytes and in infarcted mouse hearts, and that XBP1 served a protective role under these conditions (Fig. A)4. Moreover, it had been shown that ischemia/reperfusion can increase protein O-GlcNAcylation in cultured cardiac myocytes and in the mouse heart, in vivo, and that O-GlcNAcylation protected the heart from ischemia/reperfusion damage5. However, the link between the UPR and O-GlcNAcylation remained unknown (Fig. A, black box). In pursuit of finding this missing link, Wang et al observed that, in the mouse heart ischemia/reperfusion activated ER stress, XBP1, O-GlcNAcylation and GFAT1, as well as several other enzymes in the hexosamine biosynthetic pathway. They then postulated that enzymes responsible for hexosamine biosynthesis, in particular, GFAT1, which catalyzes the rate-limiting reaction, might be transcriptionally controlled by XBP1s. This was a critical insight that led to the discovery of the missing link. A most insightful leap was when Wang et al went on to show that the promoter-proximal 5’-flanking region of the GFAT1 gene has sequence through which XBP1s enhanced GFAT1 transcription in cardiac myocytes. Moreover, using combinations of XBP1 gain- and loss-of-function in the heart, in vivo, and in cultured cardiac myocytes, coupled with GFAT1 gain- and loss-of-function maneuvers, Wang et al provided clear mechanistic evidence supporting the hypothesis that XBP1s is the missing link between the UPR and protein O-GlcNAcylation. Moreover, they provided evidence supporting the idea that XBP1s-mediated increases in O-GlcNAcylation can protect the heart from ischemia/reperfusion damage, demonstrated by a reduction in infarct size.
A few questions arise from the study by Wang et al, one of which concerns the other isoform of GFAT, GFAT2, which is considered to be the major isoform of GFAT in the heart6. Other studies have shown that it is GFAT2, and not GFAT1 that is regulated in the heart by pressure overload or exercise7, 8, 9. This leads to the question of whether GFAT2 could be regulated by XBP1s? There is a sequence at -273 to -265 in the mouse GFAT2 promoter (TCACGTCT), which is close to the sequence and location of the XBP1s binding site that Wang et al found at -276 of the mouse GFAT1 promoter (CCACGTCA). Both elements have the core ACGT sequence, which was previously shown to be required for XBP1s binding10. Thus, as with GFAT1, XBP1s might bind to the GFAT2 promoter and increase GFAT2 transcription. Wang et al briefly investigated whether GFAT2 might also be regulated by XBP1, but found that, in contrast to GFAT1, GFAT2 expression was not increased by XBP1. These results are consistent with a previous study. which showed that GFAT1, but not GFAT2, was induced by XBP1 11s. Thus, even though a putative XBP1s binding site exists in GFAT2, it appears that, in contrast to GFAT1, GFAT2 does not serve as an XBP1 target.
Another question that arises is how does O-GlcNAcylation protect the heart from ischemia/reperfusion damage? Answering this question will require knowledge of the proteins that are O-GlcNAcylated, as well as an understanding of how O-GlcNAcylation alters their functions. Recently, the identities of many O-GlcNAcylated cardiac proteins were identified, but just how O-GlcNAcylation affects their functions remains to be determined 12.
Is O-GlcNAcylation always cardioprotective? Although O-GlcNAcylation protects the heart from ischemia/reperfusion damage, in other settings, including the diabetic heart, O-GlcNAcylation appears to contribute to cardiac dysfunction13. Driven mostly by elevated glucose and the resulting increase in flux through the hexosamine biosynthesis, O-GlcNAcylation of a number of proteins increases in the diabetic heart. For example, Ca2+/calmodulin-dependent kinase II (CaMKII) is O-GlcNAcylated in the diabetic heart, which leads to a hyperactivation of CaMKII, increased phosphorylation of the ryanodine receptor. Ryanodine receptor phosphorylation by CaMKII results in increased calcium leaks from the sarcoplasmic reticulum, which contributes to the arrhythmia observed in diabetic cardiomyopathy14.
What roles does O-GlcNAcylation play in other cardiac pathologies? O-GlcNAcylation is increased in mouse models of pathological cardiac hypertrophy and heart failure. In hypertrophy, NFAT activation, which is a key driver of hypertrophic growth, is inhibited by blocking O-GlcNAcylation15. Moreover, NFAT is O-GlcNAcylated, leading to speculation that cardiac hypertrophy is evoked, at least partly, by the direct O-GlcNAcylation of NFAT16. In a mouse model of heart failure induced by myocardial infarction, O-GlcNAcylation increased during heart failure17. In this study, cardiac specific deletion of OGA decreased O-GlcNAcylation, increased infarct size, and decreased survival, suggesting that O-GlcNAcylation is protective in this model of heart disease.
In summary, O-GlcNAcylation has major effects in the healthy and diseased heart. However, unlike protein phosphorylation, which governs the structure and function of a wide spectrum of hundreds of protein kinase substrates, O-GlcNAcylation addition and removal require only two enzymes; thus, the molecular mechanisms regulating the extent and determining the targets of O-GlcNAcylation must be quite different than those that regulate protein phosphorylation. The study by Wang et al has contributed significantly to our understanding of how O-GlcNAcylation can be regulated by the UPR and the transcription factor, XBP1, and that XBP1 protects the heart from ischemia/reperfusion damage, partly by increasing O-GlcNAcylation. As a result of their study, XBP1 is now also recognized for its roles as a regulator of O-glycosylation outside of the ER. These findings suggest that, by coordinating N-and O-glycosylation, XBP1 plays a pivotal role in most glycosylation events and, thus, potentially regulates the functions of a vast number of proteins. Further underscoring the potentially beneficial functions of XBP1 on an organismal level was a study that appeared in the same issue of Cell as the paper by Wang et al., which demonstrated that XBP1-mediated increases in hexosamine biosynthesis in C. elegans extended lifespan18. Taken together, these paradigm-shifting studies significantly expand our understanding of the unfolded protein response, and specifically, XBP1 as central regulators of life and death decisions in cells.
ACKNOWLEDGEMENTS
The author thanks Drs. Joseph Hill, Heinrich Taegtmeyer, Richard N. Sifers, and Shirin Doroudgar for critical reading of the manuscript and for insightful discussions.
Sources of Funding: National Institutes of Health, HL-075573, HL-085577, and HL104535
Footnotes
Disclosures: None
REFERNCES
- 1.Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PP, Ferdous A, Gillette TG, Scherer PE, Hill JA. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156(6):1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 3.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107(2):171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99(3):275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 5.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40(3):895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oki T, Yamazaki K, Kuromitsu J, Okada M, Tanaka I. cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics. 1999;57(2):227–234. doi: 10.1006/geno.1999.5785. [DOI] [PubMed] [Google Scholar]
- 7.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44(2):162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 8.Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belke DD. Swim-exercised mice show a decreased level of protein O GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol (1985) 2011;111(1):157–162. doi: 10.1152/japplphysiol.00147.2011. [DOI] [PubMed] [Google Scholar]
- 10.Kanemoto S, Kondo S, Ogata M, Murakami T, Urano F, Imaizumi K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem Biophys Res Commun. 2005;331(4):1146–1153. doi: 10.1016/j.bbrc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Zachara NE. The roles of O-linked beta-N-acetylglucosamine in cardiovascular physiology and disease. Am J Physiol Heart Circ Physiol. 2012;302(10):H1905–1918. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol. 2012;303(7):R689–699. doi: 10.1152/ajpregu.00548.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502(7471):372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2012;302(10):H2122–2130. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2013;142(1):62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107(41):17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156(6):1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]