Abstract
Drug disposition is highly regulated by membrane transporters. Some transporter-mediated drug–drug interactions (DDIs) may not manifest themselves in changes in systemic exposure but rather in changes in tissue exposure of drugs. To better assess the impact of transporter-mediated DDIs in tissues, positron emission tomography (PET)—a noninvasive imaging method—plays an increasingly important role. In this article, we provide examples of how PET can be used to assess transporter-mediated DDIs in different organs.
Transporters in Drug Development
In the past decade, an ever-increasing number of membrane transport proteins have been discovered and characterized. These membrane-bound proteins with saturable substrate-binding sites control the access of endogenous substances and drugs to various tissues within the human body. With the discovery that some of these transporters are capable of transporting a multitude of chemically diverse drug molecules, the possibility that the contribution of these transporters may exert a considerable impact on drug disposition, safety, and efficacy has been raised.1 Concomitant administration of multiple drugs that interact with the same drug transporters can lead to altered blood or tissue concentrations of the drugs compared with administration of the drugs alone (transporter-mediated drug–drug interaction (DDI)), which can result in side effects or loss of efficacy. For example, inhibition of organic anion–transporting polypeptides (OATPs), which act as basolateral uptake transporters in hepatocytes, by cyclosporin A leads to a decrease in liver concentrations and an increase in blood concentrations of statins, which may cause side effects such as myopathy or rhabdomyolysis.2,3
Considering the importance of potential transporter interactions of new drug candidates, an urgent need to provide drug developers with guidelines on how to assess the risk of potential clinically relevant transporter-mediated DDIs was recognized. In 2007, the International Transporter Consortium (ITC) was founded; it included renowned experts in the field consisting of members from industry, academia, and the US Food and Drug Administration. Workshops that were held in 2008 and 2012 focused on the identification and description of transporters that are of importance in clinical DDIs. Standards were established for in vitro–based evaluation of transporter-mediated DDIs to possibly reduce the need for in vivo studies. A series of white papers4,5,6,7,8,9,10,11 was published, which first identified seven transporters, P-glycoprotein (Pgp, encoded by ABCB1), breast cancer resistance protein (BCRP, encoded by ABCG2), OATP1B1 and OATP1B3 (encoded by SLCO1B1 and SLCO1B3, respectively), organic cation transporter 2 (OCT2, encoded by SLC22A2), and organic anion transporters 1 and 3 (OAT1 and OAT3, encoded by SLC22A6 and SLC22A7, respectively), as the clinically most relevant transporters.8 This list was later amended7 to include multidrug and toxin extrusion transporters (encoded by SLC47A), multidrug resistance proteins (MRPs, encoded by ABCCs), and the bile salt export pump (encoded by ABCB11). The white papers recommended studying the interaction of drug candidates with these transporters in vitro using, for instance, cell lines overexpressing the transporters of interest to assess the potential of clinically relevant DDIs. Moreover, decision trees were proposed to decide whether clinical studies need to be conducted to evaluate the propensity for clinically relevant DDIs.7,8 In cases in which the ratio of unbound maximum drug concentrations in blood or tissue to the half-maximal inhibitory concentration (IC50) for transporter inhibition exceeds certain thresholds, the ITC recommends in vivo DDI studies.7,8 In this respect, the availability of suitable and selective probe substrates or inhibitors that can be safely used in humans is of high importance. Both the US Food and Drug Administration and the European Medicines Agency have published guidelines on drug interactions that also deal with transporters.12,13
To date, more than 400 transporters have been identified and classified into two superfamilies, the adenosine triphosphate–binding cassette (ABC)14 and the solute carrier (SLC) transporters.15 Several ABC and SLC transporters are capable of transporting drugs. Drug transporters are classified into different functional categories. Transporters mediating the import of drugs into the cell are called uptake transporters, and those possessing export properties are referred to as efflux transporters. Previous review articles have given detailed descriptions of different transport proteins and their roles in drug disposition.1,7,8,16,17 The majority of the ABC transporters, expressed in cellular and intracellular membranes, are responsible for the efflux of substances from cells or tissues. Only in some exceptions do they act as uptake transporters. The 48 ABC transporters known today are divided into seven different families.14 Most uptake transporters were found to be members of the SLC transporter family (e.g., OATPs). SLC transporters can be subdivided into 48 transporter families and are abundantly expressed in various tissues.15 Expression of ABC and SLC transporters in different tissues was shown to be regulated by xenobiotic-activated nuclear receptors such as the pregnane X receptor.1,18
Positron Emission Tomography as a Tool to Study Drug Transporters
Several different in vitro approaches (e.g., membrane vesicle assays, transporter-overexpressing cell systems, and sandwich-cultured hepatocytes) are currently used to study the influence of transporters on drug disposition. However, in vivo studies in human subjects remain the gold standard for identifying clinically significant contributions of transport proteins to drug disposition.16 Traditionally, clinical DDI studies have been performed by treating healthy volunteers either with the drug of interest alone (i.e., victim/substrate or perpetrator/inhibitor drug) or in combination with a second drug (perpetrator/inhibitor or victim/substrate drug), followed by the assessment of the drug’s pharmacokinetics in the blood compartment, which is most readily accessible for sampling. However, it is increasingly recognized that some transporter-mediated DDIs may not manifest themselves in changes in blood pharmacokinetics but rather in changes in tissue pharmacokinetics (e.g., liver, kidney, and brain).19 For instance, inhibition of an efflux transporter at the canalicular membrane of hepatocytes may lead to changes in drug exposure in the liver and consequently to hepatotoxicity, with only minor changes in drug plasma exposure. Moreover, inhibition of an efflux transporter at the blood–brain barrier (BBB) may potentially lead to changes in brain tissue concentrations and neurotoxicity of drug candidates. To better understand the mechanistic basis and investigate the clinical relevance of such phenomena, there is an obvious need for a method that allows for assessment of drug tissue concentrations.20 Several experimental techniques are available to assess drug concentrations in tissues, such as microdialysis, magnetic resonance spectroscopy, and tissue biopsy sampling.21 Most have clear limitations for a broader use in drug development, e.g., due to their invasiveness, lack of sensitivity, or inability for sampling at multiple time points. A particularly useful tool to measure drug tissue concentrations noninvasively in both animals and humans is positron emission tomography (PET) together with radioactively labeled drugs (so-called radiotracers).22,23 Ideally, radiotracers are structurally identical to the unlabeled drug and incorporate short-lived positron-emitting radionuclides (e.g., carbon-11 (11C), half-life: 20.4 min; or fluorine-18 (18F), half-life: 109.8 min), which allow detection and quantification of the anatomical localization of these radiotracers in the living organism by means of PET cameras. PET measures radioactivity concentrations in units of, for instance, kilobecquerel (kBq) per gram of tissue, which can be converted into absolute drug concentrations (e.g., nanograms per gram of tissue) using the specific activity of the radiotracer (i.e., ratio of radioactivity to mass, usually given as gigabecquerel (GBq) per micromole of substance). In principle, PET imaging is a very useful method for investigating drug–transporter interactions in vivo.19,24,25
However, studying specific transporters with PET relies on the availability of transporter-specific, metabolically stable probe substrates that are amenable to radiolabeling. Given the promiscuity of xenobiotic transporters, the identification of suitable candidate molecules is not a trivial task. In their review article, Kannan et al.25 have proposed the following criteria for a suitable radiotracer to assess transporter function: (i) selectivity for the transporter of interest, i.e., lack of interaction with other transporters; (ii) amplitude of signal, i.e., difference in tissue uptake between a state in which the transporter is fully functional and a state in which it is completely inhibited; and (iii) chemical purity of signal, i.e., absence of radiolabeled metabolites that are taken up into the tissue of interest and may therefore confound interpretation of the PET signal. Recently published review articles have given overviews of currently available PET tracers for the assessment of ABC/SLC transporter function.19,24
PET-Based DDI Studies
In contrast to traditional DDI studies, in which plasma pharmacokinetics of unlabeled drugs are measured, a PET-based DDI study uses a drug labeled with a positron-emitting radionuclide (e.g., 11C or 18F) in conjunction with a second unlabeled drug. The radiolabeled drug is usually administered as a microdose because PET radiotracers are usually obtained in high specific activity, i.e., the mass associated with a commonly administered dose of a PET tracer is typically in the range of 1–10 µg.23 An advantage of microdosing is that less preclinical toxicity data are required to test microdoses of drugs in humans as compared with traditional phase I studies.26 However, in a few selected cases in which the drug’s clinical safety profile is well established, PET tracers may also be administered at low specific activity, i.e., at doses that may result in relevant pharmacodynamic effects. This may be of particular importance when drugs display nonlinear pharmacokinetics, which may be the case when saturable transporters or metabolizing enzymes play a critical role in drug disposition.22,23
In the case of transporter studies, the radiolabeled drug, a substrate of one or more ABC or SLC transporters, may be the drug of interest (victim drug). The unlabeled (perpetrator) drug may be a drug expected to be concomitantly used with the victim drug in the clinic and suspected to inhibit one or more ABC or SLC transporters, thereby affecting the disposition of the victim drug. Alternatively, instead of radiolabeling the drug candidate, a generic PET tracer that is a well-characterized substrate of one or several transporters may be used.19,24 This PET tracer may then be used to assess whether the drug of interest (perpetrator drug) inhibits one or more transporters at clinically obtained plasma concentrations.
PET allows the monitoring of the whole-body distribution and pharmacokinetics of a radiolabeled drug and thereby enables a simultaneous assessment of the impact of transporter inhibition on the exposure of multiple organs to a radiolabeled drug (e.g., kidney, liver, and brain). When the concentration–time profile of the radiolabeled drug in arterial plasma is measured during the PET scan, mathematical modeling approaches can be used, enabling an estimation of the exchange rate constants of radiolabeled drug between the plasma and tissue compartments. When combined with an anatomical imaging method, such as magnetic resonance tomography or computed tomography, detailed information on the intraorgan distribution of a radiotracer can be obtained (Figure 1).
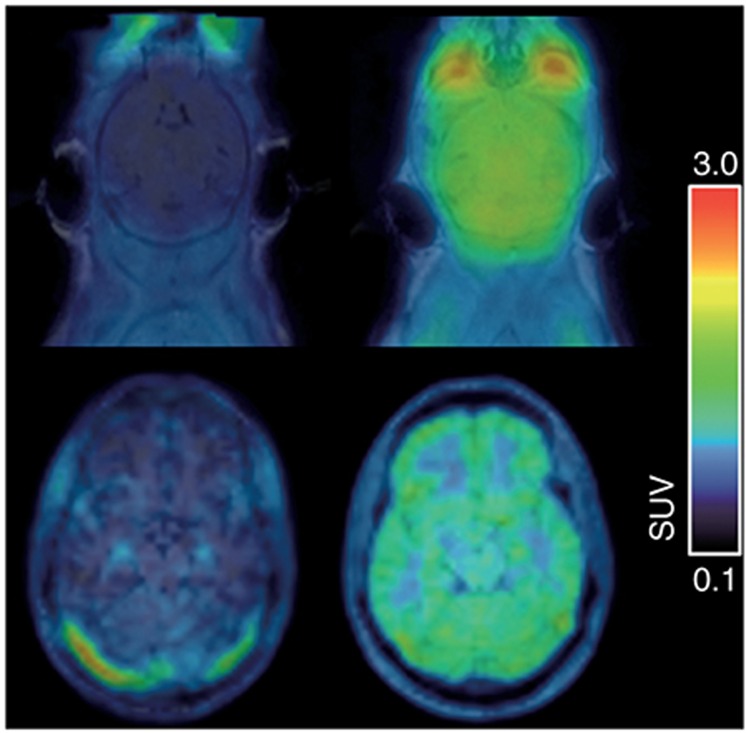
Figure 1.
Translational positron emission tomography (PET) imaging of the P-glycoprotein-mediated interaction between (R)-[11C]verapamil and tariquidar at the mouse (C57BL/6, upper row) and human blood–brain barrier (lower row). Left, magnetic resonance imaging-coregistered PET summation images (0–60 min) at baseline and, right, at 2 h (mouse) or 1 h (human) after i.v. administration of tariquidar (mouse: 15 mg/kg over 1 min, human: 8 mg/kg over 160 min) are shown. Tariquidar plasma concentrations at the end of the PET scan were similar in the shown mouse (1,297 ng/ml) and the human volunteer (1,241 ng/ml). In mice, a 4.9-fold and in humans, a 2.0-fold increase relative to baseline in the brain-to-plasma ratio of (R)-[11C]verapamil at 60 min after injection (Kp,brain) was observed. Mean baseline Kp,brain values before tariquidar administration were 0.43 ± 0.05 (n = 6) for mice and 0.55 ± 0.06 (n = 6) for humans. Radioactivity concentration is normalized to injected dose per kilogram body weight and expressed as a standardized uptake value.
In preclinical PET-based DDI studies, transgenic mice or rats in which one or several membrane transporters are genetically knocked out may be used.27 The comparison of drug tissue kinetics and effects of transporter inhibition between transporter knockout and wild-type animals may lead to an identification of specific transporters involved in drug disposition. The selection of appropriate transporter knockout animals is usually guided by a collection of in vitro data characterizing a drug’s interaction profile with different transporters. Figure 2 illustrates how the use of transgenic mice may be integrated into a preclinical PET-based DDI study at the BBB.28 In preclinical studies, at the end of the PET scan tissue and blood can be collected from animals. Obtained samples may be analyzed by radiochromatographic methods (radio-thin-layer chromatography or radio-high-performance liquid chromatography) to assess whether radiolabeled metabolites of the parent compound contribute to the measured PET signal in tissue. The great advantage of PET imaging relative to in vitro transporter assays is that PET allows capturing of the dynamic interplay of different transporters (e.g., uptake and efflux transporters) in different organ systems in the living organism, which often cannot be studied in vitro due to limited availability of suitable cellular systems expressing several different drug transporters at physiologically relevant expression levels.16 In other words, PET imaging provides the whole picture of the effect of the simultaneous actions of several different transporters on drug tissue exposure.
Figure 2.
Preclinical positron emission tomography (PET)-based drug–drug interaction study between the P-glycoprotein (Pgp) substrate (R)-[11C]verapamil and the Pgp inhibitor tariquidar using Friend virus B-type (FVB) wild-type and transporter knockout mice. Shown are coronal (R)-[11C]verapamil PET summation images (0–60 min) for paired scans acquired before (upper row) and after (lower row) i.v. pretreatment with tariquidar (15 mg/kg, 2 h before start of second PET scan). Radiation scale is expressed as a standardized uptake value. In PET scans before tariquidar pretreatment, brain uptake of (R)-[11C]verapamil was low in animals expressing Pgp (wild-type and Bcrp1−/−) and high in animals lacking Pgp (Mdr1a/b−/− and Mdr1a/b−/−Bcrp1−/−). Following Pgp inhibition with tariquidar, brain uptake of (R)-[11C]verapamil was within a comparable range in all four mouse types. Taken together, these data demonstrate that (R)-[11C]verapamil is transported by Pgp at the mouse blood–brain barrier and not by breast cancer resistance protein.
PET is a translational research tool and can be readily applied in human studies. This approach allows for direct assessment of the clinical relevance of DDIs identified in a preclinical setting. PET is an ideal tool to assess species-dependent differences in transporter specificity, expression, and function, which are of great interest for drug development (Figure 1). The recent availability of transgenic animals expressing human transporters provides a new opportunity to investigate species-dependent differences in transporters in a preclinical setting.29
Because PET is fully noninvasive and provides dynamic data, the number of animals needed in a preclinical PET study is much smaller than that in traditional preclinical DDI studies. This is in accordance with the rule of the three Rs (replacement, reduction, and refinement) and may allow for a cost reduction when expensive transgenic mouse models are used. Because animals can be scanned repeatedly, each animal may serve as its own control by facilitating assessment of drug tissue distribution both before and after administration of the transporter-inhibiting perpetrator drug, which increases the statistical power and reduces the number of animals required in the experiment (Figure 2). When short-lived 11C is used as the PET radionuclide, repeated PET scans may be performed within one imaging session. This is possible because 11C decays fast enough so that the remaining radioactivity from the first PET scan will not interfere with the second PET scan. For instance, two consecutive PET scans with an 11C-labeled radiotracer may be performed at an interval of 2–3 h between the first and second PET scans. Before the second PET scan, unlabeled transporter inhibitor may be administered (Figure 2). In the case of clinical PET studies, this experimental design requires only a single visit to the PET imaging center and may therefore increase subject throughput in clinical PET imaging studies.
Limitations of PET Imaging in Studying Transporter-Mediated DDIs
PET measures the total radioactivity in a tissue and is not able to distinguish radiolabeled metabolites from radiolabeled parent compound. In many cases, perpetrator drugs inhibit not only transporters but also inhibit or induce drug-metabolizing enzymes. When studying transporter-mediated DDIs with extensively metabolized PET tracers, it may be difficult to distinguish transporter effects from effects on metabolizing enzymes because both may lead to changes in PET signal. For instance, the Pgp substrate radiotracer (R)-[11C]verapamil is known to undergo extensive peripheral metabolism mediated mainly by cytochrome P450 3A4, which generates polar radiolabeled metabolites that were shown to be taken up into the brain independent of Pgp function.30,31 An increase in the fraction of polar radiolabeled metabolites of [11C]verapamil in plasma, for instance due an induction of drug-metabolizing enzymes, may lead to a concomitant increase in brain PET signal, which could be erroneously interpreted as an inhibition of drug efflux transporters at the BBB. It has been shown that this problem may be overcome in the case of [11C]verapamil by analyzing only the first few minutes of PET data acquired after radiotracer injection, in which peripheral metabolism is minimal.32
Another possible limitation of PET in predicting transporter-mediated DDIs is that in most cases, PET studies are performed with microdoses of radiolabeled drugs. For low-capacity drug transporters and/or high-affinity substrates, the effect of DDIs may be different when drugs are studied at microdoses and at therapeutic doses; i.e., at microdoses, active drug transport may play a role, whereas at therapeutic doses, active transport may be (partly) saturated and the DDI may no longer be relevant. For instance, for the radiolabeled third-generation Pgp inhibitor [11C]elacridar, striking differences in brain distribution were observed in rodents when the drug was studied at microdoses vs. therapeutic doses.33,34 At microdoses, brain uptake was very low, whereas at therapeutic doses, appreciable brain uptake of [11C]elacridar was observed. This was interpreted as indicating that elacridar may have inhibited its own efflux transport by Pgp and Bcrp at the BBB, leading to higher brain distribution at therapeutic doses.34,35 Because it may be difficult to predict such effects based only on in vitro transport data, it is advisable to study drug tissue distribution in preclinical PET studies at both microdoses and therapeutic doses to assess dose linearity of drug tissue distribution.23
In traditional preclinical DDI studies, tissue concentrations of drugs may be analyzed by chromatographic methods, such as liquid chromatography–mass spectrometry, before and after administration of a perpetrator drug. In a few selected cases, the extent of transporter-mediated DDIs has been measured both with liquid chromatography–mass spectrometry analysis of tissue samples and PET imaging.36,37 However, when comparing such data, caution is warranted because this can be done only for drugs that are metabolically stable; for extensively metabolized drugs, results from liquid chromatography–mass spectrometry analysis and PET analysis may differ due to possible contribution of radiolabeled metabolites to the PET signal. In addition, the administered drug doses required for liquid chromatography–mass spectrometry analysis of tissue samples are usually considerably higher than the microdoses used for PET, which may also lead to divergent results.
Selected Transporter-Mediated DDIs Assessed With PET Imaging
Blood–brain barrier
ABC and SLC transporters are abundantly expressed at the BBB to control drug access to the brain.38 Thus, transporter-mediated DDIs at the BBB may lead to unwanted clinical consequences at two different levels.39 First, DDIs may lead to an increase in side effects, especially when drugs that are not intended to exert their pharmacological effects in the central nervous system enter the brain, e.g., due to an inhibition of efflux transporters. Second, DDIs may lead to a loss of drug efficacy when penetration of neuropharmacological drug into the brain is impaired, e.g., due to an induction of efflux transporters or inhibition of uptake transporters. Hence, the ITC has recently discussed the likelihood of clinically relevant DDIs at the human BBB.6 The ITC came to the conclusion that transporter-mediated DDIs at the BBB are unlikely in clinical settings because in clinical practice most drugs do not achieve high enough unbound plasma concentrations to achieve significant transporter inhibition. However, transporter-mediated DDIs, mostly with respect to Pgp, have been extensively investigated in both preclinical and clinical PET imaging studies. Examples have been published in which the brain distribution of radiolabeled Pgp substrates, such as racemic [11C]verapamil, (R)-[11C]verapamil, or [11C]N-desmethyl-loperamide, was significantly increased following administration of potent Pgp inhibitors such as elacridar, tariquidar, valspodar, zosuquidar, or cyclosporin A.40,41,42,43,44,45,46,47 In a seminal study, Sasongko et al.41 assessed the effect of cyclosporin A on the brain distribution of [11C]verapamil in humans and found an 88% increase in [11C]verapamil brain exposure following infusion of cyclosporin A at a dose of 2.5 mg/kg/h. In another study by Kreisl et al.,45 it was shown that brain uptake of [11C]N-desmethyl-loperamide was increased by a factor of four in healthy human volunteers following i.v. infusion of the third-generation Pgp inhibitor tariquidar at a dose of 6 mg/kg body weight. The tariquidar dose used in this study was three times higher than doses in previous clinical oncology trials.48 The magnitude of the increase in brain uptake of Pgp substrates observed in humans following transporter inhibition stands in strong contrast to the several times higher increases seen in rodents after genetic or chemical transporter knockout. It is currently not entirely clear whether such differences can be solely explained by the fact that unbound plasma concentrations of Pgp inhibitors achieved in human studies were lower than those in rodent studies (Figure 1). Bauer et al.46 directly compared species differences in a Pgp-mediated DDI at the BBB by studying (R)-[11C]verapamil brain distribution in rats and in humans following administration of different tariquidar doses. They showed that the maximum effect of Pgp inhibition was different in rats and in humans. In rats, a maximum 11-fold increase was observed, whereas in humans, only a threefold increase in (R)-[11C]verapamil brain uptake was seen. This supported the concept that the consequences of transporter-mediated DDIs may be less pronounced at the human BBB than at the rodent BBB. Similarly, in mice, the magnitude of increase in (R)-[11C]verapamil brain uptake following administration of tariquidar was two to three times higher than that in humans, at tariquidar plasma concentrations comparable to those in humans (Figure 1). It cannot be excluded that for substrates whose brain distribution is more dependent on Pgp function than those of [11C]N-desmethyl-loperamide or (R)-[11C]verapamil (e.g., nelfinavir),49 higher increases in brain exposure may be observed following partial transporter inhibition at clinically relevant plasma concentrations of inhibitors. Two studies have assessed the effect of single-nucleotide polymorphisms in the ABCB1 gene in healthy volunteers on brain penetration of [11C]verapamil, and these failed to show differences in [11C]verapamil brain distribution between different ABCB1 haplotypes.50,51
Thus far, only one study has investigated whether treatment of healthy subjects with rifampicin, a pregnane X receptor activator, leads to an induction of Pgp at the BBB.52 Subjects underwent [11C]verapamil PET scans before and after treatment with rifampicin for 10–21 days. Although [11C]verapamil metabolism was significantly increased, most likely due to cytochrome P450 3A induction, no differences in [11C]verapamil brain uptake were detected following rifampicin treatment.
It has been recognized that Pgp and BCRP form a cooperative efflux system at the BBB and that dual substrates of Pgp and BCRP gain brain access only when both transporters are simultaneously inhibited.53 This is of clinical importance, given the discovery that several representatives of the class of receptor tyrosine kinase inhibitors (e.g., gefitinib, sorafenib, imatinib, and erlotinib) are hindered from entering brain tissue by Pgp and BCRP efflux.54 This has been put forward as one possible reason why these drugs have been ineffective in clinical trials in brain tumor patients.55 Several PET-labeled versions of these drugs ([11C]imatinib, [11C]erlotinib, [11C]gefitinib, [18F]gefitinib, and [11C]sorafenib) have been synthesized, and it was shown that some of these tracers allow for assessment of the functional activities of Pgp and BCRP at the BBB.56 In one study, it was shown that pretreatment of mice with elacridar, the most potent dual Pgp/BCRP inhibitor known to date, resulted in large (~10-fold) increases of brain exposure of [11C]gefitinib.36 PET imaging of the intratumoral distribution of radiolabeled tyrosine kinase inhibitors may be used in a personalized medicine approach to identify patients who will benefit from treatment with these agents.57 In addition, [11C]elacridar and [11C]tariquidar have been proposed as generic PET tracers to assess Pgp and BCRP function at the human BBB.33,58,59,60
Liver
The liver is an important organ for drug metabolism and elimination, and it has been recognized that a multitude of different SLC and ABC transporters expressed at the basolateral (sinusoidal) or canalicular membranes of hepatocytes govern the uptake into hepatocytes and the excretion into blood or bile of drugs and their metabolites.16,17 Although inhibition of a basolateral uptake transporter may primarily lead to changes in drug blood concentrations, inhibition of a canalicular efflux transporter may lead to pronounced changes in liver concentrations without changes in blood concentrations, which may have potentially life-threatening consequences (drug-induced liver injury). It has therefore been recognized that assessment of liver concentrations of drugs using noninvasive imaging methodology may be indispensable to understanding the true impact of transporter inhibition in the liver.19 In a series of pioneering studies by Yuichi Sugiyama’s group in Japan, several novel PET tracers have been developed that facilitate the study of the functional status of liver uptake and/or efflux transporters.19 Validation of these PET tracers has been performed by either using transporter knockout mice or rats or using chemical inhibitors of these transporters, some of which were substances with a potential for clinically relevant transporter-mediated DDIs. Graphical analysis approaches (“integration plots”) allowed for calculating hepatic uptake and biliary efflux clearances based on blood and tissue concentration–time curves, thereby distinguishing the effects of basolateral uptake and/or efflux transporters from the effects of canalicular efflux transporters on drug kinetics in the liver.
It has been shown that hepatobiliary clearance of telmisartan, a selective angiotensin II receptor antagonist, depends on functional activity of OATP1B3, a basolateral SLC uptake transporter in hepatocytes. PET imaging with [11C]telmisartan demonstrated a significant reduction in hepatic uptake clearance of [11C]telmisartan when rats were treated with the OATP inhibitor rifampicin, with no effect on biliary efflux clearance.61 In another study, it was shown that hepatic uptake and biliary efflux clearances of 11C-labeled (15R)-16-m-tolyl-17,18,19,20-tetranorisocarbacyclin methyl ester, a PET tracer initially developed for measurement of central nervous system–type prostacyclin receptors, were reduced in healthy human subjects after single oral treatment with rifampicin (600 mg), which was attributed to inhibition of basolateral OATPs and possibly canalicular MRP2.62 Moreover, it has been shown that PET imaging with [11C]dehydropravastatin, a derivative of the cholesterol-lowering drug pravastatin, allows for assessment of the functional activity of OATPs and MRP2 in the liver. Treatment of rats with rifampicin resulted in a 30% decrease in hepatic uptake clearance and a 60% reduction in biliary efflux clearance of [11C]dehydropravastatin, consistent with the inhibition of both Oatp and Mrp2 by rifampicin in the rat liver.63 Moreover, in Mrp2-deficient rats (Eisai hyperbilirubinemic mutant rats), the canalicular efflux clearance of [11C]dehydropravastatin was found to be decreased by 89%, as compared with that in control rats.63 In another study, pretreatment of mice with the antimalarial drug pyrimethamine resulted in markedly increased liver concentrations of the 11C-labeled oral antidiabetic drug [11C]metformin without changes in plasma concentrations,64 which was attributed to inhibition of hepatobiliary excretion by canalicular multidrug and toxin extrusion transporters.65
In another study, the interaction of glyburide, another oral antidiabetic drug, with SLC and ABC transporters was studied in vivo by performing PET experiments with [11C]glyburide in baboons with and without pretreatment with rifampicin or cyclosporin A.66 Both substances caused pronounced increases in plasma area under the concentration–time curve (AUC) values and decreases in liver-to-plasma AUC ratios of [11C]glyburide, which was attributed to inhibition of basolateral OATPs (i.e., OATP2B1) in hepatocytes (Figure 3).
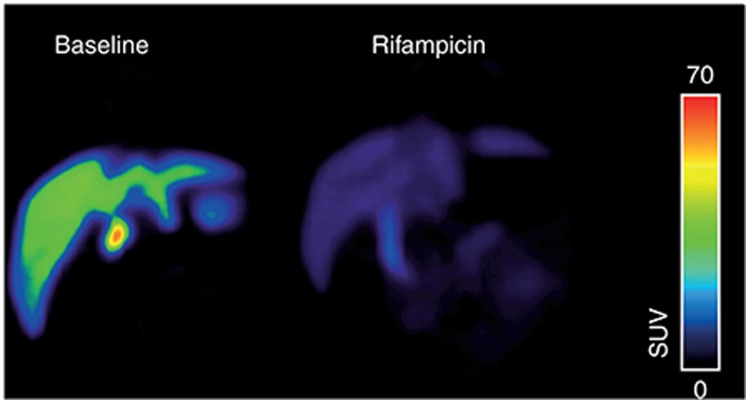
Figure 3.
Organic anion–transporting polypeptide–mediated interaction between [11C]glyburide and rifampicin in baboon (Papio anubis) liver. Shown are coronal positron emission tomography (PET) summation images (0–60 min) acquired after injection of [11C]glyburide without (left) and with (right) i.v. pretreatment with rifampicin (8.6 mg/kg over 30 min immediately before PET). Radiation scale is expressed as standardized uptake value. After rifampicin pretreatment, the area under the liver time–activity curve (AUCliver) was reduced by 2.7-fold and AUCliver/AUCplasma was reduced by 14-fold compared with baseline. PET image courtesy of N. Tournier, CEA, Service Hospitalier Frédéric Joliot, Orsay, France.
Kidney
Few examples exist for PET studies on transporter-mediated DDIs in the kidney. Measurement of drug concentrations in the kidneys might be useful for the prediction of nephrotoxicity. A study in mice showed that genetic knockout of Bcrp, which is located in the brush border membrane of proximal tubule cells, led to a decrease in renal clearance of the radiolabeled metabolite of the cyclooxygenase-2 inhibitor celecoxib [11C]SC-62807 by 99% and a concomitant (approximately sevenfold) increase in kidney exposure, which suggested that tubular secretion of [11C]SC-62807 is predominantly mediated by Bcrp.67
Placenta
The blood–placenta barrier expresses several different ABC and SLC transporters. Transporter-mediated DDIs at this barrier are clinically relevant because they may lead to changes in drug exposure of the embryo. In one study, pregnant nonhuman primates underwent [11C]verapamil PET scans before and after cyclosporin A administration (12 or 24 mg/kg/h) to measure placental Pgp activity either in mid- or late-gestational age. Percentage change in AUCfetal liver/AUCmaternal plasma ratio after cyclosporin A administration was used as a surrogate marker of placental Pgp activity and was found to significantly increase from mid- (+35 ± 25%) to late gestation (+125 ± 66%). According to the authors, the result was interpreted as indicating an increasing Pgp activity with gestational age.68,69,70
Summary and Outlook
The Food and Drug Administration and the European Medicines Agency have provided guidelines on how to assess the risk of clinically relevant transporter-mediated DDIs of new drug candidates.12,13 When the maximum unbound drug concentrations in vivo exceed defined thresholds for transporter inhibition in vitro, the ITC proposes in vivo DDI studies in human volunteers. However, it has been recognized that for some DDIs, assessment of drug plasma concentrations may not be sufficient to assess the true impact of transporter inhibition in different organ systems (e.g., liver, kidney, and brain) on drug disposition. To address these questions, nuclear imaging techniques such as PET have become an indispensable tool for noninvasive assessment of drug tissue concentrations in human subjects. Due to the requirement for specialized infrastructure (cyclotron, radiochemistry laboratory, and PET camera), PET studies are technically challenging. Nevertheless, given the growing importance of membrane transporters with respect to drug safety and efficacy, the demand for PET studies is expected to increase in future drug development. PET imaging of drug transporters offers the perspective for personalized medicine approaches in order to predict treatment failure or side effects in select patient subgroups in which transporter activity may differ due to polymorphisms in transporter genes or disease-induced alteration of transporter activity (e.g., cancer and epilepsy).71 Moreover, PET is essential to gain information on the in vivo interplay of drug uptake and efflux transporters in multiple organs and to assess differences in transporter expression/activity between preclinical species and humans.
Acknowledgments
O.L. and M.M.’s studies have been funded by the Austrian Science Fund project “Transmembrane Transporters in Health and Disease” (F 3513-B20) and by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 201380 (“Euripides”). The authors thank Nicolas Tournier (CEA, Service Hospitalier Frédéric Joliot, Orsay, France) for providing the positron emission tomography (PET) images to compose Figure 3 and Berend Oosterhuis and William Johnson for critical reading of this article and helpful discussions. Moreover, the PET group at the Department of Biomedical Imaging und Image-Guided Therapy (Medical University of Vienna) is acknowledged for continuous support.
Footnotes
The authors declared no conflict of interest.
References
- Giacomini, K.M. & Sugiyama, Y. Membrane transporters and drug response. In Goodman & Gilman’s Pharmacological Basis of Therapeutics (eds. Brunton, L., Chabner, B. & Knollman, B.) 89–121 (McGraw-Hill, New York, 2011). [Google Scholar]
- Simonson, S.G. et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin. Pharmacol. Ther. 76, 167–177 (2004). [DOI] [PubMed] [Google Scholar]
- Neuvonen, P.J., Niemi, M. & Backman, J.T. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin. Pharmacol. Ther. 80, 565–581 (2006). [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski, M.J. et al.; International Transporter Consortium. ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport-mediated PK and DDIs in humans. Clin. Pharmacol. Ther. 94, 64–79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie, D. et al.; International Transporter Consortium. Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium. Clin. Pharmacol. Ther. 94, 113–125 (2013). [DOI] [PubMed] [Google Scholar]
- Kalvass, J.C. et al.; International Transporter Consortium. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin. Pharmacol. Ther. 94, 80–94 (2013). [DOI] [PubMed] [Google Scholar]
- Hillgren, K.M. et al.; International Transporter Consortium. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin. Pharmacol. Ther. 94, 52–63 (2013). [DOI] [PubMed] [Google Scholar]
- Giacomini, K.M. et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini, K.M. et al.; International Transporter Consortium. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin. Pharmacol. Ther. 94, 23–26 (2013). [DOI] [PubMed] [Google Scholar]
- Chu, X. et al.; International Transporter Consortium. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin. Pharmacol. Ther. 94, 126–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, K.L. et al.; International Transporter Consortium. In vitro methods to support transporter evaluation in drug discovery and development. Clin. Pharmacol. Ther. 94, 95–112 (2013). [DOI] [PubMed] [Google Scholar]
- Guidance for Industry (Draft): Drug Interaction Studies - Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. <http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064982.htm>. Accessed 11 March 2014.
- European Medicines Agency’s Guideline on the Investigation of Drug Interactions (Final). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf> (2012). Accessed 11 March 2014.
- Schinkel, A.H. & Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 55, 3–29 (2003). [DOI] [PubMed] [Google Scholar]
- Hediger, M.A., Romero, M.F., Peng, J.B., Rolfs, A., Takanaga, H. & Bruford, E.A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 447, 465–468 (2004). [DOI] [PubMed] [Google Scholar]
- Köck, K. & Brouwer, K.L. A perspective on efflux transport proteins in the liver. Clin. Pharmacol. Ther. 92, 599–612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch, B. Drug uptake systems in liver and kidney: a historic perspective. Clin. Pharmacol. Ther. 87, 39–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart, B.L., Tirona, R.G. & Kim, R.B. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J. Clin. Pharmacol. 47, 566–578 (2007). [DOI] [PubMed] [Google Scholar]
- Kusuhara, H. Imaging in the study of membrane transporters. Clin. Pharmacol. Ther. 94, 33–36 (2013). [DOI] [PubMed] [Google Scholar]
- Eichler, H.G. & Müller, M. Drug distribution. The forgotten relative in clinical pharmacokinetics. Clin. Pharmacokinet. 34, 95–99 (1998). [DOI] [PubMed] [Google Scholar]
- Langer, O. & Müller, M. Methods to assess tissue-specific distribution and metabolism of drugs. Curr. Drug Metab. 5, 463–481 (2004). [DOI] [PubMed] [Google Scholar]
- Bergström, M., Grahnén, A. & Långström, B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur. J. Clin. Pharmacol. 59, 357–366 (2003). [DOI] [PubMed] [Google Scholar]
- Wagner, C.C., Langer, O. Approaches using molecular imaging technology - use of PET in clinical microdose studies. Adv. Drug. Deliv. Rev. 63, 539–546 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairinger, S., Erker, T., Müller, M. & Langer, O. PET and SPECT radiotracers to assess function and expression of ABC transporters in vivo. Curr. Drug Metab. 12, 774–792 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan, P. et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin. Pharmacol. Ther. 86, 368–377 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, P.Y. Comparative requirements for exploratory clinical trials – eIND, eCTA and microdosing. Adv. Drug Deliv. Rev. 63, 511–517 (2011). [DOI] [PubMed] [Google Scholar]
- Lagas, J.S., Vlaming, M.L. & Schinkel, A.H. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol. Interv. 9, 136–145 (2009). [DOI] [PubMed] [Google Scholar]
- Römermann, K. et al. (R)-[(11)C]verapamil is selectively transported by murine and human P-glycoprotein at the blood-brain barrier, and not by MRP1 and BCRP. Nucl. Med. Biol. 40, 873–878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, N., Balimane, P., Hayward, M.D., Buechel, S., Kauselmann, G. & Wolf, C.R. Generation and characterization of a novel multidrug resistance protein 2 humanized mouse line. Drug Metab. Dispos. 40, 2212–2218 (2012). [DOI] [PubMed] [Google Scholar]
- Luurtsema, G., Molthoff, C.F., Schuit, R.C., Windhorst, A.D., Lammertsma, A.A. & Franssen, E.J. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood-brain barrier: kinetics and metabolism in the rat. Nucl. Med. Biol. 32, 87–93 (2005). [DOI] [PubMed] [Google Scholar]
- Abrahim, A. et al. Peripheral metabolism of (R)-[11C]verapamil in epilepsy patients. Eur. J. Nucl. Med. Mol. Imaging 35, 116–123 (2008). [DOI] [PubMed] [Google Scholar]
- Muzi, M. et al. Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J. Nucl. Med. 50, 1267–1275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, K. et al. Evaluation of limiting brain penetration related to P-glycoprotein and breast cancer resistance protein using [11C]GF120918 by PET in mice. Mol. Imaging Biol. 13, 152–160 (2011). [DOI] [PubMed] [Google Scholar]
- Bankstahl, J.P. et al. Tariquidar and elacridar are dose-dependently transported by p-glycoprotein and bcrp at the blood-brain barrier: a small-animal positron emission tomography and in vitro study. Drug Metabol. Dispos. 41, 754–762 (2013). [DOI] [PubMed] [Google Scholar]
- Sane, R., Agarwal, S., Mittapalli, R.K. & Elmquist, W.F. Saturable active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J. Pharmacol. Exp. Ther. 345, 111–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, K. et al. In vivo evaluation of P-glycoprotein and breast cancer resistance protein modulation in the brain using [(11)C]gefitinib. Nucl. Med. Biol. 36, 239–246 (2009). [DOI] [PubMed] [Google Scholar]
- Agarwal, S., Sane, R., Gallardo, J.L., Ohlfest, J.R. & Elmquist, W.F. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J. Pharmacol. Exp. Ther. 334, 147–155 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher, W. & Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 76, 22–76 (2005). [DOI] [PubMed] [Google Scholar]
- Eyal, S., Hsiao, P. & Unadkat, J.D. Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol. Ther. 123, 80–104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen, S. et al. Duration and degree of cyclosporin induced P-glycoprotein inhibition in the rat blood-brain barrier can be studied with PET. Neuroimage 32, 1134–1141 (2006). [DOI] [PubMed] [Google Scholar]
- Sasongko, L. et al. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin. Pharmacol. Ther. 77, 503–514 (2005). [DOI] [PubMed] [Google Scholar]
- Liow, J.S. et al. P-glycoprotein function at the blood-brain barrier imaged using 11C-N-desmethyl-loperamide in monkeys. J. Nucl. Med. 50, 108–115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.J. et al. In vivo evaluation of P-glycoprotein function at the blood-brain barrier in nonhuman primates using [11C]verapamil. J. Pharmacol. Exp. Ther. 316, 647–653 (2006). [DOI] [PubMed] [Google Scholar]
- Kuntner, C. et al. Dose-response assessment of tariquidar and elacridar and regional quantification of P-glycoprotein inhibition at the rat blood-brain barrier using (R)-[(11)C]verapamil PET. Eur. J. Nucl. Med. Mol. Imaging 37, 942–953 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl, W.C. et al. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J. Nucl. Med. 51, 559–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, M. et al. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: a comparison with rat data. Clin. Pharmacol. Ther. 91, 227–233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart, J. et al. Quantitative assessment of P-glycoprotein function in the rat blood-brain barrier by distribution volume of [11C]verapamil measured with PET. Neuroimage 20, 1775–1782 (2003). [DOI] [PubMed] [Google Scholar]
- Fox, E. & Bates, S.E. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 7, 447–459 (2007). [DOI] [PubMed] [Google Scholar]
- Hsiao, P. & Unadkat, J.D. Predicting the outer boundaries of P-glycoprotein (P-gp)-based drug Interactions at the human blood-brain barrier based on rat studies. Mol. Pharm. 11, 436–444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, A. et al. Evaluation of in vivo P-glycoprotein function at the blood-brain barrier among MDR1 gene polymorphisms by using 11C-verapamil. J. Nucl. Med. 47, 1427–1433 (2006). [PubMed] [Google Scholar]
- Brunner, M. et al. Influence of functional haplotypes in the drug transporter gene ABCB1 on central nervous system drug distribution in humans. Clin. Pharmacol. Ther. 78, 182–190 (2005). [DOI] [PubMed] [Google Scholar]
- Liu, L. et al. Can P-glycoprotein at the blood-brain barrier be induced by rifampin? A PET imaging study. 17th North American Regional ISSX Meeting, Atlanta, GA, 16–20 October 2011. ISSX Online Abstracts (http://www.issx.org), Supplement 6, No. 2, 2011. [Google Scholar]
- Kodaira, H., Kusuhara, H., Ushiki, J., Fuse, E. & Sugiyama, Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 333, 788–796 (2010). [DOI] [PubMed] [Google Scholar]
- Agarwal, S., Hartz, A.M., Elmquist, W.F. & Bauer, B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr. Pharm. Des. 17, 2793–2802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, S., Sane, R., Oberoi, R., Ohlfest, J.R. & Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 13, e17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobbe, P., Poot, A.J., Windhorst, A.D. & van Dongen, G.A. PET imaging with small-molecule tyrosine kinase inhibitors: TKI-PET. Drug Discov. Today 17, 1175–1187 (2012). [DOI] [PubMed] [Google Scholar]
- Poot, A.J., Slobbe, P., Hendrikse, N.H., Windhorst, A.D. & van Dongen, G.A. Imaging of TKI-target interactions for personalized cancer therapy. Clin. Pharmacol. Ther. 93, 239–241 (2013). [DOI] [PubMed] [Google Scholar]
- Kawamura, K. et al. Synthesis and evaluation of [11C]XR9576 to assess the function of drug efflux transporters using PET. Ann. Nucl. Med. 24, 403–412 (2010). [DOI] [PubMed] [Google Scholar]
- Bauer, M. et al. Interaction of 11C-tariquidar and 11C-elacridar with P-glycoprotein and breast cancer resistance protein at the human blood-brain barrier. J. Nucl. Med. 54, 1181–1187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanek, T. et al. A novel PET protocol for visualization of breast cancer resistance protein function at the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 2002–2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima, T. et al. The involvement of organic anion transporting polypeptide in the hepatic uptake of telmisartan in rats: PET studies with [¹¹C]telmisartan. Mol. Pharm. 8, 1789–1798 (2011). [DOI] [PubMed] [Google Scholar]
- Takashima, T. et al. PET imaging-based evaluation of hepatobiliary transport in humans with (15R)-11C-TIC-Me. J. Nucl. Med. 53, 741–748 (2012). [DOI] [PubMed] [Google Scholar]
- Shingaki, T. et al. Evaluation of Oatp and Mrp2 activities in hepatobiliary excretion using newly developed positron emission tomography tracer [11C]dehydropravastatin in rats. J. Pharmacol. Exp. Ther. 347, 193–202 (2013). [DOI] [PubMed] [Google Scholar]
- Hume, W.E. et al. The synthesis and biodistribution of [(11)C]metformin as a PET probe to study hepatobiliary transport mediated by the multi-drug and toxin extrusion transporter 1 (MATE1) in vivo. Bioorg. Med. Chem. 21, 7584–7590 (2013). [DOI] [PubMed] [Google Scholar]
- Ito, S. et al. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J. Pharmacol. Exp. Ther. 333, 341–350 (2010). [DOI] [PubMed] [Google Scholar]
- Tournier, N. et al. Effects of selected OATP and/or ABC transporter inhibitors on the brain and whole-body distribution of glyburide. AAPS J. 15, 1082–1090 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima, T. et al. Evaluation of breast cancer resistance protein function in hepatobiliary and renal excretion using PET with 11C-SC-62807. J. Nucl. Med. 54, 267–276 (2013). [DOI] [PubMed] [Google Scholar]
- Ke, A.B. et al. Modeling cyclosporine A inhibition of the distribution of a P-glycoprotein PET ligand, 11C-verapamil, into the maternal brain and fetal liver of the pregnant nonhuman primate: impact of tissue blood flow and site of inhibition. J. Nucl. Med. 54, 437–446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal, S. et al. Simultaneous PET imaging of P-glycoprotein inhibition in multiple tissues in the pregnant nonhuman primate. J. Nucl. Med. 50, 798–806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, F.S. et al. Positron emission tomography imaging of tissue P-glycoprotein activity during pregnancy in the non-human primate. Br. J. Pharmacol. 159, 394–404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, M. et al. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 12, 777–785 (2013). [DOI] [PubMed] [Google Scholar]