Summary
This review addresses the possible role of the insulin-like growth factor (IGF)-axis in normal glucose homoeostasis and in the etiopathogenesis of type 2 diabetes. IGF-I, a peptide hormone, shares amino acid sequence homology with insulin and has insulin-like activity; most notably, the promotion of glucose uptake by peripheral tissues. Type 2 diabetes as well as pre-diabetic states, including impaired fasting glucose and impaired glucose tolerance, are associated cross-sectionally with altered circulating levels of IGF-I and its binding proteins (IGFBPs). Administration of recombinant human IGF-I has been reported to improve insulin sensitivity in healthy individuals as well as in patients with insulin resistance and type 2 diabetes. Further, IGF-I may have beneficial effects on systemic inflammation, a risk factor for type 2 diabetes, and on pancreatic β-cell mass and function. There is considerable inter-individual heterogeneity in endogenous levels of IGF-I and its binding proteins; however, the relationship between these variations and the risk of developing type 2 diabetes has not been extensively investigated. Large prospective studies are required to evaluate this association.
Keywords: insulin-like growth factor (IGF)-I, glucose, diabetes, IGFBP
Introduction
The insulin-like growth factor (IGF)-axis is an evolutionarily conserved system involved in the regulation of cell growth, proliferation, and survival that affects nearly every organ system in the body. This axis includes two growth factors, IGF-I and IGF-II, six IGF-binding proteins (IGFBP-1 to -6), and nine IGFBP-related proteins (IGFBP-rPs) [1–3]. IGF-I mediates many of the somatic effects of growth hormone (GH) and most cells express the IGF-I receptor (IGF-IR) [4]. Indeed, laboratory and epidemiological studies suggest that the IGF-axis may be involved in the pathogenesis of a wide range of health conditions, including several common cancers [5], osteoporosis [6], and possibly coronary heart disease [7].
IGF-I may also have a role in regulating glucose and lipid metabolism. In vitro studies conducted as early as the 1960s observed that insulin did not account for all the insulin-like activity (ILA) detected in human serum [8]. Research at the time found that a significant fraction of ILA could not be suppressed by antibodies targeted against insulin, and was instead attributed to a newly isolated molecule which was later named IGF-I [9]. IGF-I shares structural homology and downstream signaling pathways with insulin, and laboratory data have shown that IGF-I has insulin-like effects on peripheral uptake of glucose and fatty acids [1,10]. Further, exogenous administration of recombinant human IGF-I enhances insulin sensitivity in healthy adults [11,12] as well as those with type 2 diabetes [13]. Insulin resistance states including obesity, a strong risk factor for type 2 diabetes, have been associated with altered levels of IGF-I and its binding proteins in circulation [14]. Against this background, the purpose of this review, therefore, is to critically evaluate the existing evidence for a role of the IGF-axis in the maintenance of normal glucose homeostasis as well as in the etiology of type 2 diabetes.
Overview of the IGF-axis
IGF-I is a peptide hormone that shares nearly 50% amino acid sequence homology with proinsulin, and like insulin, is composed of an alpha and a beta chain connected by disulfide bonds [10]. Most IGF-I in circulation is produced by the liver, with IGF-I levels largely regulated by GH via a negative feedback mechanism [15]. However, other factors may also affect hepatic IGF-I synthesis, including nutrition (e.g. caloric intake and protein consumption), insulin, and inflammatory cytokines [16–21]. IGF-I levels are relatively low in fetal life, peak at the time of the adolescent growth spurt, and then undergo a slow decline during adulthood [22–25].
The effects of IGF-I are primarily mediated by its binding to the IGF-IR [26]. The IGF-IR, like the insulin receptor, is comprised of two membrane-spanning alpha subunits and two intracellular beta subunits. Insulin and IGF-I can bind to each other’s receptors, albeit with low affinity, and only at high (non-physiological) levels [27,28]. IGF-II can bind the IGF-IR as well its own receptor, IGF-IIR [29,30], however, the function of IGF-II in adults is not well understood. The binding of both IGF-I and insulin to their respective receptors results in the activation of the tyrosine kinase domain present in these receptors, and post-receptor phosphorylation of members of the insulin receptor substrate (IRS) family [31]. The insulin receptor preferentially phosphorylates IRS-1, whereas IGF-IR preferentially phosphorylates IRS-2, which may partly correspond to the differences in their activity [26]. IGF-I is a more potent mitogen with stronger anti-apoptotic activity than insulin, and plays a major role in regulating cell replication, differentiation, and survival [32], whereas insulin has stronger metabolic activity than IGF-I [33].
In addition to the IGF-IR and the insulin receptor, there are hybrid receptors composed of one alpha and one beta subunit of the IGF-IR, and one alpha and one beta subunit of the insulin receptor. Depending on the insulin receptor isoform present (either IR-A or IR-B), these hybrid receptors may vary both in their relative affinity for IGF-I, IGF-II, and insulin, as well as in their activity [34–36]. A hybrid receptor containing isoform IR-A, for example, strongly binds both insulin and IGF-II and is most extensively expressed by embryonic, hematopoietic, central nervous system, and tumor cells. In contrast, a hybrid receptor containing the IR-B isoform is expressed predominantly in muscle and adipose tissue [37,38], which are important target tissues for insulin action. These IR-B isoform hybrid receptors represent a possible signaling pathway for the insulin-like effects of IGF-I. A recent laboratory study, in fact, suggested that the binding of IGF-I with these hybrid receptors may be as potent in stimulating glucose uptake as insulin binding with its receptor [39]. It is possible, therefore, that tissue-specific differences in the insulin-like activity of IGF-I could at least be partly attributable to the differences in receptor distribution.
In contrast to insulin, which is largely unbound to any transport molecules, as much as 99% of IGF-I in circulation is bound to one of the six IGF-binding proteins (IGFBPs) [40]. By binding IGF-I, IGFBPs can prevent its proteolysis and extend its half-life in serum, while also reducing its bio-availability. For example, the most abundant IGFBP in circulation, IGFBP-3, forms a ternary complex with IGF-I and an acid-labile subunit (ALS). The half-life of this ternary complex is 12–15 hours, whereas it is ≤10 minutes for ‘free’ or unbound IGF-I [41]. These relationships are complex, however, and certain IGFBPs may additionally act as transport molecules helping to transport IGF-I to its target cells, and some may even modulate the interaction of IGF-I with its cellular receptors.
Furthermore, IGFBPs have biological activity that is independent of IGF-I [42]. IGFBP-3, for example, can directly bind to cellular targets involved in the cell-cycle, including the ribonucleic acid polymerase II binding subunit 3 (Rpb3), suggesting a possible role of IGFBP-3 in directly regulating gene transcription [43]. The effects of IGFBP-3 on cell-cycle are largely opposite to those of IGF-I since IGFBP-3 is pro-apoptotic [44–53] and anti-proliferative [52,54–58]. Although the IGFBP family was recently expanded to include nine IGFBP-rPs that can bind IGF-I and IGF-II [3,59], some investigators have challenged their inclusion due to absence of clear phylogenetic relationships between the IGFBP-rPs and the IGFBPs [60], and the limited understanding of IGFBP-rP function.
Total circulating IGF-I and IGFBP-3 levels appear to have little or no detectable diurnal or circadian variation [61,62]. However, there is extensive inter-individual variation in levels of total IGF-I [62–65] and the IGFBPs [7,62–64,66]. It is reasonable, therefore, to assume that these inter-individual differences could play a role in the risk of disease. Only approximately 1% of IGF-I in circulation is reported to be unbound to IGFBPs [33], but this ‘free’ IGF-I levels, like total IGF-I levels, are heterogeneous across individuals [41,67]. Free IGF-I has been proposed by some investigators to be the main bioactive component of IGF-I in circulation (as is the case for several other hormones; e.g. estradiol, thyroid hormone). Unlike total IGF-I, though, free IGF-I levels may vary significantly in the post-prandial state, largely due to the regulation of IGFBP-1 by insulin (see below). While the importance of circulating free IGF-I to IGF activity is debated, laboratory data suggest that free, and not total, IGF-I levels in circulation regulate the negative feedback loop with GH [68]. Currently, several methods are available to estimate free IGF-I levels [33,69–71]. The most commonly employed methods, based on enzyme-linked immunoassays, likely detect the easily dissociable as well as the free IGF-I fraction. The relevance of the easily dissociable fraction, if any, and its bioactivity is yet unknown.
The IGF-axis and glucose and lipid metabolism
Insulin is the primary regulator of glucose metabolism, but it is reasonable to hypothesize that the IGF-axis might also play a role in maintaining glucose homeostasis. As mentioned, laboratory studies have found that IGF-I can promote glucose uptake in certain peripheral tissues [11,12,72,73]. Although the magnitude of this effect is only 4–7% of that of insulin [33,74], the molar concentration of IGF-I in human plasma is 100-fold greater than concentration of insulin. Of the two important peripheral tissues involved in glucose homeostasis, skeletal muscle and adipose tissue, muscle has been shown to have much higher IGF-IR expression [4,75]. Under normal physiologic conditions, therefore, it has been hypothesized that IGF-I might influence glucose homeostasis largely through its insulin-like effects on muscle. In the presence of insulin resistance, though, there is up-regulation of insulin/IGF hybrid receptor expression in both muscle and fat tissue [76–80]. Studies have also shown that IGF-I can suppress hepatic glucose production [73,81–83]. In short, there are at least several mechanisms through which IGF-I could, theoretically, affect glucose homeostasis.
In keeping with the above, a significant positive correlation between insulin sensitivity and endogenous IGF-I concentration among patients with varying degrees of glucose intolerance was reported [84], and the investigators estimated that as much as 11% of the variation in insulin sensitivity could relate to circulating IGF-I levels. Although this was a cross-sectional study, other studies provide additional relevant evidence. Exogenous IGF-I administration, for example, has been shown to reduce serum glucose levels [33,85,86], an effect not only observed among healthy individuals [12,33,87,88] but also in those with insulin resistance [89,90], type 1 [91–93] and type 2 diabetes [13,83,94]. Interestingly, in several of these studies, investigators further demonstrated that these exogenous IGF-I-induced reductions in serum glucose levels were accompanied by an improvement in insulin sensitivity [83,88,93]. There is also some genetic evidence for a role of IGF-I in glucose metabolism. In particular, a rare state of IGF-I deficiency related to a homozygous partial deletion in the IGF-I gene (IGF1) has been associated with severe insulin resistance, which is normalized by IGF-I therapy [95,96]. This insulin-sensitizing effect of IGF-I may not only be due to its GH-suppressing effect but also due to independent IGF-I effects [97]. In addition, similar to IGF-I, congenital GH deficiency is also associated with insulin resistance and chronic complications of hyperglycaemia [98,99]. It is also important to note, however, that some of the metabolic actions of IGF-I are opposite to that of GH, for example, IGF-I decreases glucose and insulin whereas GH raises both [100,101].
IGFBPs are also hypothesized to have a role in glucose metabolism. In particular, IGFBP-1 may acutely regulate glucose levels through its effects on free IGF-I [102]. Several sources of evidence point to this possibility: (i) insulin suppresses IGFBP-1 gene transcription [103], and changes in insulin levels are correlated with relatively acute changes in circulating IGFBP-1 levels [104,105]; (ii) endogenous IGFBP-1 levels correlate inversely with free IGF-I levels [63,104,106]; (iii) exogenous IGFBP-1 injection has been shown to cause reductions in free IGF-I levels [107] and circulating glucose levels [108]; (iv) reduced IGFBP-1 synthesis and circulating levels are observed in states of insulin resistance, such as obesity (a potential compensatory mechanism; see next section) [109–112]. Although insulin may additionally regulate IGFBP-2, its relationship with IGFBP-2 is not as strong as that with IGFBP-1 [113]. IGFBP-2 levels are thought to be affected by chronically high insulin levels, but not by acute changes in insulin as observed with IGFBP-1. Nonetheless, the relation of IGFBP-2 with insulin may be important. Indeed, IGFBP-2 is the principal binding protein secreted by differentiating white pre-adipocytes [114], suggesting a role for IGFBP-2 in the adipocyte self-regulation (autocrine control). To our knowledge, though, there have been no prospective human data to distinguish the effects of IGFBP-2 on obesity from the effects of obesity-induced hyperinsulinemia on IGFBP-2.
Less well known is that, IGFBP-3, the most abundant IGFBP in circulation, may play a role in glucose homeostasis. It has been reported that IGFBP-3 binds to a nuclear receptor, 9-cis retinoic acid receptor-alpha (RXR-α), which interacts with peroxisome proliferator activated receptor-gamma (PPAR-γ), a nuclear protein involved in the regulation of glucose and lipid metabolism [115,116]. Overall, the metabolic effects of IGFBP-3, like its cell-cycle effects, are largely opposite to those of IGF-I [117]. Recent transgenic animal data, for example, demonstrate that overexpression of IGFBP-3 is associated with fasting hyperglycemia and impaired glucose tolerance (IGT) in mice [118,119]. The role of the remaining IGFBPs and IGFBP-rPs in glucose regulation have been little studied, albeit, it was recently reported that circulating IGFBP-rP1 levels are elevated in the presence of insulin resistance [120]. In addition, another study reported a significant correlation (r = 0.4, p < 0.0001) between fasting glucose levels and IGFBP-rP1 levels in cancer tissues [121].
The IGF-axis may also affect lipid metabolism. Specifically, in vitro studies have shown that IGF-I may have insulin-like effects in promoting the uptake of free fatty acids (FFA) into adipocytes, hepatocytes, and other tissues, and secondly, in promoting lipogenesis [83,122]. Consistent with this, several human studies [12,83,123,124], albeit, not all [11,33,72], reported that exogenous IGF-I administration significantly lowered serum FFA levels. In one typical study, for example, recombinant IGF-I administration reduced serum FFA levels from a mean of 411 ± 58 µM at baseline to just 165 ± 36 µM (p < 0.001), with similar effects observed among patients with and without type 2 diabetes [83]. In general, FFA uptake is thought to be the predominant mechanism, with promotion of lipogenesis playing only a minor role in the IGF-axis’s effects on FFAs. It is also interesting to note that while IGF-I mediates many of the cell-cycle effects of GH, the two hormones have sometimes opposing metabolic effects, including in relation to FFA homeostasis. That is, while IGF-I may reduce serum FFA levels, as above, GH promotes lipolysis [125]. As discussed below, however, there may be a disconnect between GH and IGF-I levels in the face of increasing obesity and insulin resistance, and the effects of IGF-I in reducing serum FFA may be important in improving insulin sensitivity related to the ‘lipotoxic’ effect of FFA (e.g., on pancreatic β-cells) [126–128].
The IGF-axis and type 2 diabetes
The evidence summarized above, suggesting that the IGF-axis plays a role in maintaining glucose homeostasis, provides a biological rationale for further hypothesizing that it may also have a role in the etiopathogenesis of type 2 diabetes. Indeed, cross-sectional data have repeatedly shown that obesity and insulin resistance are associated with a number of alterations in the IGF-axis and related factors.
Obesity, for example, is associated with hyposecretion of GH, the major regulator of IGF-I secretion by the liver, but circulating levels of total IGF-I are not low, and free IGF-I levels may actually be elevated in obesity [14,129]. How might this be explained? First, adipocytes can produce IGF-I, and in obese individuals, may significantly contribute to circulating IGF-I levels [130]. Second, insulin, as mentioned, stimulates hepatic IGF-I synthesis [21] which may partly offset the impact of GH hyposecretion on IGF-I production by the liver. Third, insulin increases the fraction of circulating free IGF-I by down-regulating hepatic synthesis of IGFBP-1 and to a lesser extent, the hepatic secretion of IGFBP-2 [131,132]. Indeed, several studies have reported that free IGF-I levels are elevated in obesity [14,67,132–135], while total IGF-I levels remain within normal range [14,135]. It may be reasonable, therefore, to additionally hypothesize that high free IGF-I levels themselves play a role in GH hyposecretion in obese patients.
Similarly, cross-sectional studies have found that free IGF-I levels are, on average, elevated in patients with IGT and type 2 diabetes [14], and our data [136] and that of others [110,137,138], have shown that IGFBP-1 levels are low in people with IGT and type 2 diabetes. IGFBP-3, in contrast, has a positive cross-sectional correlation with fasting insulin and C-peptide levels [139–141].
Taken as a whole, these data lead us to speculate that low IGFBP-1, possibly low IGFBP-2, and high free IGF-I represent compensatory mechanisms in response to increasing insulin resistance, whereas high IGFBP-3 may be a risk factor for insulin resistance and type 2 diabetes. These hypotheses, however, can only be assessed in appropriate prospective studies.
Prospective data
Cross-sectional studies are limited in their ability to assess causality. That is, the diabetic state itself may be the cause of changes in the IGF-axis rather than the other way around – a situation appropriately termed ‘reverse’ causality. Cross-sectional data cannot, for example, distinguish between the effects of hyperinsulinemia on IGFBP-1 versus the effects of IGFBP-1 on insulin resistance. Furthermore, in patients with overt diabetes, the use of pharmacologic agents (e.g. insulin therapy oral medications) may also affect the IGF-axis [142–144]. In short, we expect the prospective analysis to conflict with prior cross-sectional data (Table 1).
Table 1.
Current cross-sectional vs predicted prospective associations between the IGF-axis and Type 2 diabetesa
| Cross-sectional associations |
Predicted prospective association with type 2 diabetes |
|||
|---|---|---|---|---|
| Obesity/pre-diabetesb | Type 2 diabetes | Remark | ||
| Total IGF-I | Normal levels | Normal levels | Low levels associated with increased risk of diabetes | IGF-I has insulin-like effects on glucose and FFA uptake as well as other effects that may compensate for increasing insulin resistance. Total IGF-I levels are normal despite GH hyposecretion, due to production by adipocytes, and insulin stimulation of hepatic IGF-I synthesis. |
| Free IGF-I | Elevated levels | Elevated levels | Low levels associated with increased diabetes risk (stronger association than that for total IGF-I) | Free IGF-I may be more bio-available than bound IGF-I, and may, as above, compensate for insulin resistance. If correct, those with insulin resistance who have low free IGF-I levels will be at an increased diabetes risk. |
| IGFBP-1 | Reduced levels | Reduced levels | High levels associated with increased risk of diabetes | IGFBP-1 reduces the bio-availability of IGF-I. Insulin, however, down-regulates IGFBP-1, increasing free IGF-I levels in the face of insulin resistance; a potential compensatory mechanism. Cross-sectionally, though, low IGFBP-1 falsely appears to be associated with diabetes. |
| IGFBP-2 | Reduced levels | Reduced levels | High levels associated with increased risk of diabetes | Similar to IGFBP-1. |
| IGFBP-3 | Elevated levels | Elevated levels | High levels associated with increased risk of diabetes | IGFBP-3 reduces the bio-availability of IGF-I, increasing risk of diabetes. Insulin does not, however, regulate IGFBP-3; hence, reverse causality is not an issue with IGFBP-3 (unlike with IGFBP-1 and −2, above). |
Cross-sectional data reflect not only the effects of the IGF-axis parameter (e.g. IGF-I) on diabetes, but also the effects of the diabetes on the parameter (reverse causality). For example, the high free IGF-I levels observed in people with type 2 diabetes may not be because high IGF-I causes diabetes but because increases in free IGF-I are, in theory, a compensatory (protective) mechanism in the face of increasing insulin resistance.
Prediabetes includes impaired glucose tolerance and impaired fasting glucose.
However, prospective data regarding the IGF-axis and its association with the risk of type 2 diabetes are sparse. The human data that do exist are small metabolic studies and just two prospective epidemiologic studies, only one of which was conducted among healthy adults.
The metabolic data were mentioned in earlier sections. Briefly, administration of recombinant IGF-I was found to reduce serum glucose levels and improve insulin sensitivity in healthy individuals [12,33,87] as well as among those with existing insulin resistance [89,90], or type 2 diabetes [13,94]. These studies of exogenous IGF-I administration provide indirect evidence that relatively high endogenous levels of IGF-I may reduce insulin resistance and, thereby, lower the risk of type 2 diabetes.
In one prospective epidemiologic study among healthy adults, Sandhu et al. [145] studied total IGF-I and IGFBP-1 levels among 615 women and men 45–65 years of age. Consistent with the above hypotheses, the study found an inverse association between IGF-I levels and the risk of developing IGT/type 2 diabetes after an average of 4.5 years of follow-up. Specifically, the risk of incident type 2 diabetes in adults with IGF-I levels above the median was approximately half that in those with IGF-I levels below the median (odds ratio [OR] = 0.50; 95% CI: 0.26–0.95). In addition, there was an inverse association between total IGF-I levels at baseline and the 2-hour post-load glucose concentration measured at the end of follow-up; albeit, only among subjects with low IGFBP-1 concentrations (≤25 µg/mL). These data are fairly consistent with the hypothesis that IGFBP-1, which is down-regulated by insulin, may affect glucose metabolism by regulating IGF-I bio-availability. Nonetheless, the interpretation of these findings is somewhat limited by the relatively small sample size of the study (number of cases: IGT = 44 and diabetes = 7), the use of a combined outcome (IGT/type 2 diabetes), and the absence of data regarding free IGF-I or additional IGFBPs, including IGFBP-2 and IGFBP-3. A second prospective study was conducted among patients hospitalized with acute myocardial infarction (n = 186). While this was a highly selected population, as in the above prospective study, the risk of subsequent IGT/type 2 diabetes was lower among those with high baseline IGF-I levels compared to those with low levels (OR: 0.29; 95% CI: 0.09, 0.91) [146].
Thus, the limited prospective epidemiologic data that exist at this time suggest a protective effect of IGF-I against the development of type 2 diabetes. A more comprehensive assessment of the IGF-axis with adequate control for relevant cofactors is now warranted.
Summary of potential mechanisms
Insulin-like IGF-I activity
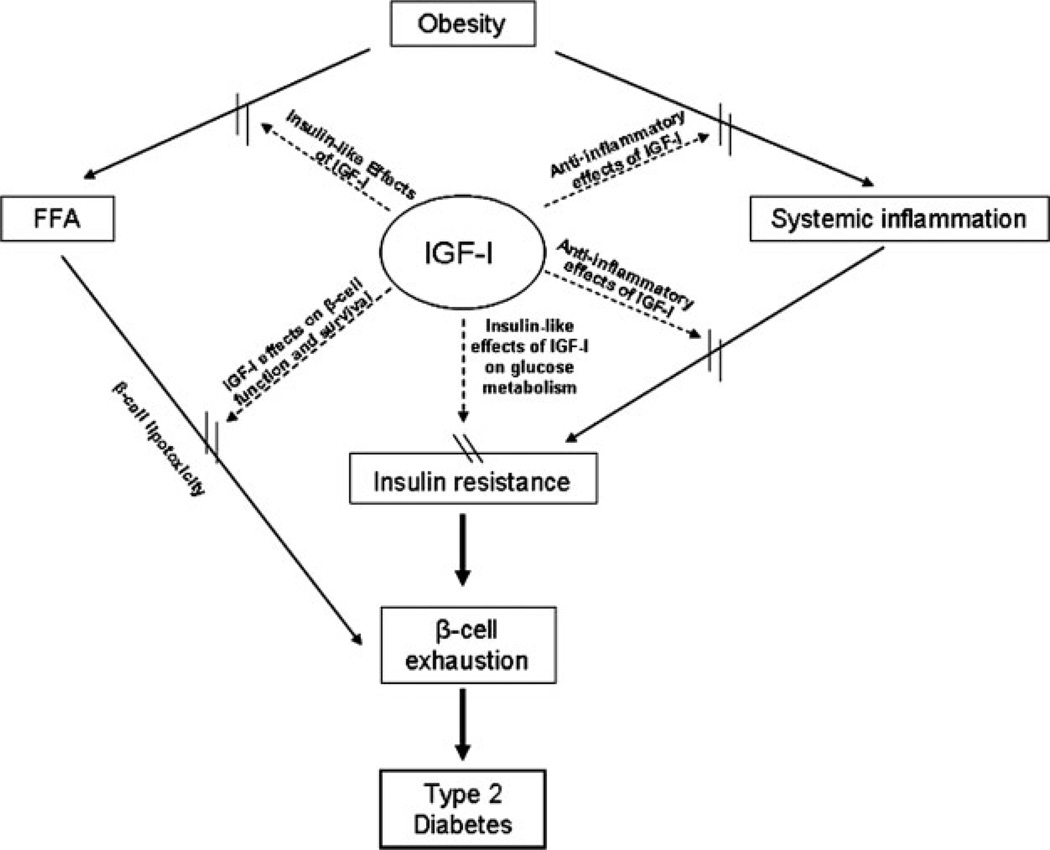
Figure 1 shows a schematic representation of the hypothesized role of the IGF-axis in the pathogenesis of type 2 diabetes. Specifically, we propose that circulating IGF-I (most importantly, free IGF-I) helps maintain euglycemia in the face of increasing insulin resistance, largely by impacting the peripheral uptake of glucose. This at first involves mainly the insulin-like effects of IGF-I on the IGF-IR present in muscle tissue, but with worsening of insulin resistance there is an increased expression of the hybrid receptors in muscle as well as in adipose tissue leading to increased glucose uptake in both these tissues [77–80]. The insulin-like effects of IGF-I and increases in hybrid receptor expression, also result in greater uptake of FFA [83], reducing the negative impact of FFA on insulin sensitivity, as well as the ‘lipotoxic’ effect of FFA on pancreatic β-cells [126–128]. Furthermore, as insulin resistance worsens, and insulin levels rise, these higher insulin levels result in lower serum IGFBP-1 levels, up-regulation of hepatic IGF-I production, and higher levels of free (presumably, bioactive) IGF-I levels.
Figure 1.
Schematic representation for the role of IGF-axis in the pathogenesis of type 2 diabetes
Additional mechanisms
Although the insulin-like effects of IGF-I are likely the major contribution of the IGF-axis to glucose homeostasis, IGF-I may additionally influence the risk of type 2 diabetes through its effects on pancreatic β-cells [147–153]. β-cells express the IGF-IR, and the tyrosine kinase activity of these receptors on the IRS pathways could potentially alter insulin secretion by influencing cell replication and survival [154,155]. For example, the reintroduction of β-cell IRS-2 in irs-2 knockout mice was found to result in an increase in β-cell number and volume (consistent with the mitogenic and anti-apoptotic activity of IGF-I and its signaling pathway), as well as the resolution of diabetes in these animals [156]. Disruption of IGF-IR function on β-cells, in contrast, impaired the insulin response to glucose [150,152]. Certain laboratory data have complicated this view, however. In contrast to the hormonal effects of IGF-I, these laboratory studies suggested that locally produced IGF-I might have a paradoxical effect, actually inhibiting islet cell growth [157,158]. The most recent data from these same laboratories, though, promote the idea that while IGF-I may not directly increase β-cell replication, IGF-I does have a positive survival (anti-apoptotic) effect [159] In any event, taken as a whole, these data to date suggest that the IGF-axis may play a role in early stages of the development of type 2 diabetes [160–162], helping to preserve β-cell mass and function.
Lastly, the IGF-axis may influence the risk of type 2 diabetes through the anti-inflammatory effects of IGF-I. Recent data suggest that high levels of C-reactive protein (CRP) and inflammatory cytokines are associated with insulin resistance and increase the risk of type 2 diabetes [20,163,164]. Data from our group [136] and others [18,19,165], have shown a significant inverse correlation between levels of IGF-I with CRP and other cytokine levels; laboratory data indicate that this may involve the effects of IGF-I on cytokines [166], the effects of cytokines on the IGF-axis [167–170], or more likely both. Thus, these data raise the possibility that the IGF-axis may alter inflammatory cytokine levels and, thereby, affect insulin resistance and its progression to type 2 diabetes.
Conclusion
Several lines of evidence suggest that the IGF-axis has an important role in the maintenance of normal glucose homeostasis and may contribute to the etiopathogenesis of type 2 diabetes. In vitro and animal data have demonstrated that IGF-I has insulin-like effects in peripheral tissues. Cross-sectional studies in humans indicate that levels of IGF-I and its binding proteins are altered in adults with obesity, insulin resistance, and type 2 diabetes. Only one prospective cohort study, however, has evaluated the association between the IGF-axis and risk of type 2 diabetes in healthy individuals, and that study, while reporting intriguing data consistent with an IGF-axis–type 2 diabetes relationship, was limited by its small sample size and by incomplete assessment of the IGF-axis. Prospective cohort studies of appropriate size, and with relevant data to control for other major risk factors, are needed to assess the fundamental question of whether the IGF-axis plays a significant etiopathogenic role in the development of type 2 diabetes.
Acknowledgements
This work was supported by NIH grants 1R01DK08792 and P60DK20541.
Footnotes
Conflict of interest
None declared.
References
- 1.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 2.Froesch ER, Schmid C, Schwander J, Zapf J. Actions of insulinlike growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld RG, Hwa V, Wilson E, Plymate SR, Oh Y. The insulin-like growth factor-binding protein superfamily. Growth Horm IGF Res. 2000;10(Suppl. A):S16–S17. doi: 10.1016/s1096-6374(00)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins P, Bustin S. Evidence for a link between IGF-I and cancer 10.1530/eje.0.151S017. Eur J Endocrinol. 2004;151:S17–S22. doi: 10.1530/eje.0.151s017. [DOI] [PubMed] [Google Scholar]
- 6.Zofkova I. Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res. 2003;52:657–679. [PubMed] [Google Scholar]
- 7.Kaplan RC, Strickler HD, Rohan TE, Muzumdar R, Brown DL. Insulin-like growth factors and coronary heart disease. Cardiol Rev. 2005;13:35–39. doi: 10.1097/01.crd.0000134914.10407.40. [DOI] [PubMed] [Google Scholar]
- 8.Froesch ER, Muller WA, Burgi H, Waldvogel M, Labhart A. Non-suppressible insulin-like activity of human serum. II. Biological properties of plasma extracts with non-suppressible insulin-like activity. Biochim Biophys Acta. 1966;121:360–374. doi: 10.1016/0304-4165(66)90125-5. [DOI] [PubMed] [Google Scholar]
- 9.Froesch ER, Buergi H, Ramseier EB, Bally P, Labhart A. Antibody-suppressible and nonsuppressible Insulin-like activities in human serum and their physiologic significance. An insulin assay with adipose tissue of increased precision and specificity. J Clin Invest. 1963;42:1816–1834. doi: 10.1172/JCI104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- 11.Russell-Jones DL, Bates AT, Umpleby AM, et al. A comparison of the effects of IGF-I and insulin on glucose metabolism, fat metabolism and the cardiovascular system in normal human volunteers. Eur J Clin Invest. 1995;25:403–411. doi: 10.1111/j.1365-2362.1995.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 12.Boulware SD, Tamborlane WV, Rennert NJ, Gesundheit N, Sherwin RS. Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin. Dose-response relationships in healthy young and middle-aged adults. J Clin Invest. 1994;93:1131–1139. doi: 10.1172/JCI117065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses AC, Young SC, Morrow LA, O’Brien M, Clemmons DR. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45:91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Bak JF, Moller N, Schmitz O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol. 1991;260:E736–E742. doi: 10.1152/ajpendo.1991.260.5.E736. [DOI] [PubMed] [Google Scholar]
- 16.Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res. 1994;42:145–151. doi: 10.1159/000184187. [DOI] [PubMed] [Google Scholar]
- 17.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 18.Kaushal K, Heald AH, Siddals KW, et al. The impact of abnormalities in IGF and inflammatory systems on the metabolic syndrome. Diabetes Care. 2004;27:2682–2688. doi: 10.2337/diacare.27.11.2682. [DOI] [PubMed] [Google Scholar]
- 19.Eivindson M, Nielsen JN, Gronbaek H, Flyvbjerg A, Hey H. The insulin-like growth factor system and markers of inflammation in adult patients with inflammatory bowel disease. Horm Res. 2005;64:9–15. doi: 10.1159/000087190. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 21.Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–E851. doi: 10.1152/ajpendo.1991.260.6.E846. [DOI] [PubMed] [Google Scholar]
- 22.Luna AM, Wilson DM, Wibbelsman CJ, et al. Somatomedins in adolescence: a cross-sectional study of the effect of puberty on plasma insulin-like growth factor I and II levels. J Clin Endocrinol Metab. 1983;57:268–271. doi: 10.1210/jcem-57-2-268. [DOI] [PubMed] [Google Scholar]
- 23.Juul A, Bang P, Hertel NT, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 24.Kelijman M. Age-related alterations of the growth hormone/insulin-like-growth-factor I axis. J Am Geriatr Soc. 1991;39:295–307. doi: 10.1111/j.1532-5415.1991.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Mistry J, Nicar MJ, et al. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J Clin Lab Anal. 1999;13:166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JJ, Accili D. Signalling through IGF-I and insulin receptors: where is the specificity? Growth Horm IGF Res. 2002;12:84–90. doi: 10.1054/ghir.2002.0265. [DOI] [PubMed] [Google Scholar]
- 27.Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6:620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not Insulin-like growth factor I (IGF-I) or Insulin/IGF-I hybrid receptors in endothelial cells 10.1210/en.2005-0505. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 29.Ellis MJ, Leav BA, Yang Z, et al. Affinity for the insulin-like growth factor-II (IGF-II) receptor inhibits autocrine IGF-II activity in MCF-7 breast cancer cells. Mol Endocrinol. 1996;10:286–297. doi: 10.1210/mend.10.3.8833657. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen FC, Wang E, Gammeltoft S. Receptor binding, endocytosis, and mitogenesis of insulin-like growth factors I and II in fetal rat brain neurons. J Neurochem. 1991;56:12–21. doi: 10.1111/j.1471-4159.1991.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 31.Holzenberger M, Kappeler L, De Magalhaes Filho C. IGF-1 signaling and aging. Exp Gerontol. 2004;39:1761–1764. doi: 10.1016/j.exger.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Zapf J, Schmid C, Froesch ER. Biological and immunological properties of insulin-like growth factors (IGF) I and II. Clin Endocrinol Metab. 1984;13:3–30. doi: 10.1016/s0300-595x(84)80006-7. [DOI] [PubMed] [Google Scholar]
- 33.Guler HP, Zapf J, Froesch ER. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. 1987;317:137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]
- 34.Sakai K, Lowman HB, Clemmons DR. Increases in free, unbound insulin-like growth factor I enhance insulin responsiveness in human hepatoma G2 cells in culture. J Biol Chem. 2002;277:13620–13627. doi: 10.1074/jbc.M107771200. [DOI] [PubMed] [Google Scholar]
- 35.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 36.Denley A, Carroll JM, Brierley GV, et al. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007;27:3569–3577. doi: 10.1128/MCB.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moller DE, Yokota A, Caro JF, Flier JS. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989;3:1263–1269. doi: 10.1210/mend-3-8-1263. [DOI] [PubMed] [Google Scholar]
- 38.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9:2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modan-Moses D, Janicot M, McLenithan JC, Lane MD, Casella SJ. Expression and function of insulin/insulin-like growth factor I hybrid receptors during differentiation of 3T3-L1 preadipocytes. Biochem J. 1998;333(Pt 3):825–831. doi: 10.1042/bj3330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 41.Janssen JA, Lamberts SW. Is the measurement of free IGF-I more indicative than that of total IGF-I in the evaluation of the biological activity of the GH/IGF-I axis? J Endocrinol Invest. 1999;22:313–315. doi: 10.1007/BF03343563. [DOI] [PubMed] [Google Scholar]
- 42.Lee KW, Cohen P. Nuclear effects: unexpected intracellular actions of insulin-like growth factor binding protein-3. J Endocrinol. 2002;175:33–40. doi: 10.1677/joe.0.1750033. [DOI] [PubMed] [Google Scholar]
- 43.Oufattole M, Lin SW, Liu B, Mascarenhas D, Cohen P, Rodgers BD. Ribonucleic acid polymerase II binding subunit 3 (Rpb3), a potential nuclear target of insulin-like growth factor binding protein-3. Endocrinology. 2006;147:2138–2146. doi: 10.1210/en.2005-1269. [DOI] [PubMed] [Google Scholar]
- 44.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 45.Rajah R, Katz L, Nunn S, Solberg P, Beers T, Cohen P. Insulinlike growth factor binding protein (IGFBP) proteases: functional regulators of cell growth. Prog Growth Factor Res. 1995;6:273–284. doi: 10.1016/0955-2235(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 46.Rajah R, Khare A, Lee PD, Cohen P. Insulin-like growth factor-binding protein-3 is partially responsible for high-serum-induced apoptosis in PC-3 prostate cancer cells. J Endocrinol. 1999;163:487–494. doi: 10.1677/joe.0.1630487. [DOI] [PubMed] [Google Scholar]
- 47.Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth Differ. 2002;13:163–171. [PubMed] [Google Scholar]
- 48.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 49.Cobb LJ, Liu B, Lee KW, Cohen P. Phosphorylation by DNA-dependent protein kinase is critical for apoptosis induction by insulin-like growth factor binding protein-3. Cancer Res. 2006;66:10878–10884. doi: 10.1158/0008-5472.CAN-06-0585. [DOI] [PubMed] [Google Scholar]
- 50.Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem. 2005;280:16942–16948. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- 51.Butt AJ, Fraley KA, Firth SM, Baxter RC. IGF-binding protein-3-induced growth inhibition and apoptosis do not require cell surface binding and nuclear translocation in human breast cancer cells. Endocrinology. 2002;143:2693–2699. doi: 10.1210/endo.143.7.8876. [DOI] [PubMed] [Google Scholar]
- 52.Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. J Biol Chem. 2002;277:10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharyya N, Pechhold K, Shahjee H, et al. Nonsecreted insulin-like growth factor binding protein-3 (IGFBP-3) can induce apoptosis in human prostate cancer cells by IGF-independent mechanisms without being concentrated in the nucleus. J Biol Chem. 2006;281:24588–24601. doi: 10.1074/jbc.M509463200. [DOI] [PubMed] [Google Scholar]
- 54.Hochscheid R, Jaques G, Wegmann B. Transfection of human insulin-like growth factor-binding protein 3 gene inhibits cell growth and tumorigenicity: a cell culture model for lung cancer. J Endocrinol. 2000;166:553–563. doi: 10.1677/joe.0.1660553. [DOI] [PubMed] [Google Scholar]
- 55.Valentinis B, Bhala A, DeAngelis T, Baserga R, Cohen P. The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Mol Endocrinol. 1995;9:361–367. doi: 10.1210/mend.9.3.7539889. [DOI] [PubMed] [Google Scholar]
- 56.Fanayan S, Firth SM, Butt AJ, Baxter RC. Growth inhibition by insulin-like growth factor-binding protein-3 in T47D breast cancer cells requires transforming growth factor-beta (TGF-beta) and the type II TGF-beta receptor. J Biol Chem. 2000;275:39146–39151. doi: 10.1074/jbc.M006964200. [DOI] [PubMed] [Google Scholar]
- 57.Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Barreca A, Artini PG, Cesarone A, et al. Interrelationships between follicle stimulating hormone and the growth hormone-insulin-like growth factor-IGF-binding proteins axes in human granulosa cells in culture. J Endocrinol Invest. 1996;19:35–42. doi: 10.1007/BF03347856. [DOI] [PubMed] [Google Scholar]
- 59.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 60.Rodgers BD, Roalson EH, Thompson C. Phylogenetic analysis of the insulin-like growth factor binding protein (IGFBP) and IGFBP-related protein gene families. Gen Comp Endocrinol. 2008;155(1):201–207. doi: 10.1016/j.ygcen.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 62.Goodman-Gruen D, Barrett-Connor E. Epidemiology of Insulinlike Growth Factor-I in elderly men and women: The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- 63.Janssen JA, Stolk RP, Pols HA, Grobbee DE, de Jong FH, Lamberts SW. Serum free IGF-I, total IGF-I, IGFBP-1 and IGFBP-3 levels in an elderly population: relation to age and sex steroid levels. Clin Endocrinol (Oxf) 1998;48:471–478. doi: 10.1046/j.1365-2265.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 64.Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–1228. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 65.Rosen CJ. Serum insulin-like growth factors and insulin-like growth factor-binding proteins: clinical implications. Clin Chem. 1999;45:1384–1390. [PubMed] [Google Scholar]
- 66.Jenab M, Riboli E, Cleveland RJ, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121(2):368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 67.Frystyk J. Free insulin-like growth factors - measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14:337–375. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Chen JW, Hojlund K, Beck-Nielsen H, Sandahl Christiansen J, Orskov H, Frystyk J. Free rather than total circulating insulinlike growth factor-I determines the feedback on growth hormone release in normal subjects. J Clin Endocrinol Metab. 2005;90:366–371. doi: 10.1210/jc.2004-0039. [DOI] [PubMed] [Google Scholar]
- 69.Lieberman SA, Bukar J, Chen SA, et al. Effects of recombinant human insulin-like growth factor-I (rhIGF-I) on total and free IGF-I concentrations, IGF-binding proteins, and glycemic response in humans. J Clin Endocrinol Metab. 1992;75:30–36. doi: 10.1210/jcem.75.1.1377706. [DOI] [PubMed] [Google Scholar]
- 70.Mukku V. A 96-well microtiter plate assay for free IGF-I in plasma using immobilized IGFBP-3; 73rd Annual Meeting of The Endocrine Society; Washington. 1991. [Google Scholar]
- 71.Lee P. Characterization of a direct, non-extraction imunoradiometric assay for free IGF-I; 76th Annual Meeting of the Endocrine Society; Anaheim. 1994. [Google Scholar]
- 72.Elahi D, McAloon-Dyke M, Fukagawa NK, et al. Effects of recombinant human IGF-I on glucose and leucine kinetics in men. Am J Physiol. 1993;265:E831–E838. doi: 10.1152/ajpendo.1993.265.6.E831. [DOI] [PubMed] [Google Scholar]
- 73.Laager R, Ninnis R, Keller U. Comparison of the effects of recombinant human insulin-like growth factor-I and insulin on glucose and leucine kinetics in humans. J Clin Invest. 1993;92:1903–1909. doi: 10.1172/JCI116783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frystyk J, Grofte T, Skjaerbaek C, Orskov H. The effect of oral glucose on serum free insulin-like growth factor-I and -II in health adults. J Clin Endocrinol Metab. 1997;82:3124–3127. doi: 10.1210/jcem.82.9.4259. [DOI] [PubMed] [Google Scholar]
- 75.Alexandrides T, Moses AC, Smith RJ. Developmental expression of receptors for insulin, insulin-like growth factor I (IGF-I), and IGF-II in rat skeletal muscle. Endocrinology. 1989;124:1064–1076. doi: 10.1210/endo-124-2-1064. [DOI] [PubMed] [Google Scholar]
- 76.Federici M, Giaccari A, Hribal ML, et al. Evidence for glucose/hexosamine in vivo regulation of insulin/IGF-I hybrid receptor assembly. Diabetes. 1999;48:2277–2285. doi: 10.2337/diabetes.48.12.2277. [DOI] [PubMed] [Google Scholar]
- 77.Federici M, Lauro D, D’Adamo M, et al. Expression of insulin/IGF-I hybrid receptors is increased in skeletal muscle of patients with chronic primary hyperinsulinemia. Diabetes. 1998;47:87–92. doi: 10.2337/diab.47.1.87. [DOI] [PubMed] [Google Scholar]
- 78.Federici M, Porzio O, Lauro D, et al. Increased abundance of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of obese subjects is correlated with in vivo insulin sensitivity. J Clin Endocrinol Metab. 1998;83:2911–2915. doi: 10.1210/jcem.83.8.4935. [DOI] [PubMed] [Google Scholar]
- 79.Federici M, Porzio O, Zucaro L, et al. Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol Cell Endocrinol. 1997;135:41–47. doi: 10.1016/s0303-7207(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 80.Federici M, Zucaro L, Porzio O, et al. Increased expression of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of noninsulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;98:2887–2893. doi: 10.1172/JCI119117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacob R, Barrett E, Plewe G, Fagin KD, Sherwin RS. Acute effects of insulin-like growth factor I on glucose and amino acid metabolism in the awake fasted rat. Comparison with insulin. J Clin Invest. 1989;83:1717–1723. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moxley RT3rd, Arner P, Moss A, et al. Acute effects of insulinlike growth factor I and insulin on glucose metabolism in vivo. Am J Physiol. 1990;259:E561–E567. doi: 10.1152/ajpendo.1990.259.4.E561. [DOI] [PubMed] [Google Scholar]
- 83.Pratipanawatr T, Pratipanawatr W, Rosen C, et al. Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2002;282:E1360–E1368. doi: 10.1152/ajpendo.00335.2001. [DOI] [PubMed] [Google Scholar]
- 84.Sesti G, Sciacqua A, Cardellini M, et al. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. 2005;28:120–125. doi: 10.2337/diacare.28.1.120. [DOI] [PubMed] [Google Scholar]
- 85.Zapf J, Hauri C, Waldvogel M, Froesch ER. Acute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest. 1986;77:1768–1775. doi: 10.1172/JCI112500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zenobi PD, Guler HP, Zapf J, Froesch ER. Insulin-like growth factors in the Gottinger miniature-pig. Acta Endocrinol (Copenh) 1988;117:343–352. doi: 10.1530/acta.0.1170343. [DOI] [PubMed] [Google Scholar]
- 87.Zenobi PD, Graf S, Ursprung H, Froesch ER. Effects of insulinlike growth factor-I on glucose tolerance, insulin levels, and insulin secretion. J Clin Invest. 1992;89:1908–1913. doi: 10.1172/JCI115796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmid C, Bianda T, Zwimpfer C, Zapf J, Wiesli P. Changes in insulin sensitivity induced by short-term growth hormone (GH) and insulin-like growth factor I (IGF-I) treatment in GH-deficient adults are not associated with changes in adiponectin levels. Growth Horm IGF Res. 2005;15:300–303. doi: 10.1016/j.ghir.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Zenobi PD, Glatz Y, Keller A, et al. Beneficial metabolic effects of insulin-like growth factor I in patients with severe insulin-resistant diabetes type A. Eur J Endocrinol. 1994;131:251–257. doi: 10.1530/eje.0.1310251. [DOI] [PubMed] [Google Scholar]
- 90.Morrow LA, O’Brien MB, Moller DE, Flier JS, Moses AC. Recombinant human insulin-like growth factor-I therapy improves glycemic control and insulin action in the type A syndrome of severe insulin resistance. J Clin Endocrinol Metab. 1994;79:205–210. doi: 10.1210/jcem.79.1.8027228. [DOI] [PubMed] [Google Scholar]
- 91.Cheetham TD, Holly JM, Clayton K, Cwyfan-Hughes S, Dunger DB. The effects of repeated daily recombinant human insulin-like growth factor I administration in adolescents with type 1 diabetes. Diabet Med. 1995;12:885–892. doi: 10.1111/j.1464-5491.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 92.Carroll PV, Umpleby M, Alexander EL, et al. Recombinant human insulin-like growth factor-I (rhIGF-I) therapy in adults with type 1 diabetes mellitus: effects on IGFs, IGF-binding proteins, glucose levels and insulin treatment. Clin Endocrinol (Oxf) 1998;49:739–746. doi: 10.1046/j.1365-2265.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 93.Saukkonen T, Amin R, Williams RM, et al. Dose-dependent effects of recombinant human insulin-like growth factor (IGF)-I/IGF binding protein-3 complex on overnight growth hormone secretion and insulin sensitivity in type 1 diabetes. J Clin Endocrinol Metab. 2004;89:4634–4641. doi: 10.1210/jc.2004-0243. [DOI] [PubMed] [Google Scholar]
- 94.Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Roder ME, Froesch ER. Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest. 1992;90:2234–2241. doi: 10.1172/JCI116109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woods KA, Camacho-Hubner C, Barter D, Clark AJ, Savage MO. Insulin-like growth factor I gene deletion causing intrauterine growth retardation and severe short stature. Acta Paediatr Suppl. 1997;423:39–45. doi: 10.1111/j.1651-2227.1997.tb18367.x. [DOI] [PubMed] [Google Scholar]
- 96.Woods KA, Camacho-Hubner C, Bergman RN, Barter D, Clark AJ, Savage MO. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab. 2000;85:1407–1411. doi: 10.1210/jcem.85.4.6495. [DOI] [PubMed] [Google Scholar]
- 97.O’Connell T, Clemmons DR. IGF-I/IGF-binding protein-3 combination improves insulin resistance by GH-dependent and independent mechanisms. J Clin Endocrinol Metab. 2002;87:4356–4360. doi: 10.1210/jc.2002-020343. [DOI] [PubMed] [Google Scholar]
- 98.Laron Z, Weinberger D. Diabetic retinopathy, nephropathy and cardiovascular disease in a patient with GH gene deletion. Clin Endocrinol (Oxf) 2005;63:699–700. doi: 10.1111/j.1365-2265.2005.02402.x. [DOI] [PubMed] [Google Scholar]
- 99.Laron Z, Weinberger D. Diabetic retinopathy in two patients with congenital IGF-I deficiency (Laron syndrome) Eur J Endocrinol. 2004;151:103–106. doi: 10.1530/eje.0.1510103. [DOI] [PubMed] [Google Scholar]
- 100.Norrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15:95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am. 2007;36:75–87. doi: 10.1016/j.ecl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Katz LE, DeLeon DD, Zhao H, Jawad AF. Free and total insulinlike growth factor (IGF)-I levels decline during fasting: relationships with insulin and IGF-binding protein-1. J Clin Endocrinol Metab. 2002;87:2978–2983. doi: 10.1210/jcem.87.6.8601. [DOI] [PubMed] [Google Scholar]
- 103.Luo J, Murphy LJ. Differential expression of the insulin-like growth factor binding proteins in spontaneously diabetic rats. J Mol Endocrinol. 1992;8:155–163. doi: 10.1677/jme.0.0080155. [DOI] [PubMed] [Google Scholar]
- 104.Nyomba BL, Berard L, Murphy LJ. Free insulin-like growth factor I (IGF-I) in healthy subjects: relationship with IGF-binding proteins and insulin sensitivity. J Clin Endocrinol Metab. 1997;82:2177–2181. doi: 10.1210/jcem.82.7.4070. [DOI] [PubMed] [Google Scholar]
- 105.Murphy LJ, Seneviratne C, Moreira P, Reid RE. Enhanced expression of insulin-like growth factor-binding protein-I in the fasted rat: the effects of insulin and growth hormone administration. Endocrinology. 1991;128:689–696. doi: 10.1210/endo-128-2-689. [DOI] [PubMed] [Google Scholar]
- 106.Thierry van Dessel HJ, Lee PD, Faessen G, Fauser BC, Giudice LC. Elevated serum levels of free insulin-like growth factor I in polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:3030–3035. doi: 10.1210/jcem.84.9.5941. [DOI] [PubMed] [Google Scholar]
- 107.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulinlike growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- 108.Lewitt MS, Denyer GS, Cooney GJ, Baxter RC. Insulin-like growth factor-binding protein-1 modulates blood glucose levels. Endocrinology. 1991;129:2254–2256. doi: 10.1210/endo-129-4-2254. [DOI] [PubMed] [Google Scholar]
- 109.Manetta J, Brun JF, Maimoun L, Callis A, Prefaut C, Mercier J. Effect of training on the GH/IGF-I axis during exercise in middle-aged men: relationship to glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;283:E929–E936. doi: 10.1152/ajpendo.00539.2001. [DOI] [PubMed] [Google Scholar]
- 110.Heald AH, Cruickshank JK, Riste LK, et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–339. doi: 10.1007/s001250051623. [DOI] [PubMed] [Google Scholar]
- 111.Mohamed-Ali V, Pinkney JH, Panahloo A, Cwyfan-Hughes S, Holly JM, Yudkin JS. Insulin-like growth factor binding protein-1 in NIDDM: relationship with the insulin resistance syndrome. Clin Endocrinol (Oxf) 1999;50:221–228. doi: 10.1046/j.1365-2265.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 112.Liew CF, Wise SD, Yeo KP, Lee KO. Insulin-like growth factor binding protein-1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy, glucose-tolerant young men. J Clin Endocrinol Metab. 2005;90:1483–1488. doi: 10.1210/jc.2004-1501. [DOI] [PubMed] [Google Scholar]
- 113.Clemmons DR, Snyder DK, Busby WH., Jr Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J Clin Endocrinol Metab. 1991;73:727–733. doi: 10.1210/jcem-73-4-727. [DOI] [PubMed] [Google Scholar]
- 114.Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamanaka Y, Fowlkes JL, Wilson EM, Rosenfeld RG, Oh Y. Characterization of insulin-like growth factor binding protein-3 (IGFBP-3) binding to human breast cancer cells: kinetics of IGFBP-3 binding and identification of receptor binding domain on the IGFBP-3 molecule. Endocrinology. 1999;140:1319–1328. doi: 10.1210/endo.140.3.6566. [DOI] [PubMed] [Google Scholar]
- 116.Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and −5 is mediated by the importin beta subunit. J Biol Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- 117.Muzumdar RH, Ma X, Fishman S, et al. Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action. Diabetes. 2006;55:2788–2796. doi: 10.2337/db06-0318. [DOI] [PubMed] [Google Scholar]
- 118.Modric T, Silha JV, Shi Z, et al. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–1967. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- 119.Silha JV, Gui Y, Murphy LJ. Impaired glucose homeostasis in insulin-like growth factor-binding protein-3-transgenic mice. Am J Physiol Endocrinol Metab. 2002;283:E937–E945. doi: 10.1152/ajpendo.00014.2002. [DOI] [PubMed] [Google Scholar]
- 120.Lopez-Bermejo A, Khosravi J, Fernandez-Real JM, et al. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25) Diabetes. 2006;55:2333–2339. doi: 10.2337/db05-1627. [DOI] [PubMed] [Google Scholar]
- 121.Shao L, Huang Q, Luo M, Lai M. Detection of the differentially expressed gene IGF-binding protein-related protein-1 and analysis of its relationship to fasting glucose in Chinese colorectal cancer patients. Endocr Relat Cancer. 2004;11:141–148. doi: 10.1677/erc.0.0110141. [DOI] [PubMed] [Google Scholar]
- 122.Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab. 2004;89:3543–3553. doi: 10.1210/jc.2003-031682. [DOI] [PubMed] [Google Scholar]
- 123.Laager R, Keller U. Effects of recombinant human insulin-like growth factor I and insulin on counterregulation during acute plasma glucose decrements in normal and type 2 (non-insulin-dependent) diabetic subjects. Diabetologia. 1993;36:966–971. doi: 10.1007/BF02374481. [DOI] [PubMed] [Google Scholar]
- 124.Turkalj I, Keller U, Ninnis R, Vosmeer S, Stauffacher W. Effect of increasing doses of recombinant human insulin-like growth factor-I on glucose, lipid, and leucine metabolism in man. J Clin Endocrinol Metab. 1992;75:1186–1191. doi: 10.1210/jcem.75.5.1430077. [DOI] [PubMed] [Google Scholar]
- 125.Moller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res. 2003;13(Suppl. A):S18–S21. doi: 10.1016/s1096-6374(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 126.Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- 127.Poitout V, Robertson RP. Minireview: Secondary {beta}-Cell Failure in Type 2 Diabetes-A Convergence of Glucotoxicity and Lipotoxicity 10.1210/en.143.2.339. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 128.Cousin SP, Hugl SR, Wrede CE, Kajio H, Myers MG, Jr, Rhodes CJ. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
- 129.Williams T, Berelowitz M, Joffe SN, et al. Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med. 1984;311:1403–1407. doi: 10.1056/NEJM198411293112203. [DOI] [PubMed] [Google Scholar]
- 130.Wabitsch M, Heinze E, Debatin KM, Blum WF. IGF-I- and IGFBP-3-expression in cultured human preadipocytes and adipocytes. Horm Metab Res. 2000;32:555–559. doi: 10.1055/s-2007-978685. [DOI] [PubMed] [Google Scholar]
- 131.Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab. 1994;79:872–878. doi: 10.1210/jcem.79.3.7521354. [DOI] [PubMed] [Google Scholar]
- 132.Conover CA, Lee PD, Kanaley JA, Clarkson JT, Jensen MD. Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab. 1992;74:1355–1360. doi: 10.1210/jcem.74.6.1375600. [DOI] [PubMed] [Google Scholar]
- 133.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44:37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 134.Rasmussen MH, Frystyk J, Andersen T, Breum L, Christiansen JS, Hilsted J. The impact of obesity, fat distribution, and energy restriction on insulin-like growth factor-1 (IGF-1), IGF-binding protein-3, insulin, and growth hormone. Metabolism. 1994;43:315–319. doi: 10.1016/0026-0495(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 135.Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- 136.Rajpathak SN, McGinn AP, Strickler HD, et al. Insulin-like growth factor (IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm IGF Res. 2008;18(2):166–173. doi: 10.1016/j.ghir.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heald AH, Siddals KW, Fraser W, et al. Low circulating levels of insulin-like growth factor binding protein-1 (IGFBP-1) are closely associated with the presence of macrovascular disease and hypertension in type 2 diabetes. Diabetes. 2002;51:2629–2636. doi: 10.2337/diabetes.51.8.2629. [DOI] [PubMed] [Google Scholar]
- 138.Gibson JM, Westwood M, Young RJ, White A. Reduced insulinlike growth factor binding protein-1 (IGFBP-1) levels correlate with increased cardiovascular risk in non-insulin dependent diabetes mellitus (NIDDM) J Clin Endocrinol Metab. 1996;81:860–863. doi: 10.1210/jcem.81.2.8636318. [DOI] [PubMed] [Google Scholar]
- 139.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voskuil DW, Bueno de Mesquita HB, Kaaks R, et al. Determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1–3 in premenopausal women: physical activity and anthropometry (Netherlands) Cancer Causes Control. 2001;12:951–958. doi: 10.1023/a:1013708627664. [DOI] [PubMed] [Google Scholar]
- 141.Bezemer ID, Rinaldi S, Dossus L, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2005;16:561–572. doi: 10.1007/s10552-004-7472-9. [DOI] [PubMed] [Google Scholar]
- 142.Clauson PG, Brismar K, Hall K, Linnarsson R, Grill V. Insulinlike growth factor-I and insulin-like growth factor binding protein-1 in a representative population of type 2 diabetic patients in Sweden. Scand J Clin Lab Invest. 1998;58:353–360. doi: 10.1080/00365519850186544. [DOI] [PubMed] [Google Scholar]
- 143.Gibson JM, Westwood M, Crosby SR, et al. Choice of treatment affects plasma levels of insulin-like growth factor-binding protein-1 in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1369–1375. doi: 10.1210/jcem.80.4.7536208. [DOI] [PubMed] [Google Scholar]
- 144.Tan K, Baxter RC. Serum insulin-like growth factor I levels in adult diabetic patients: the effect of age. J Clin Endocrinol Metab. 1986;63:651–655. doi: 10.1210/jcem-63-3-651. [DOI] [PubMed] [Google Scholar]
- 145.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 146.Wallander M, Brismar K, Ohrvik J, Ryden L, Norhammar A. Insulin-like growth factor I: a predictor of long-term glucose abnormalities in patients with acute myocardial infarction. Diabetologia. 2006;49:2247–2255. doi: 10.1007/s00125-006-0386-1. [DOI] [PubMed] [Google Scholar]
- 147.Ripa P, Robertson I, Cowley D, Harris M, Masters IB, Cotterill AM. The relationship between insulin secretion, the insulin-like growth factor axis and growth in children with cystic fibrosis. Clin Endocrinol (Oxf) 2002;56:383–389. doi: 10.1046/j.1365-2265.2002.01484.x. [DOI] [PubMed] [Google Scholar]
- 148.Rennert NJ, Caprio S, Sherwin RS. Insulin-like growth factor I inhibits glucose-stimulated insulin secretion but does not impair glucose metabolism in normal humans. J Clin Endocrinol Metab. 1993;76:804–806. doi: 10.1210/jcem.76.3.8445040. [DOI] [PubMed] [Google Scholar]
- 149.Porksen N, Hussain MA, Bianda TL, et al. IGF-I inhibits burst mass of pulsatile insulin secretion at supraphysiological and low IGF-I infusion rates. Am J Physiol Endocrinol Metab. 1997;272:E352–E358. doi: 10.1152/ajpendo.1997.272.3.E352. [DOI] [PubMed] [Google Scholar]
- 150.Xuan S, Kitamura T, Nakae J, et al. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest. 2002;110:1011–1019. doi: 10.1172/JCI15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kulkarni RN. New insights into the roles of insulin/IGF-I in the development and maintenance of beta-cell mass. Rev Endocr Metab Disord. 2005;6:199–210. doi: 10.1007/s11154-005-3051-y. [DOI] [PubMed] [Google Scholar]
- 152.Ueki K, Okada T, Hu J, et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- 153.van Haeften TW, Twickler TB. Insulin-like growth factors and pancreas beta cells. Eur J Clin Invest. 2004;34:249–255. doi: 10.1111/j.1365-2362.2004.01337.x. [DOI] [PubMed] [Google Scholar]
- 154.Jones PM, Persaud SJ. Tyrosine kinase inhibitors inhibit glucose-stimulated insulin secretion. Biochem Soc Trans. 1994;22:209S. doi: 10.1042/bst022209s. [DOI] [PubMed] [Google Scholar]
- 155.Persaud SJ, Harris TE, Burns CJ, Jones PM. Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J Mol Endocrinol. 1999;22:19–28. doi: 10.1677/jme.0.0220019. [DOI] [PubMed] [Google Scholar]
- 156.Hennige AM, Burks DJ, Ozcan U, et al. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest. 2003;112:1521–1532. doi: 10.1172/JCI18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu Y, Herrera PL, Guo Y, et al. Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes. 2004;53:3131–3141. doi: 10.2337/diabetes.53.12.3131. [DOI] [PubMed] [Google Scholar]
- 158.Liu JL. Does IGF-I stimulate pancreatic islet cell growth? Cell Biochem Biophys. 2007;48:115–125. doi: 10.1007/s12013-007-0016-7. [DOI] [PubMed] [Google Scholar]
- 159.Robertson K, Lu Y, De Jesus K, et al. A general and islet cell-enriched overexpression of IGF-I results in normal islet cell growth, hypoglycemia, and significant resistance to experimental diabetes. Am J Physiol Endocrinol Metab. 2008;294:E928–E938. doi: 10.1152/ajpendo.00606.2007. [DOI] [PubMed] [Google Scholar]
- 160.Owens DR, Cozma LS, Luzio SD. Early-phase prandial insulin secretion: its role in the pathogenesis of type 2 diabetes mellitus and its modulation by repaglinide. Diabetes Nutr Metab. 2002;15:19–27. [PubMed] [Google Scholar]
- 161.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia. 2001;44:929–945. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- 162.Szoke E, Gerich JE. Role of impaired insulin secretion and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Compr Ther. 2005;31:106–112. doi: 10.1007/s12019-005-0005-y. [DOI] [PubMed] [Google Scholar]
- 163.Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 164.Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. Elevated C-reactive protein is a risk factor for the development of type 2 diabetes in Japanese Americans. Diabetes Care. 2003;26:2754–2757. doi: 10.2337/diacare.26.10.2754. [DOI] [PubMed] [Google Scholar]
- 165.Heald AH, Anderson SG, Ivison F, Laing I, Gibson JM, Cruickshank K. C-reactive protein and the insulin-like growth factor (IGF)-system in relation to risk of cardiovascular disease in different ethnic groups. Atherosclerosis. 2003;170:79–86. doi: 10.1016/s0021-9150(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 166.Renier G, Clement I, Desfaits AC, Lambert A. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production. Endocrinology. 1996;137:4611–4618. doi: 10.1210/endo.137.11.8895324. [DOI] [PubMed] [Google Scholar]
- 167.Wang P, Li N, Li JS, Li WQ. The role of endotoxin, TNF-alpha, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol. 2002;8:531–536. doi: 10.3748/wjg.v8.i3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lang CH, Nystrom GJ, Frost RA. Regulation of IGF binding protein-1 in hep G2 cells by cytokines and reactive oxygen species. Am J Physiol. 1999;276:G719–G727. doi: 10.1152/ajpgi.1999.276.3.G719. [DOI] [PubMed] [Google Scholar]
- 169.Fan J, Char D, Bagby GJ, Gelato MC, Lang CH. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204–R1212. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- 170.Samstein B, Hoimes ML, Fan J, Frost RA, Gelato MC, Lang CH. IL-6 stimulation of insulin-like growth factor binding protein (IGFBP)-1 production. Biochem Biophys Res Commun. 1996;228:611–615. doi: 10.1006/bbrc.1996.1705. [DOI] [PubMed] [Google Scholar]