Summary
A central goal of regenerative medicine is to generate transplantable organs from cells derived or expanded in vitro. Although numerous studies have demonstrated production of defined cell-types in vitro1, creation of a fully intact organ has not been reported. The transcription factor Forkhead box N1 (FOXN1) is critically required for development of thymic epithelial cells (TECs)2,3 a key cell-type of the thymic stroma4. Here, we show that enforced Foxn1 expression is sufficient to reprogramme fibroblasts into functional TECs, an unrelated cell-type across a germ-layer boundary. These Foxn1-induced TECs (iTECs) supported efficient development of both CD4+ and CD8+ T cells in vitro. Upon transplantation, iTEC established a complete, fully organized and functional thymus, that contained all of the TEC sub-types required to support T cell differentiation and populated the recipient immune system with T cells. iTEC thus demonstrate that cellular reprogramming approaches can be used to generate an entire organ, and open the possibility of widespread use of thymus transplantation to boost immune function in patients.
Results and discussion
The thymus, the primary lymphoid organ crucially required for generation of a functional T cell repertoire5, is the first organ to undergo age-related degeneration (thymic involution) during normal ageing6. Thymic involution is a critical factor in the impaired capacity of adult patients to recover adaptive immunity following therapeutic immune depletion7. Thus, development of improved thymus-based therapies for enhancing immune system function in patients is of broad interest. TECs are critical effectors in the intrathymic microenvironments required for T cell development. Two distinct TEC sub-lineages exist, located in the cortical and medullary compartments of the thymus8. These cortical (c) and medullary (m) TEC mediate discrete aspects of T cell development, and their segregation into distinct compartments is thought vital for accurate and efficient production of a self-restricted, self-tolerant T cell repertoire. Despite their functional differences, cTEC and mTEC initially arise from a common progenitor TEC, and the forkhead transcription factor FOXN1, expressed exclusively in thymic and cutaneous epithelia, is required at multiple stages for differentiation of both sub-lineages2,3,9,10. Neonatal thymus transplantation can confer adaptive immunity to congenitally athymic patients11 but its widespread use is limited by donor tissue supply and histocompatibility; these limitations would be overcome if functional TECs could be generated or expanded in vitro. Several investigators have reported derivation of TEC-like cells from pluripotent cells by directed differentiation using growth factors12–16, however, neither generation from these TEC-like cells of an organized thymus containing all TEC subtypes, nor their capacity to support T cell development in vitro has been demonstrated. Although in one report TEC expressing the transcription factor Autoimmune Regulator (AIRE), which are critical for the establishment of self-tolerance 17, were detected after transplantation of the pluripotent cell-derived TEC, there, no demarcation of cortical and medullary compartments was evident16. In sum, the challenge of generating therapeutically useful TEC in vitro, that can form a fully functional thymus upon transplantation, remains unsolved. Here, we have examined two questions: first, whether the alternative approach of direct reprogramming can be used to generate functional TEC in vitro, and second, whether artificial TEC derived by this method can generate a complete thymus upon transplantation.
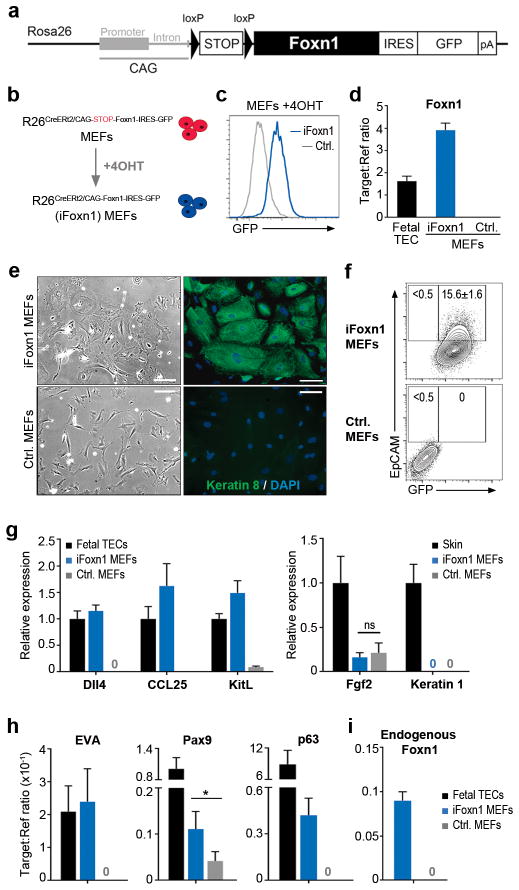
To address the first question, we tested the outcome of enforcing expression of FOXN1, our candidate reprogramming factor, in primary mouse embryonic fibroblasts (MEFs). For this purpose, we developed a transgenic mouse line in which a full length Foxn1 cDNA under control of the CAG promoter was knocked in to the Rosa26 locus, with a LoxP-flanked transcriptional STOP cassette inserted between the CAG promoter and the Foxn1 cDNA (Rosa26CAG-STOP-Foxn1-IRES-GFP; Fig. 1a and Supplementary Fig. 1). Crossing with Rosa26CreERt2 mice generated Rosa26CreERt2/CAG-STOP-Foxn1-IRES-GFP embryos, from which we derived primary MEFs. Tamoxifen-induced Cre-mediated excision of the STOP cassette in these MEFs generated Rosa26CreERt2/CAG-Foxn1-IRES-GFP (iFoxn1) MEFs, in which Foxn1 expression was induced at levels comparable to fetal TEC (Fig. 1b– d); tamoxifen-independent Cre-mediated excision was not detected (Supplementary Fig. 2).
Figure 1. Enforced Foxn1 expression induces epithelial identity in fibroblasts.
a, iFoxn1 transgene. b, Schematic showing procedure for generating iFoxn1 MEFs. c, GFP expression in iFoxn1 and Rosa26CreERt2/+ (control) MEFs 10 days after 4OHT treatment. d, Foxn1 mRNA expression in iFoxn1 and Rosa26CreERt2/+control MEFs 10 days after 4OHT treatment, and E15.5 wild-type EpCAM+ fetal TEC, normalized to the geometric mean of two housekeeping (2HK) genes. Data shown are mean ± s.d. from n=3 independent experiments. e, Bright field and immunofluorescence images showing morphology and Keratin 8 staining, 10 days after 4OHT treatment. Scale bar, 100μm. f, EpCAM and GFP expression 10 days after 4OHT treatment, after gating on live cells. Values shown are mean ± s.d. from n=3 independent experiments. g-i, mRNA expression levels of the genes shown in purified EpCAM+ iFoxn1 and control MEFs normalized to the geometric mean of 2HK. Data shown are mean ± s.d. from n=3 independent experiments (ns, not significant; *p<0.05). g, Expression level is shown relative to E15.5 EpCAM+ fetal TEC or skin. Blue bars, Rosa26CreERt2/CAG-Foxn1-IRES-GFP (iFoxn1); grey bars, Rosa26CreERt2/+ (control); black bars, fetal TEC, throughout. n≥3 independent experiments for panels (c-i). (d,g-i) 3 technical replicates were run for each n. cDNA was generated from 200 cells using the cells direct (amplification) method (d,g,h) or from 50,000 cells without amplification (i). Error bars or values show mean ± s.d. See also Supplementary Fig. 1 and Supplementary Fig. 2.
By ten days after initiation of Foxn1 expression, the morphology of iFoxn1 MEFs had changed from an elongated, bipolar shape characteristic of fibroblasts, to a broader, polygonal shape, characteristic of epithelial cells (Fig. 1e). The identity of these cells was probed using the epithelial-specific markers, Keratin 8 (K8) and Epithelial Cell Adhesion Molecule (EpCAM), which are expressed by all TECs during early thymus development9,18. Most if not all iFoxn1 MEFs, but no control MEFs, expressed K8 (Fig. 1e) and approximately 15% expressed EpCAM (Fig. 1f), suggesting that Foxn1 induction had converted the fibroblasts to an epithelial-like state.
To investigate the identity of the epithelial-like iFoxn1 MEFs, we isolated EpCAM+ cells and analyzed them for expression of TEC- (Dll4, Ccl25 and Kitl)9,19 and cutaneous epithelium- (Fgf2 and Keratin 1)20 specific FOXN1-regulated genes. The iFoxn1 MEFs but not control MEFs expressed Dll4, Ccl25 and Kitl at levels comparable to fetal TEC, but did not express cutaneous epithelium-associated genes (Fig. 1g). Other TEC-associated genes not previously implicated as FOXN1 targets, Epithelial V-like antigen (EVA), which is broadly expressed in TEC, and Pax9 and p6319, which are differentially expressed among TEC subsets, were also induced in iFoxn1 MEFs (Fig. 1h). We consistently detected low levels of endogenous Foxn1 (Fig. 1i), indicating a direct auto-regulatory mechanism and/or indirect activation of endogenous Foxn1 as part of an initiated TEC differentiation programme. Collectively, enforced FOXN1 expression in MEFs induces genes involved in TEC development and function, suggesting a FOXN1-mediated conversion of MEFs into TEC-like cells (designated iTEC hereafter). Foxn1 is also expressed in cutaneous epithelium, yet here clearly induces a transcriptional programme characteristic of TEC rather than cutaneous epithelium. While the reasons for this are not understood, the gene expression programme in TEC clearly shares some elements with that of cutaneous epithelium (p63, keratins, some claudins). This may relate to the high levels of FOXN1 induced in our system, and to the intracellular context provided by the iTEC-induction protocol.
To test the functional attributes of the iTEC, we determined their capacity to support T cell development in vitro. A monolayer of iTEC, in which FOXN1 had been induced for seven days, was seeded with fetal Lin−CD25−C-Kit+ early T lineage progenitors (ETPs21). Analysis after twelve days of co-culture (Fig. 2a) revealed the presence of CD4+CD8+ double positive (CD4+CD8+ DP), CD4+ single positive (CD4+ SP) and CD8+ single positive (CD8+ SP) cells, with the subset distribution on the iTEC monolayers closely resembling that of the adult mouse thymus (Fig. 2b). As expected, the T cells generated on the iTEC monolayer expressed both CD3ε and T cell receptor beta (TCRβ) (Fig. 2c,d). In contrast, ETP seeded onto control MEFs did not enter thymopoiesis (Fig. 2b); instead, most hematopoietic cells remaining after co-culture on MEFs expressed the B-cell marker B220 (Fig. 2c). Consistent with the requirement for MHC Class II on TEC for positive selection and therefore efficient generation of CD4+ SP thymocytes, EpCAM+ iTEC showed robust expression of MHC Class II after co-culture with thymocytes (Fig. 2e). Surface MHC Class II was not evident in iTEC prior to co-culture, demonstrating that the cells generated by the initial reprogramming step differentiated upon exposure to developing T cells, as observed for TEC within the native thymus in vivo22. Interestingly, the capacity of iTEC to support thymocyte development was dependent on their plating density, with a high density (>500 cells/mm2) producing >3 times more CD4+CD8+ T cells within twelve days than a lower density (<250 cells/mm2) (Supplementary Fig. 3). However, the addition of Fibroblast Growth Factor 7 (FGF7)23 to low density iTEC substantially improved their ability to support T cell development (Supplementary Fig. 3), consistent with the established role of FGF7 as mitogen for fetal and postnatal TEC23. Of note, FGF7 does not affect fibroblast proliferation24, while FgfR2IIIb, the receptor for FGF7 and FGF10 is required for normal thymus development23. The kinetics of T cell development were similar when ETP were seeded onto iTEC or the stromal cell line OP9-DL1 (Supplementary Fig. 4), although as expected, development of CD4+ SP thymocytes was typically more efficient in iTEC cultures (Supplementary Fig. 4). Collectively, iTEC can robustly support T cell development in vitro, with thymocyte differentiation to the CD4+ and CD8+ SP stages closely mirroring in vivo differentiation. Although further work is required to test the capacity of iTEC to support T cell development from the circulating lympho-myeloid proliferating progenitor (LMPP), which is considered to be the thymus-seeding hematopoietic progenitor that gives rise to mature T cells25,26, we anticipate that iTEC will have this capacity as it is well established that LMPP differentiate into T cells in co-culture with OP9-DL1 cells25,27,28.
Figure 2. iTEC can support T cell development in vitro.
a, Strategy for in vitro T cell differentiation assay. Monolayers of iTEC or control MEFs, 7 days following 4OHT treatment, were seeded with ETP (Lin-CD25−C-Kit+) isolated from E14.5 wild-type thymus lobes and were analyzed 12 days later (Lin: CD3ε, CD4, CD8, CD11b, CD11c, B220, Gr-1, NK1.1, Ter119). Right panel, bright field image of iTEC/thymocyte co-culture. Scale bar, 50μm. b-d, Plots show staining after gating on CD45+ cells following 12 days co-culture with iTEC or control MEFs. Values shown are mean ± s.d. from n=4 independent experiments. d, TCRβ expression on CD4+ and CD8+ cells from iTEC co-cultures or wild-type (WT) thymus. e, Control MEFs or iTEC after gating on CD45- cells, and EpCAM+ cells for iTEC, following 12 days co-culture with thymocytes. Values shown are mean ± s.d. from n=3 independent experiments. See also Supplementary Fig. 3 and Supplementary Fig. 4.
To address our second question, of whether the iTEC, an artificial cell type generated in vitro, could form an organized and functional thymus, we aggregated iTEC (5 days after induction of Foxn1) or control MEFs with immature thymocytes (CD45+Lin−) and fetal thymic mesenchyme from embryos at day 12.5 to 13.5 of development (E12.5–13.5) (CD45−PDGFRαβ+), and grafted the resulting cell aggregate under the kidney capsule of syngeneic adult mice29,30 (Fig. 3a). Fetal thymic mesenchyme was included in order to ensure that growth factors essential for expansion of the thymus, including Fgf10 and IGF 23,31,32, were available within the graft. Macroscopic, well-formed organs were recovered 4 weeks post-grafting from recipients of iTEC, but not control MEF-only, grafts (Fig. 3b). These iTEC-derived organs exhibited a characteristic thymus morphology, with multiple clearly defined cortical and medullary regions evident in all grafts (Fig. 3c). Pan-cytokeratin staining revealed a reticular network of epithelial cells throughout the organs (Fig. 3c), while flow cytometric analysis indicated that haematopoietic and epithelial cells were present in similar proportions in the recovered iTEC grafts to in the native adult thymus (Fig. 3d). All EpCAM+ cells in the iTEC grafts expressed GFP, reporting the transgenic iFoxn1-IRES-GFP mRNA, confirming they were derived from the input iTEC (Fig. 3e).
Figure 3. iTEC form a functional thymus in vivo.
a, Schematic of grafting assay. iTEC (5 days following Foxn1induction) or control MEFs were aggregated with wild-type CD45+Lin− thymocytes and E12.5-13.5 wild-type CD45−PDGFRαβ+ thymic mesenchymal cells, and grafted under the kidney capsule of adult mice. Lin: CD3ε, CD4, CD8, CD11b, CD11c, B220, Gr-1, NK1.1, Ter119. b, Summary of recovered grafts. c, Haematoxylin and eosin staining, and pan-cytokeratin (panK) staining of iTEC-derived kidney graft. m, medulla; c, cortex; scale bar, 500μm. d–e, Analysis of iTEC grafts, and wildtype (WT) thymi from 8 week old mice. e, shows CD45−EpCAM+ cells from gate defined in (d); GFP expression reports CAG-iFoxn1. Data shown are representative and are from one of at least 3 independent experiments.
Thymus architecture and function are intimately linked, and furthermore, the presence of discrete TEC subsets within the cortical and medullary compartments is required for fully functionality of the organ9, including for inducing central tolerance in the emerging T cell repertoire33. Correctly compartmentalized regions of cTECs and mTECs were evident in the iTEC-derived grafts, and expressed region-appropriate markers (CD205 and K14 respectively18,34) (Fig. 4a). The functional cTEC marker β5t35, and the UEA-1hi sub-population of mTECs9, were also evident (Fig. 4a). Functional competence was further demonstrated by the presence of AIRE+ mTEC33 and MHC Class IIlo and MHC Class IIhi TEC populations (Fig. 4b,c). iTEC recovered from the grafts expressed a range of markers associated with TEC differentiation and function at or close to the levels normally present in TEC (Fig. 4d). Thus, iTEC were able to differentiate to generate a functional thymus containing all of the major TEC populations and exhibiting the architectural characteristics diagnostic of a fully functional native thymus.
Figure 4. Intrathymic T cell development in iTEC-derived grafts.
a,b, Immunohistochemical analyses of iTEC grafts and wild-type (WT) thymi using cTEC (CD205 and β5t) and mTEC (Keratin 14 (K14) and UEA-1) specific markers, and α-AIRE. Scale bars (a), 150μm; scale bar (b), 25μm. c, Flow cytometry of MHC Class II expression for CD45-EpCAM+ cells defined in (Fig. 3d). Gates show MHC Class IIlo and MHC Class IIhi populations defined using WT TEC. (a–c) Data shown are representative and are from one of at least 4 independent experiments. (d) RT-qPCR analysis of 50 CD45-EpCam+MHC Class II+ iTEC, recovered from a single graft 7 weeks post-transplantation, for the markers shown. Data are shown relative to expression in E15.5 WT total EpCam+ TEC after normalization to 2HK (HMBS and TBP); expression level in E15.5 WT is 1 for all samples. Values shown are from 1 experiment. (e–h) Analysis of 8 week-old WT mouse thymus, iTEC grafts or cells collected from control MEFs graft site at 4 weeks post-transplantation. (f,g,h) show thymocyte populations defined in (e). e, CD4 and CD8 expression after gating on CD45+ cells. f, Percentage of CD4+CD8+ cells. g, TCRβ expression. h, TCRγδ expression on CD4-CD8- thymocytes. (e,g) Data shown are representative and are from one of at least 4 independent experiments, (f) each data point represents a separate graft from n=4 independent experiments, (h) data shown are from 1 experiment.
A crucial question was whether the iTEC-derived grafts supported normal T cell development, and hence population of the peripheral immune system with newly generated naïve T cells. Thymocyte subset distribution in the iTEC-derived grafts closely matched that of the native adult thymus (Fig. 4e, f). Distribution of TCRβ expression within iTEC grafts was near identical to that observed within the native thymus, with TCRβhi cells restricted to CD4+ SP and CD8+ SP populations (Fig. 4g). TCRγδ+ T cells were present within CD4−CD8− DN populations, again at a comparable proportion in the iTEC grafts and native thymus (Fig. 4h). No macroscopic grafts were recovered from MEFs-graft recipients, and no evidence of thympoiesis could be found in tissue adjacent to the graft site in any MEFs-graft recipient mice (Fig. 4e, f). The T cells generated in the iTEC grafts were exported to populate the peripheral immune system. Peripheral blood analysis revealed the presence of CD4+ and CD8+ T cells, and Foxp3+ regulatory T cells in two of three nu/nu iTEC recipient mice by 8 weeks post grafting, with numbers continuing to rise over time, while no donor-derived peripheral T cells were detected in control mice (Fig. 5a-f, Supplementary Fig. 5). These peripheral T cells expressed normal levels of TCRβ and exhibited CD62LCD44 subset phenotypes consistent with the presence of both naïve and activated cells (shown for splenocytes in Fig. 5d, e). Analysis of both CD44loVβ+ and total Vβ+ cells present in the spleen and lymph node of iTEC-grafted mice indicated a diverse T cell repertoire (Fig. 5g), the functionality of which was demonstrated by cytokine secretion in response to CD3/CD28 cross-linking (Fig. 5h,i).
Figure 5. A diverse and functional peripheral T cell repertoire is generated by iTEC-derived grafts.
a, Schematic showing experimental design for (b-i). (b-f) Representative analysis of spleen and lymph node cells or peripheral blood (PB) from nude mice, using the T cell markers indicated. T cells were detected in the PB of two of three iTEC recipients. Splenocytes (b,d) or peripheral blood (c,e,f) from 6 weeks (f), 8 weeks (e), 14 weeks (b,d) or 2-weekly intervals, as shown (c) post-transplantation. (b,d,e) Data shown are representative and are from one of at least 3 independent experiments, (c) values shown are mean from n=2 independent experiments. Values used to derive mean in (c): iTEC graft recipient mice at 0 weeks, 0; 4 weeks, 1.03 and 0.61; 6 weeks, 1.40 and 1.10; 8 weeks, 2.02 and 1.46, and control graft recipient mice at 0 weeks, 0; 4 weeks, 0.02 and 0.09; 6 weeks, 0.07 and 0.19; 8 weeks, 0.22 and 0.15. (f) data shown are from 1 experiment. (g) Analysis of CD3+ cells from pooled spleen and lymph nodes of recipient nude mice 20 weeks post-transplantation. Data shown are representative and are from one of 2 independent experiments,. (h,i) Analysis of IL2 expression in CD4+ cells from lymph nodes of recipient nude mice 20 weeks post-transplantation. Values represent mean ± s.d. for cells from n=3 separate culture dish wells from 1 experiment. Values used to derive mean and s.d. in (i): wildtype, 55, 33, 75 (unstimulated) and 700, 931, 898 (stimulated); iTEC, 61, 22, 42 (unstimulated) and 572, 403, 780 (stimulated); control, 11, 20, 8 (unstimulated) and 50, 27, 65 (stimulated). See also Supplementary Fig. 5.
The data present herein establish that enforced expression of Foxn1 is sufficient to convert fibroblasts into iTEC, an in vitro generated cell type that exhibits phenotypic and functional properties of in vivo TEC. They further show that iTEC are able to promote full T cell development in vitro, potentially offering an improved system for in vitro T cell development over currently existing models due to their capacity to express MHC Class II and thus for selection of CD4+ SP thymocytes, a characteristic not observed in other models of in vitro T cell differentiation36,37. iTEC thus represent a clear and essential step towards generation of patient-specific T cells in culture. They further demonstrate that iTEC generate a properly patterned, functional organ upon transplantation in vivo, composed of cTEC and mTECs that express markers diagnostic of each cell type, including markers critical for T cell lineage differentiation and repertoire selection. To our knowledge this is the first demonstration of generation of a complete, organized and functional, complex organ from reprogrammed cells. Our data thus identify iTEC as a novel and readily available source of TEC, that may provide the basis for thymus transplantation therapies aimed at boosting adaptive immune system function in immunocompromised patients.
Supplementary Material
Acknowledgments
We thank O. Rodrigues and C. Cryer for cell sorting, V Berno for imaging, F.H. Stenhouse and C.D. Peddie for technical assistance and J. Mee for kidney grafting, R. Zamoyska, Becky Brownlie and Graeme Cowan for advice, V. Wilson, K. Kaji, A.G. Smith, I. Chambers, and N. Hastie for comments on the manuscript, and the Biological Research Facility staff for animal care. The research leading to these results received funding Leukaemia and Lymphoma Research (CCB and NB), the Darwin Trust of Edinburgh (SU), the School of Biological Sciences, University of Edinburgh (HV), the Medical Research Council (CCB), the European Union Seventh Framework Programme (FP7/2007-2013) collaborative projects EuroSyStem (CCB and NB) and ThymiStem (CCB, SU, KO’N) under grant agreement numbers (200720 and 602587 respectively), and NIH/NIAID grant # R01 AI082127 (NRM).
Footnotes
Authorship Contributions
NB generated the iFoxn1 allele, conceived and designed experiments, performed experiments, analyzed the data and contributed to writing the manuscript; NRM contributed to experimental design and to writing the manuscript; SU, KO’N and HV performed experiments and contributed to analysis of the data; CCB conceived the original idea, designed experiments, contributed to analysis of the data and wrote the manuscript.
See also Supplementary Information.
References
- 1.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 2.Nehls M, et al. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn CC, et al. The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. Proc Natl Acad Sci USA. 1996;93:5742– 5746. doi: 10.1073/pnas.93.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritter MA, Boyd RL. Development in the thymus: it takes two to tango. Immunol Today. 1993;14:462–469. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- 5.Miller JFAP. Immunological function of the thymus. Lancet. 1961;2:748– 749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 6.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch HE, et al. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manley NR, Richie ER, Blackburn CC, Condie BG, Sage J. Structure and function of the thymic microenvironment. Frontiers Biosci. 2011;16:2461–2477. doi: 10.2741/3866. [DOI] [PubMed] [Google Scholar]
- 9.Nowell CS, et al. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi: 10.1371/journal.pgen.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul CC, et al. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 11.Markert ML, et al. Transplantation of thymus tissue in complete DiGeorge Syndrome. The New England J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 12.Lai L, Jin J. Generation of thymic epithelial cell progenitors by mouse embryonic stem cells. Stem Cells. 2009;27:3012–3020. doi: 10.1002/stem.238. [DOI] [PubMed] [Google Scholar]
- 13.Lai L, et al. Mouse embryonic stem cell-derived thymic epithelial cell progenitors enhance T-cell reconstitution after allogeneic bone marrow transplantation. Blood. 2011;118:3410–3418. doi: 10.1182/blood-2011-03-340794. [DOI] [PubMed] [Google Scholar]
- 14.Inami Y, et al. Differentiation of induced pluripotent stem cells to thymic epithelial cells by phenotype. Immunol Cell Biol. 2011;89:314–321. doi: 10.1038/icb.2010.96. [DOI] [PubMed] [Google Scholar]
- 15.Parent AV, et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, et al. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell. 2013;13:230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 18.Klug DB, et al. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci USA. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manley NR, Condie BG. Transcriptional regulation of thymus organogenesis and thymic epithelial cell differentiation. Prog Mol Biol Transl Sci. 2010;92:103–120. doi: 10.1016/S1877-1173(10)92005-X. [DOI] [PubMed] [Google Scholar]
- 20.Weiner L, et al. Dedicated epithelial recipient cells determine pigmentation patterns. Cell. 2007;130:932–942. doi: 10.1016/j.cell.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Allman D, et al. Thymopoiesis independent of common lymphoid progenitors. Nature Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 22.Klug DB, Carter C, Gimenez-Conti IB, Richie ER. Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol. 2002;169:2842–2845. doi: 10.4049/jimmunol.169.6.2842. [DOI] [PubMed] [Google Scholar]
- 23.Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–1961. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- 24.Rubin JS, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Luc S, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nature Immunol. 2012;13:412–419. doi: 10.1038/ni.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nature Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 28.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 29.Bennett AR, et al. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan JM, Taoudi S, Medvinsky A, Blackburn CC. A novel method for the generation of reaggregated organotypic cultures that permits juxtaposition of defined cell populations. Genesis. 2009;47:346–351. doi: 10.1002/dvg.20505. [DOI] [PubMed] [Google Scholar]
- 31.Auerbach R. Morphogenetic interactions in the development of the mouse thymus gland. Dev Biol. 1960;2:271– 284. doi: 10.1016/0012-1606(60)90009-9. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G. PDGFRalpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood. 2007;109:954–960. doi: 10.1182/blood-2006-05-023143. [DOI] [PubMed] [Google Scholar]
- 33.Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Shakib S, et al. Checkpoints in the development of thymic cortical epithelial cells. J Immunol. 2009;182:130–137. doi: 10.4049/jimmunol.182.1.130. [DOI] [PubMed] [Google Scholar]
- 35.Murata S, et al. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 37.Dervovic DD, et al. Cellular and molecular requirements for the selection of in vitro-generated CD8 T cells reveal a role for Notch. J Immunol. 2013;191:1704–1715. doi: 10.4049/jimmunol.1300417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hameyer D, et al. Toxicity of ligand-dependent Cre recombinases and generation of a conditional Cre deleter mouse allowing mosaic recombination in peripheral tissues. Physiol Genomics. 2007;31:32–41. doi: 10.1152/physiolgenomics.00019.2007. [DOI] [PubMed] [Google Scholar]
- 39.Wallace HA, et al. Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence. Cell. 2007;128:197–209. doi: 10.1016/j.cell.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 40.Gray DH, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.