Abstract
Purpose
To compare the proteomic profile of a clinical isolate of Pseudomonas aeruginosa (P. aeruginosa) obtained from an infected cornea of a contact lens wearer and the laboratory strain P. aeruginosa ATCC 10145.
Methods
Antibiotic sensitivity, motility, biofilm formation, and virulence tests were performed using standard methods. Whole protein lysates were analyzed with liquid chromatography/ tandem mass spectrometry (LC-MS/MS) in triplicate, and relative protein abundances were determined with spectral counting. The G test followed by a post hoc Holm-Sidak adjustment was used for the statistical analyses to determine significance in the differential expression of proteins between the two strains.
Results
A total of 687 proteins were detected. One-hundred thirty-three (133) proteins were significantly different between the two strains. Among these, 13 were upregulated, and 16 were downregulated in the clinical strain compared to ATCC 10145, whereas 57 were detected only in the clinical strain. The upregulated proteins are associated with virulence and pathogenicity.
Conclusions
Proteins detected at higher levels in the clinical strain of P. aeruginosa were proteins known to be virulence factors. These results confirm that the keratitis-associated P. aeruginosa strain is pathogenic and expresses a higher number of virulence factors compared to the laboratory strain ATCC 10145. Identification of the protein profile of the corneal strain of P. aeruginosa in this study will aid in elucidating novel intervention strategies for reducing the burden of P. aeruginosa infection in keratitis.
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a ubiquitous Gram-negative bacterium associated with microbial keratitis and one of the most destructive of all opportunistic pathogens. P. aeruginosa keratitis (PA keratitis) progresses rapidly and is characterized by the infiltration of inflammatory cells and tissue destruction, which can lead to corneal perforation [1]. Recent reports confirm that contact lens wear is the most common risk factor for keratitis and that the most commonly isolated organism is P. aeruginosa [2-4], even in patients who follow routine disinfection procedures [5]. In 2002, it was reported that 25,000–30,000 contact lens wearers developed microbial keratitis annually in the United States [6] and that up to 39% of these cases are caused by P. aeruginosa [7,8]. Although relatively rare, bacterial keratitis remains a serious complication of contact lens wear. Currently, there are at least 34 million contact lens users in the United States and 140 million worldwide [9].
In addition to the effect on the cornea, other sight-threatening problems such as development of cataracts have also been reportedly linked to PA keratitis [10]. This was attributed to the host’s inflammatory response and possibly the influence of the bacterial toxins and toxicity from antibiotic and topical steroid treatment [10]. Secondary glaucoma can also be a sequela of bacterial keratitis.
The pathogenesis of PA keratitis is a complex process that is not completely understood. It depends on the interaction between the bacterium and the host as well as the virulence of the bacterium. The latter is multifactorial and involves different components, including pili, flagella, outer membrane proteins, and lipopolysaccharides, in addition to several secreted products such as exotoxins and proteases. Cytotoxins include ExoU and ExoS [11,12] that belong to the type III secretion system. Proteases include elastase B (LasB) [13,14], alkaline protease (AprA), protease IV (PrpL) [15], and P. aeruginosa small protease (Pasp). The current treatment for PA keratitis consists of multiple antibiotics that are used simultaneously with frequent dosing and which must be introduced rapidly following the onset of symptoms to minimize corneal damage [16]. However, such shotgun therapy results in corneal toxicity [17,18] and selection of antibiotic-resistant bacterial strains [19,20], leading to failure of treatment [21]. Because of the increasing number of contact lens wearers [9], and an increase in antibiotic-resistant bacteria including P. aeruginosa [22], a better understanding of the mechanism(s) is critical for developing improved therapeutic strategies. Therefore, identification of novel virulence factors used to initiate and maintain infection will provide additional insights into the pathophysiology of the disease.
In this study, using liquid chromatography followed by tandem mass spectrometry (LC-MS/MS), we sought to identify the proteomic profile of a clinical isolate of P. aeruginosa obtained from an active human ulcerative keratitis and compared it to the non-clinical laboratory strain ATCC 10145 [23]. The goal of this study was to identify missing or upregulated proteins in the clinical isolate of P. aeruginosa that may potentially contribute to its virulence.
Methods
Bacterial strains and growth conditions
P. aeruginosa ATCC 10145 [23] (American Type Culture Collection, Manassas, VA) and a clinical isolate of P. aeruginosa (obtained from active ulcerative keratitis) were used and grown under the same conditions. Strains were cultured in triplicate in 50 ml of salt modified Luria-Bertani (LB) broth and grown to stationary phase (OD600 nm approximately 1.0) with incubation at 37 °C and shaking at 250 × rpm. Cultures were harvested and washed three times with PBS (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4±0.2). Cells were collected by centrifugation at 6,000 ×g for 10 min at 4 °C. The resulting bacterial cell pellets were frozen and stored at −80 °C until processing.
Antibiotic susceptibility
All assays were performed in triplicate at least three times on at least three different occasions. Antibiotic sensitivity tests for the two bacterial strains were performed using the Kirby Bauer disk diffusion method on LB agar. The antibiotics tested were Gentamicin (10 μg), moxifloxacin (5 μg), levofloxacin (5 μg), ciprofloxacin (5 μg), ceftazidime (30 μg), vancomycin (30 μg), ampicillin (30 μg), kanamycin (30 μg), penicillin/streptomycin (pen/strep; 10 μg), tetracycline μg), and chloramphenicol (30 μg). All antibiotics were purchased from Thermo Fisher Scientific, Waltham, MA, except Moxifloxacin which was purchased from Cardinal Health Medical, Dublin, OH.
Motility assays
Twitching and swimming motility assays were performed using standard methods. Swimming motility was assayed by spotting a single colony onto a 0.3% LB agar plate and incubating for 24 h at 37 °C. Twitching motility was assayed by stabbing a colony into the bottom of a 10 ml 1% LB agar plate and incubating for 24 h at 37 °C. In both cases, motility was measured by the diameter of the resulting growth zones. Biofilm formation was measured as previously described [24] using a 1:100 dilution of an overnight LB broth culture in fresh LB medium. Briefly, 100 l of culture was added to each well of a flat-bottom MicroTest tissue culture plate (BD, Franklin Lakes, NJ) and incubated in a moist environment at 37 °C for 24 h. The bacterial suspensions were then removed, and the wells were rinsed three times with H2O. Wells were stained with 200 μl 0.5% crystal violet for 3 h before dissolving in 200 μl 30% (v/v) acetic acid. Absorbance was read at 550 nm.
Virulence assay
General virulence of the two strains was determined by their ability to produce pyocyanin, pyorubin, and pyoverdine on King’s A and B agar as previously described [25] with some modifications. Briefly, isolated colonies of ATCC 10145 and the clinical strain were grown on King’s A at 37 °C overnight in the dark followed by incubation at room temperature for 48 h. Five (5) ml of H2O was added to each plate. The plates were shaken gently for 30 min. The 100 slurry, containing cells, was removed, and the agar was cut into small pieces and collected in 50 ml tubes. Ten ml of chloroform was added to each 12.5 g of agar and vortexed. Pyorubin was measured from the chloroform phase at 520 nm, whereas pyocyanin was extracted using 0.2 N HCl. The concentration of pyocyanin (mg/ml) was determined by multiplying the OD492 by 17.072 [26]. For pyoverdine, 5 µl of overnight grown cultures of ATCC 10145 and the clinical strain was spotted in the center of King’s B agar followed by incubation overnight at 37 °C. The amount of pyoverdine produced was determined by measuring the diameter of the growth zone visualized under an ultraviolet (UV) light.
Contact lens adhesion assay
The ability of ATCC 10145 and the clinical strain to adhere to contact lenses was determined as previously described [27]. Briefly, isolated colonies of ATCC 10145 and the clinical strain were grown overnight on LB broth at 37 °C. Before the cells were collected, the cultures were adjusted to optical density (OD) = 0.1 at 550 nm. Cells were removed with centrifugation and washed three times with PBS. Cells were adjusted to OD 0.1 at 595 nm and incubated with the contact lenses (Acuvue 2, Johnson & Johnson) for 2 h at 37 °C with shaking at 120 rpm. Adhered cells were then removed from the contact lenses by stirring for 10 s, serially diluted in PBS, and plated on LB agar plates. Colony-forming units (CFU/ml) were then determined the next day.
Sample preparation and liquid chromatography/tandem mass spectrometry
Samples were prepared as previously described with some minor modifications [28]. Briefly, cells (25 ml) were pelleted and resuspended in 500 μl of 0.2 M Tris-HCl pH 8, 1 M sucrose, and 1 mM EDTA. One hundred microliters of lysozyme (Sigma-Aldrich; 5 mg/ml in dH2O) was added, and the cells were vortexed and incubated for 5 min at room temperature. Two milliliters of distilled water was added to cells and incubated again for 20 min at room temperature until spheroblast formation was observed under the microscope. Then 3 mL of 50 mM Tris-HCl pH 8, 2% (w/v) Triton X-100, 10 mM MgCl2, and 50 μl DNase I (Applichem; 1 mg/ ml in dH2O) were added and mixed until the suspension was clear. Suspensions were then processed individually as described previously [29,30]. Samples were prepared for in-gel digest by mixing 150 μg of protein with 100 µl acrylamide/bis (30%T/2.67%C), 10 µl of 10% sodium hydroxide (NaOH), 10 µl of 10% ammonium persulfate, and 5 µl of N,N,N′,N′-Tetramethylethylenediamine (TEMED) in the lid of a microcentrifuge tube. Gel pieces were transferred into the tubes, fixed in 1 ml of 40% methanol, 7% acetic acid for 30 min, washed twice with water, twice with 50% acetonitrile, and once with 50 mM ammonium bicarbonate in acetonitrile, once with 100 mM ammonium bicarbonate in 50% acetonitrile, and then dried under vacuum using a SpeedVac. Two hundred microliters of 100 mM ammonium bicarbonate (pH 8.0) containing 1.0 µg trypsin (Promega; Madison, WI) was added to each gel piece and incubated overnight at 37 °C. Peptides in each gel piece were extracted with three washes of 70% acitonitrile and 0.1% formic acid. The extracts were then dried. Twenty microliters of 6 M guanidine-HCl in 5 mM potassium phosphate and 1 mM dithiothreitol (DTT; pH 6.5) was added to each dried sample and sonicated. Peptides were then extracted using a Dionex 15 cm × 75 μm id Acclaim Pepmap C18, 2 μm, 100 A° reversed-phase capillary chromatography column (Thermo Scientific, Sunnyvale, CA), and subjected to nanospray LC-MS/MS analysis. The microelectrospray ion source was operated at 2.5 kV. The digests were analyzed using the data-dependent multitask capability of the instrument acquiring full-scan mass spectra to determine peptide molecular weights and product ion spectra to determine amino acid sequence in successive instrument scans. This mode of analysis produces approximately 15,000 collisionally induced dissociation (CID) spectra of ions ranging in abundance over several orders of magnitude. Data were analyzed using all CID spectra collected in the experiment to search all P. aeruginosa databases.
Data analysis
Relative protein abundances were determined with spectral counting. Quantitative comparisons of normalized scan counts were performed for all proteins detected by at least two unique peptides in at least two replicates from either the clinical isolate or the ATCC 10145 group. Proteins that were detected with one peptide were eliminated. A normalized p value for each protein was calculated using the G test as previously described [31]. The p values were adjusted according to the Holm-Sidak method of correction for multiple comparisons. Proteins with an adjusted p value <0.05 were considered significant. Additionally, proteins were eliminated from the list, despite their statistical significance, if the normalized log ratio of the scan counts did not represent at least a twofold increase or decrease in the treated group relative to control. These methods for comparative analysis of proteomic data sets have been successfully used in our laboratory as previously described [30,32].
Statistical analysis
The Mann–Whitney U test was used to determine the significance in strain capability to adhere to contact lenses; the G test followed by the Holm-Sidak test was used to determine the significance in protein abundance between the two strains. P<0.05 was considered statistically significant, no variance, no SEM in proteomics.
Results
Phenotypic assays
Antibiotic susceptibility
Both strains showed 100% resistance to ampicillin, kanamycin, pen/strep, and vancomycin. ATCC 10145 strain was more sensitive to moxifloxacin than the clinical strain (2.7±0.1 cm versus 1.8±0.05 cm) and more resistant to tetracycline and gentamycin when compared to the clinical strain that showed an intermediate resistance to tetracycline and was susceptible to gentamycin. Both strains were susceptible to levofloxacin, ceftazidime, and tobramycin.
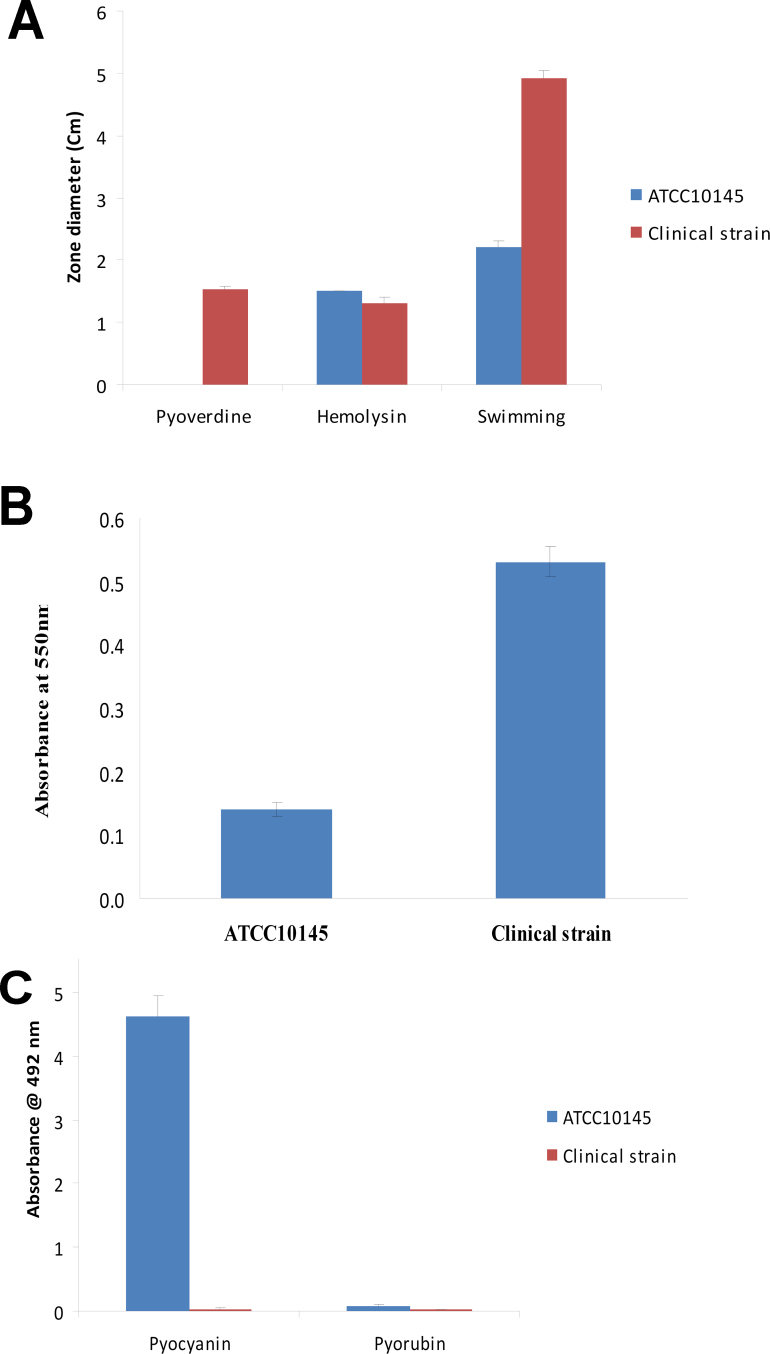
On the agar surfaces, both strains showed spreading, yellow colonies, and the clinical isolate colonies were larger (2 mm) and softer than the ATCC 10145 colonies (1 mm). Swimming motility was higher in the clinical strain compared to ATCC 10145 strain (Figure 1); whereas twitching was higher in the ATCC 10145 strain compared to the clinical strain. Biofilm formation was four times higher in the clinical strain compared to the ATCC 10145 strain (Figure 1).
Figure 1.
Bar graphs showing pyoverdine, hemolysin, and swimming motility (A), biofilm formation after 24 h incubation at 37 °C (B), and pyocyanin and pyorubin production (C) by ATCC 10145 and the clinical strain. Error bars indicate the standard deviation. The bars represent the average of three to five replicates per group.
For the virulence assays, the clinical strain produced hemolysin, pyoverdine, and small amounts of pyorubin but no pyocyanin, while the ATCC 10145 strain produced hemolysin, pyocyanin, and similar amounts of pyorubin but no pyoverdine (Figure 1). When assayed for the ability to adhere to contact lenses, the clinical strain had greater adherence capability than that of ATCC 10145 (p = 0.025; Figure 2).
Figure 2.
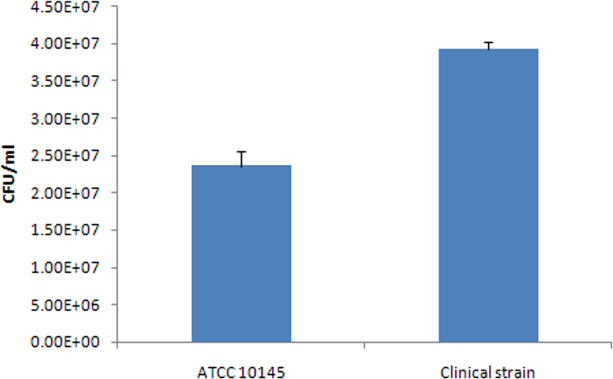
A bar graph showing the adhesion of ATCC 10145 and the clinical strain to contact lenses. Error bars indicate the standard deviation. The bars represent the average of three replicates per group.
Liquid chromatography/ tandem mass spectrometry
A total of 687 proteins were detected in both strains (Appendix 1). One-hundred thirty-three (133) proteins were significantly altered in the corneal strain compared to the ATCC 10145 strain, 13 were detected at higher levels (Table 1), with the top ones being flagellin B (FlgB, 14 fold, p = 0), hypothetical protein PA14_33310 (6.8-fold, p = 0.013), hypothetical protein PA3691 (8.9-fold, p =0.000038), and lipotoxin F (LptF, about threefold, p = 0.000002). Sixteen were detected at lower levels; the top proteins included a putative ClpA/B protease ATP binding subunit (16-fold, p=0), dihydrolipoamide dehydrogenase (14-fold, p = 0), phosphate ABC transporter substrate-binding protein (14-fold, p =0), and several hypothetical proteins. Fifty-five proteins were detected only in the clinical strain and not in the ATCC 10145 strain with two non-ribosomal peptide synthetases (NRPSs) the most abundant (NCBI reference sequences # 218891724 and 386058801). These proteins were the fourth and fifth most abundant proteins, respectively, in the clinical strain proteome and the first and second, respectively, in the proteins that were present in the clinical strain but missing in the ATCC 10145 strain.
Table 1. Significantly upregulated proteins in the clinical strain compared to ATCC10145 strain.
| RefSep accession | Description | Clinical Isolate SC | ATCC10145 SC | NLOG-FoldR* | Np | Adj-Np |
|---|---|---|---|---|---|---|
| 15596289 |
Flagellin type B |
252 |
12 |
4.0551 |
2.81E-49 |
0.00E+00 |
| 15598888 |
Lipotoxon F, LptF |
158 |
54 |
1.3040 |
2.79E-09 |
0.000002 |
| 15599933 |
Hypothetical protein PA4739 |
138 |
51 |
1.1910 |
2.27E-07 |
0.000139 |
| 116050368 |
Hypothetical protein PA14_33310 |
85 |
8 |
3.0288 |
3.46E-14 |
2.25E-11 |
| 15598887 |
Hypothetical protein PA3691 |
70 |
4 |
3.6003 |
8.69E-14 |
5.65E-11 |
| 15599895 |
Hypothetical protein PA4701 |
66 |
12 |
2.1381 |
4.17E-08 |
0.000026 |
| 116049276 |
Hypothetical protein PA14_47120 |
65 |
4 |
3.4950 |
1.24E-12 |
8.01E-10 |
| 116049954 |
3-hydroxybutyrate dehydrogenase |
50 |
12 |
1.7445 |
0.000030 |
0.016786 |
| 15595257 |
Osmotically inducible protein OsmC |
45 |
11 |
1.7111 |
0.000092 |
0.049631 |
| 15597196 |
Dehydrocarnitine CoA transferase subunit B |
40 |
6 |
2.3227 |
0.000006 |
0.003651 |
| 15597527 |
Hypothetical protein PA2331 |
39 |
3 |
3.0944 |
1.74E-07 |
0.000107 |
| 15600253 |
Polyhydroxyalkanoate synthesis protein PhaF |
33 |
3 |
2.8600 |
0.000004 |
0.002163 |
| 15595792 | Organic solvent tolerance protein OstA | 27 | 1 | 3.5799 | 0.000003 | 0.001770 |
Abbreviations: RefSeq: Reference sequence, SC: scan count, NLOG-FoldR: normalized log ratio, Np: normalized p value, Adj-NP: adjusted normalized p value *Normalized ratios are calculated from the ratio of the scans (clinical/ATCC10145) adjusted to the ratio of the total scans for all proteins detected in each group.
Discussion
In this paper, we discuss the proteomic profile of a clinical strain of P. aeruginosa isolated from an active corneal ulcer of a contact lens wearer compared to that of the laboratory strain ATCC 10145. Using stringent filtering criteria described previously [30,32], 133 proteins were significantly altered: 13 were upregulated in the clinical strain, and 55 proteins were not detected in ATCC 10145. The upregulated proteins are associated with virulence and play roles in pathogenesis, which indicates that the clinical strain is more virulent than the laboratory strain ATCC 10145. The clinical strain also produced more hemolysin and pyoverdine, was able to form biofilm four times higher, and had a higher swimming ability than ATCC 10145 strain. The strain ATCC 10145 produced hemolysin at relatively lower levels than the clinical strain and did not produce pyoverdine. Hemolysin, pyoverdine production, biofilm formation, and swimming motility all play major roles in virulence [33-39]. Both strains were resistant to most of the antibiotics tested, and the strain ATCC 10145 had higher twitching motility compared to that of the clinical strain (data not shown). When tested for their ability to adhere to contact lenses, the clinical strain had a greater adherence capability than that of ATCC 10145. This indicates that the clinical strain expresses adhesion proteins that the strain ATCC 10145 may not; some are described below.
Among the proteins that were detected at higher levels was flagellin B (the major structural protein of flagella, FlgB). FlgB was 21-fold higher in the clinical strain compared to ATCC 10145. Changes in flagellar expression are associated with decreased virulence in several animal models of bacterial pathogenesis, including P. aeruginosa in corneal [40] and lung infections [41-43]. Upregulation of FlgB in the clinical strain indicates that flagellin is a virulence factor used by P. aeruginosa in keratitis.
Lipotoxin F (LptF) was also one of the most abundant proteins in the clinical strain, and was detected threefold higher compared to the ATCC 10145 strain. Lipotoxins have been shown to stimulate inflammatory responses in cystic fibrosis (CF) [44,45]. LptF is an outer membrane protein [46] that is upregulated in mucoid cells that cause chronic infection in CF [44,45,47,48] and has been suggested to have roles in establishing mucoid biofilms. Upregulation of LptF in this study indicates the mucoidity of the clinical strain and the increased virulence of this strain compared to the ATCC 10145 strain. Similar to P. aeruginosa strains infecting patients with CF, P. aeruginosa in keratitis may also go through the process of conversion to mucoidy where they mutate into a mucoid, exopolysaccharide alginate-overproducing form [49]. Other upregulated proteins included several hypothetical proteins that had similarities to several lipoproteins. Some of these had conserved known domains such as the Clustered Regularly Interspaced Short Palindromic Repeats (CRSPR)/Cas domain that belongs to the CRISPR/Cas system-associated Repair Associated Mysterious Proteins (RAMP) superfamily protein (hypothetical protein PA14_33310). CRISPR loci are composed of short DNA repeat sequences separated by stretches of variable spacer sequences [50]. The loci are located near clusters of CRISPR-associated (cas) genes that, together with the RNA transcribed from the CRISPR loci, mediate resistance to viruses, conjugative plasmids, and transposable elements [51,52]. CRISPR regions are considered part of the accessory genome of P. aeruginosa present in some strains and not in others [53]. CRISPR loci have been described in clinical isolates of P. aeruginosa obtained from the eye, among other clinical strains tested [54], but no studies have investigated the role of CRISPR in keratitis. Other conserved domains detected include gluconate and ATP binding sites (hypothetical protein PA14_47120), alkylhydroperoxidase (PA2331), and the bacterial OsmY and nodulation (BON) superfamily. Proteins containing these conserved domains could be also be used by P. aeruginosa as virulence factors in keratitis.
The remaining upregulated proteins consist of organic solvent tolerant protein (OstA) for resistance to organic solvents and antibiotics [55], polyhydroxyalkanoate synthesis protein (PhaF), and dehydrocarnitine CoA transferase subunit B. The latter is required for cartinine metabolism. Carnitine is a quaternary amine compound prevalent in animal tissues, and a potential carbon, nitrogen, and energy source for pathogens during infection [56,57], which could be the case in keratitis. Other proteins included 3-hydroxybutyrate dehydrogenase (PHB), which is used as intracellular carbon source by bacteria when oxygen, phosphorus, or nitrogen is limited in the environment [58,59] and osmotically inducible protein OsmC. The OsmC homolog was initially identified in Escherichia coli as a protein responding to osmotic stress [60] and has been shown to share structural and functional similarity to organic hydroperoxide reductase (Ohr) [61]. Expression of these proteins at high levels by the clinical strain indicates its high resistance to the outside environment, including infection by viruses, H2O2, antibiotics, and osmotic stress, making it more virulent than the ATCC 10145 strain. These could all be mechanisms of virulence that P. aeruginosa uses in its infection of the cornea.
In addition to these upregulated proteins that all appear to play roles in bacterial virulence, 55 proteins were detected in the clinical strain but not in ATCC 10145. Among the proteins that were highly abundant in the clinical strain, and were not detected in the ATCC 10145 strain, were two putative NRPSs (NCBI reference sequences # 218891724 and 386058801). These proteins were the fourth and fifth most abundant proteins, respectively, in the clinical strain proteome and first and second, respectively, in the proteins that were present in the clinical strain but missing in the ATCC 10145 strain. These proteins have a 99% similarity to a protein designated AmbE from PA2302. The latter is part of a gene cluster responsible for the production of the secondary metabolite l-2-amino-4-methoxy-trans-3-butenoic acid (AMB) [62]. AMB, a potent toxin produced by P. aeruginosa [63], was originally isolated from a fermentation broth of P. aeruginosa ATCC 7700 [64] in P. aeruginosa PAO1, and is synthesized by the gene cluster ambABCDE [62]. Interestingly, AmbC and AmbB were detected in the clinical strain in this study and not in the ATCC 10145 strain, suggesting that in addition to the known virulence factors, the clinical strain used in this study expresses genes and proteins responsible for the biosynthesis of AMB. It is possible that P. aeruginosa uses this NRPS as a virulence factor to synthesize and secrete AMB to invade the cornea in keratitis. Further studies are necessary to confirm this hypothesis.
Members from the quinolone signaling (PQS) system PqsB, C, and D were also detected in the clinical strain and not in the ATCC 10145 strain. The PQS system has been shown to be produced by P. aeruginosa under stressful conditions to survive and to have antioxidant activity [65]. This system controls the rhl quorum-sensing system [66] that controls the cell density. The latter controls the secretion of several virulence factors suggesting that the PQS system could be regulating the secretion of virulence factors in keratitis.
Elongation factors (EFs) Ts and G were also detected at higher levels in the corneal strain and were missing in ATCC 10145. These proteins, along with EF-Tu and EF-P, belong to the superfamily of GTPase switch proteins and promote the elongation phase of protein synthesis [67]. Elongation factor proteins have been shown to be critical for bacterial virulence [68], including P. aeruginosa in keratitis. Other proteins that were detected at higher levels in the clinical strain and were not detected in ATCC 10145 included hypothetical proteins that had similarities to the yeast N-terminal acetyltransferase, which plays a role in cell division, acyl CoA dehydrogenase (PA2330), fimbrial proteins (PilA), outer membrane proteins (porin), and siderophore synthesis (pyochelin synthetase), all of which have been shown to contribute to bacterial virulence [69-75], some of which must be investigated in keratitis.
In conclusion, in this study we used a proteomic approach to quantitatively compare the proteomes of a P. aeruginosa strain obtained from an infected eye and the laboratory strain ATCC 10145. The protein profile of each strain reflects its adaptation to its niche. The upregulated proteins in the clinical strain were associated with virulence, suggesting that this strain is highly virulent and produces a plethora of virulence factors to invade and establish its infection in the cornea, whereas the upregulated proteins in ATCC 10145 were associated with the citric acid (TCA) cycle, pyruvate metabolism, and acetate utilization. Although ATCC 10145 also expresses some virulence factors, these factors were detected at lower levels than those in the clinical strain. Identification of the proteins in PA keratitis will aid in the elucidation of novel virulence factors, thus contributing to the development of novel intervention strategies to reduce the burden of P. aeruginosa infections in contact lens wearers.
Appendix 1. Proteins detected in the clinical strain and ATCC10145 strain by LC-MS/MS along with their RefSeq accession number, scan count, fold change and p values, sorted based on their scan count from highest to lowest.
To access the data, click or select the words “Appendix 1.” Abbreviations: RefSeq: reference sequence, SC: scan count, NLOG-FoldR: normalized log ratio, Np: normalized p value, Adj-NP: adjusted normalized p value *Normalized ratios are calculated from the ratio of the scans (clinical/ATCC10145) adjusted to the ratio of the total scans for all proteins detected in each group.
References
- 1.Rattanatam T, Heng WJ, Rapuano CJ, Laibson PR, Cohen EJ. Trends in contact lens-related corneal ulcers. Cornea. 2001;20:290–4. doi: 10.1097/00003226-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Schornack MM, Faia LJ, Griepentrog GJ. Pseudomonas keratitis associated with daily wear of silicone hydrogel contact lenses. Eye Contact Lens. 2008;34:124–8. doi: 10.1097/ICL.0b013e318126c0ee. [DOI] [PubMed] [Google Scholar]
- 3.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–7. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 4.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–8. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 5.Najjar DM, Aktan SG, Rapuano CJ, Laibson PR, Cohen EJ. Contact lens-related corneal ulcers in compliant patients. Am J Ophthalmol. 2004;137:170–2. doi: 10.1016/s0002-9394(03)00722-0. [DOI] [PubMed] [Google Scholar]
- 6.Khatri S, Lass JH, Heinzel FP, Petroll WM, Gomez J, Diaconu E, Kalsow CM, Pearlman E. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–84. [PubMed] [Google Scholar]
- 7.Forster RK. Conrad Berens Lecture. The management of infectious keratitis as we approach the 21st century. CLAO J. 1998;24:175–80. [PubMed] [Google Scholar]
- 8.Varaprasathan G, Miller K, Lietman T, Whitcher JP, Cevallos V, Okumoto M, Margolis TP, Yinghui M, Cunningham ET., Jr Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–4. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton F, Keay L, Edwards K, Naduvilath T, Brian G, Jacobs R. Studies of contact lens-related microbial keratitis in Australia and New Zealand: new learnings. Eye Contact Lens. 2007;33:354–7. doi: 10.1097/ICL.0b013e318157c57e. [DOI] [PubMed] [Google Scholar]
- 10.Lotti R, Dart JK. Cataract as a complication of severe microbial keratitis. Eye (Lond) 1992;6:400–3. doi: 10.1038/eye.1992.82. [DOI] [PubMed] [Google Scholar]
- 11.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–86. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–69. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 13.Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13:389–97. doi: 10.1016/j.tim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–23. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobden JA. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 2002;21:391–6. doi: 10.1089/10445490260099674. [DOI] [PubMed] [Google Scholar]
- 16.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–8. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AM. Ocular toxicity of fluoroquinolones. Clin Experiment Ophthalmol. 2007;35:566–77. doi: 10.1111/j.1442-9071.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Pennefather PM, Kaye SB, Hart CA. Fluoroquinolones: place in ocular therapy. Drugs. 2001;61:747–61. doi: 10.2165/00003495-200161060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Moshirfar M, Meyer JJ, Espandar L. Fourth-generation fluoroquinolone-resistant mycobacterial keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:1978–81. doi: 10.1016/j.jcrs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106:1313–8. [PubMed] [Google Scholar]
- 21.Mannis MJ. The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc. 2002;100:243–71. [PMC free article] [PubMed] [Google Scholar]
- 22.Jhanji V, Sharma N, Satpathy G, Titiyal J. Fourth-generation fluoroquinolone-resistant bacterial keratitis. J Cataract Refract Surg. 2007;33:1488–9. doi: 10.1016/j.jcrs.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 23.OPINION 36: Designation of Strain ATCC 10145 as the Neotype Strain of Pseudomonas aeruginosa (Schroeter) Migula. Int J Syst Bacteriol. 1970;20:15–6. [Google Scholar]
- 24.O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:pii 2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44:301–7. [PubMed] [Google Scholar]
- 26.Kurachi M. Studies on the biosynthesis of pyocyanine. Bull Inst Chem Res Kyoto Univ. 1958;36:174–187. [Google Scholar]
- 27.Dutta D, Willcox M. A Laboratory Assessment of Factors That Affect Bacterial Adhesion to Contact Lenses. Biology. 2013;2:1268–81. doi: 10.3390/biology2041268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thein M, Sauer G, Paramasivam N, Grin I, Linke D. Efficient subfractionation of gram-negative bacteria for proteomics studies. J Proteome Res. 2010;9:6135–47. doi: 10.1021/pr1002438. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Wakim B, Li M, Halligan B, Tint GS, Patel SB. Quantifying raft proteins in neonatal mouse brain by 'tube-gel' protein digestion label-free shotgun proteomics. Proteome Sci. 2007;5:17. doi: 10.1186/1477-5956-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhenni RA, Al Shahwan S, Morales J, Wakim BT, Chomyk AM, Alkuraya FS, Edward DP. Identification of differentially expressed proteins in the aqueous humor of primary congenital glaucoma. Exp Eye Res. 2011;92:67–75. doi: 10.1016/j.exer.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Hendrickson EL, Xia Q, Wang T, Leigh JA, Hackett M. Comparison of spectral counting and metabolic stable isotope labeling for use with quantitative microbial proteomics. Analyst. 2006;131:1335–41. doi: 10.1039/b610957h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunmire JJ, Bouhenni R, Hart ML, Wakim BT, Chomyk AM, Scott SE, Nakamura H, Edward DP. Novel serum proteomic signatures in a non-human primate model of retinal injury. Mol Vis. 2011;17:779–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 34.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 35.Davey ME, O'Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MK, Allen JH. The role of hemolysin in corneal infections with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1978;17:480–3. [PubMed] [Google Scholar]
- 37.Manos J, Hu H, Rose BR, Wainwright CE, Zablotska IB, Cheney J, Turnbull L, Whitchurch CB, Grimwood K, Harmer C, Anuj SN, Harbour C. Virulence factor expression patterns in Pseudomonas aeruginosa strains from infants with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2013;32:1583–92. doi: 10.1007/s10096-013-1916-7. [DOI] [PubMed] [Google Scholar]
- 38.O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–23. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleiszig SM, Arora SK, Van R, Ramphal R. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect Immun. 2001;69:4931–7. doi: 10.1128/IAI.69.8.4931-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–10. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramphal R, Arora SK, Ritchings BW. Recognition of mucin by the adhesin-flagellar system of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1996;154:S170–4. doi: 10.1164/ajrccm/154.4_Pt_2.S170. [DOI] [PubMed] [Google Scholar]
- 44.Firoved AM, Boucher JC, Deretic V. Global genomic analysis of AlgU (sigma(E))-dependent promoters (sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J Bacteriol. 2002;184:1057–64. doi: 10.1128/jb.184.4.1057-1064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Firoved AM, Ornatowski W, Deretic V. Microarray analysis reveals induction of lipoprotein genes in mucoid Pseudomonas aeruginosa: implications for inflammation in cystic fibrosis. Infect Immun. 2004;72:5012–8. doi: 10.1128/IAI.72.9.5012-5018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damron FH, Napper J, Teter MA, Yu HD. Lipotoxin F of Pseudomonas aeruginosa is an AlgU-dependent and alginate-independent outer membrane protein involved in resistance to oxidative stress and adhesion to A549 human lung epithelia. Microbiology. 2009;155:1028–38. doi: 10.1099/mic.0.025833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firoved AM, Deretic V. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J Bacteriol. 2003;185:1071–81. doi: 10.1128/JB.185.3.1071-1081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood LF, Leech AJ, Ohman DE. Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: Roles of sigma (AlgT) and the AlgW and Prc proteases. Mol Microbiol. 2006;62:412–26. doi: 10.1111/j.1365-2958.2006.05390.x. [DOI] [PubMed] [Google Scholar]
- 49.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–74. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakata A, Amemura M, Makino K. Unusual nucleotide arrangement with repeated sequences in the Escherichia coli K-12 chromosome. J Bacteriol. 1989;171:3553–6. doi: 10.1128/jb.171.6.3553-3556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, van der Oost J, Koonin EV. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–77. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–4. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2010;74:621–41. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cady KC, White AS, Hammond JH, Abendroth MD, Karthikeyan RS, Lalitha P, Zegans ME, O'Toole GA. Prevalence, conservation and functional analysis of Yersinia and Escherichia CRISPR regions in clinical Pseudomonas aeruginosa isolates. Microbiology. 2011;157:430–7. doi: 10.1099/mic.0.045732-0. [DOI] [PubMed] [Google Scholar]
- 55.Chiu HC, Lin TL, Wang JT. Identification and characterization of an organic solvent tolerance gene in Helicobacter pylori. Helicobacter. 2007;12:74–81. doi: 10.1111/j.1523-5378.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 56.Wargo MJ, Hogan DA. Identification of genes required for Pseudomonas aeruginosa carnitine catabolism. Microbiology. 2009;155:2411–9. doi: 10.1099/mic.0.028787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bremer J. Carnitine–metabolism and functions. Physiol Rev. 1983;63:1420–80. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 58.Anderson AJ, Dawes EA. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–72. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawes EA, Senior PJ. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 60.Gutierrez C, Devedjian JC. Osmotic induction of gene osmC expression in Escherichia coli K12. J Mol Biol. 1991;220:959–73. doi: 10.1016/0022-2836(91)90366-e. [DOI] [PubMed] [Google Scholar]
- 61.Atichartpongkul S, Loprasert S, Vattanaviboon P, Whangsuk W, Helmann JD, Mongkolsuk S. Bacterial Ohr and OsmC paralogues define two protein families with distinct functions and patterns of expression. Microbiology. 2001;147:1775–82. doi: 10.1099/00221287-147-7-1775. [DOI] [PubMed] [Google Scholar]
- 62.Lee X, Fox A, Sufrin J, Henry H, Majcherczyk P, Haas D, Reimmann C. Identification of the biosynthetic gene cluster for the Pseudomonas aeruginosa antimetabolite L-2-amino-4-methoxy-trans-3-butenoic acid. J Bacteriol. 2010;192:4251–5. doi: 10.1128/JB.00492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahm U, Knobloch G, Wagner F. Isolation and characterization of the methionine antagonist L-2-amino-4-methoxy-trans-3-butenoic acid from Pseudomonas aeruginosa grown on n-paraffin. J Antibiot (Tokyo) 1973;26:389–90. doi: 10.7164/antibiotics.26.389. [DOI] [PubMed] [Google Scholar]
- 64.Scannel JP, Pruess DL, Demny TC, Sello LH, Williams T. Antimetabolites produced by microorganisms. V. L-2-amino-4-methoxy-trans-3-butenoic acid. J Antibiot (Tokyo) 1972;25:122–7. doi: 10.7164/antibiotics.25.122. [DOI] [PubMed] [Google Scholar]
- 65.Häussler S, Becker T. The pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog. 2008;4:e1000166. doi: 10.1371/journal.ppat.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–8. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nyborg J. Possible evolution of factors involved in protein biosynthesis. Acta Biochim Pol. 1998;45:883–94. [PubMed] [Google Scholar]
- 68.Zou SB, Roy H, Ibba M, Navarre WW. Elongation factor P mediates a novel post-transcriptional regulatory pathway critical for bacterial virulence. Virulence. 2011;2:147–51. doi: 10.4161/viru.2.2.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pereira MP, Blanchard JE, Murphy C, Roderick SL, Brown ED. High-throughput screening identifies novel inhibitors of the acetyltransferase activity of Escherichia coli GlmU. Antimicrob Agents Chemother. 2009;53:2306–11. doi: 10.1128/AAC.01572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doig P, Todd T, Sastry PA, Lee KK, Hodges RS, Paranchych W, Irvin RT. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988;56:1641–6. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johanson WG, Jr, Higuchi JH, Chaudhuri TR, Woods DE. Bacterial adherence to epithelial cells in bacillary colonization of the respiratory tract. Am Rev Respir Dis. 1980;121:55–63. doi: 10.1164/arrd.1980.121.1.55. [DOI] [PubMed] [Google Scholar]
- 72.Chatfield SN, Dorman CJ, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect Immun. 1991;59:449–52. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonasaeruginosa. Proc Natl Acad Sci USA. 2002;99:7072–7. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feinbaum RL, Urbach JM, Liberati NT, Djonovic S, Adonizio A, Carvunis AR, Ausubel FM. Genome-wide identification of Pseudomonas aeruginosa virulence-related genes using a Caenorhabditis elegans infection model. PLoS Pathog. 2012;8:e1002813. doi: 10.1371/journal.ppat.1002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zolfaghar I, Evans DJ, Fleiszig SM. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infect Immun. 2003;71:5389–93. doi: 10.1128/IAI.71.9.5389-5393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]