Although uncommon, malignant pleural mesothelioma is being diagnosed at an increasing rate worldwide due to continued workplace exposure to asbestos and other potentially carcinogenic inhaled silicates in developing countries. This article emphasizes the need for multidisciplinary evaluation at diagnosis to identify appropriate candidates for multimodality therapy and to optimize survival outcomes for this deadly disease.
Keywords: Mesothelioma, Pleural neoplasms, Review, Clinical trials, Asbestos adverse effects
Abstract
Malignant pleural mesothelioma (MPM) is an uncommon disease most often associated with occupational asbestos exposure and is steadily increasing in worldwide incidence. Patients typically present at an older age, with advanced clinical stage and other medical comorbidities, making management quite challenging. Despite great efforts, the prognosis of MPM remains poor, especially at progression after initial treatment. Macroscopic complete resection of MPM can be achieved through extrapleural pneumonectomy (EPP) or extended (ie, radical) pleurectomy (e-P/D) in selected patients and can result in prolonged survival when incorporated into a multimodality approach. Given the morbidity associated with surgical resection of MPM, optimizing identification of appropriate patients is essential. Unfortunately, most patients are not candidates for EPP or e-P/D due to advanced stage, age, and/or medical comorbidity. Pemetrexed and platinum combination chemotherapy has become the cornerstone of therapy for patients with unresectable disease because the combination is associated with improved survival and quality of life in treated patients. However, MPM eventually becomes resistant to initial therapy, and benefit to further lines of therapy has not been substantiated in randomized clinical trials. Translational research has provided exciting insights into tumorigenesis, biomarkers, and immune response in MPM, leading to the development of multiple novel therapeutic agents that are currently in clinical trials. These advances hold the promise of a new era in the treatment of MPM and suggest that this disease will not be left behind in the war on cancer.
Implications for Practice:
Although uncommon, malignant pleural mesothelioma (MPM) is being diagnosed at an increasing rate worldwide due to continued workplace exposure in developing countries to asbestos and other potentially carcinogenic inhaled silicates. This article emphasizes the need for multidisciplinary evaluation at diagnosis to identify appropriate candidates for multimodality therapy and to optimize survival outcomes for this deadly disease. A growing body of data suggests that lung-sparing extended pleurectomy is the option of choice for most patients who are surgical candidates. Insights into altered molecular pathways and the immunology of MPM have led to clinical trials of novel drugs.
Introduction
Malignant pleural mesothelioma (MPM) is a highly lethal disease with 5-year overall survival (OS) of less than 10%, which has not changed for the past four decades [1]. Treatment-related mortality and morbidity continue to pose unique challenges. In this paper, we review the current epidemiology, diagnosis, and treatment of MPM, with a focus on multimodality therapy and novel agents.
Epidemiology
The annual incidence of MPM in the United States is estimated to be 1 in every 100,000, with approximately 3,000 new cases per year [1]. It is more common in men, and the majority of patients are over the age of 65 years.
The incidence of MPM in the U.S. peaked around the turn of this century and has since slowly started to decline, mainly in male patients [1]. Worldwide, however, MPM rates are still increasing. In developed countries, such as the U.K. and Australia, the peak incidence is expected to occur before 2030 [2]. In contrast, the incidence of mesothelioma is predicted to increase dramatically in developing countries where asbestos is still used in the workplace [3, 4]. Furthermore, the burden from the high mortality rate of mesothelioma is heavy. The mortality rate in the U.K., for example, has risen rapidly since 1968. Between 1968 and 2050 it is expected that there will have been approximately 91,000 deaths from mesothelioma in U.K., with 61,000 occurring after 2007 [4].
Occupational exposure to asbestos is the single most important risk factor associated with MPM. Asbestos is used in cement, ceiling and pool tiles, and automobile brake linings and in shipbuilding. The lifetime risk of developing MPM among asbestos workers was thought to be as high as 10% [5]. Family members of asbestos workers also have increased risk from second-hand exposure. There is a long latency (at least 20–30 years) from the time of asbestos exposure to the development of mesothelioma [6], and the two events appear to have a dose-response association [7]. Nonoccupational exposure to asbestos (e.g., in areas with asbestos-rich soil or inhalation of other fibrous silicates) can also contribute to an increased risk of MPM [8–10].
Ionizing radiation (therapeutic or nontherapeutic) to the upper body may be a risk factor for the subsequent development of MPM, again, with a long latent period [11–13]. Oncogenic viral infections, such as Simian virus 40 infections, have been implicated in the etiology of MPM [14, 15], although a clear relationship has yet to be established [16, 17].
Inactivation of the nuclear deubiquitinase BRCA1-associated protein 1 (BAP1), an important regulator of transcription factors related to tumorigenesis, has been associated with MPM [18, 19]. Germline mutations in BAP1 were identified in two families with high incidence of MPM [20], and BAP1 inactivation through somatic mutations was detected in 23% of MPM tumor tissues [21]. These emerging data suggest individuals with loss of BAP1 may have higher risk of developing MPM, especially after asbestos exposure; close monitoring and early intervention might be warranted, although genetic screening strategies have yet to be identified.
Diagnosis and Staging
Pulmonary symptoms (e.g., chest pain, dyspnea, cough) with unilateral large-volume pleural effusion in a patient with history of asbestos exposure should raise the suspicion of MPM; however, pleural fluid cytology from thoracentesis is often nondiagnostic, even after repeated attempts. More invasive procedures, such as core needle biopsy or video-assisted thoracic surgery, have higher diagnostic yields and are frequently needed [22].
There are three major histologic subtypes of MPM: epithelioid, sarcomatoid, and mixed-type (biphasic). The epithelioid subtype is associated with the best outcomes, whereas the sarcomatoid subtype typically has a poor prognosis [23]. Further histologic features may provide additional prognostic value. It was suggested, for example, that the pleomorphic subtype predicts aggressive behavior in epithelioid MPM with no survival difference from biphasic or sarcomatoid MPM [24], whereas a high degree of chronic inflammation in stroma is associated with improved survival in epithelioid MPM [25]. On immunohistochemical (IHC) staining, MPM is often positive for pan-cytokeratin, calretinin, cytokeratin 5/6, and Wilms’ tumor 1 (WT1; nuclear staining) but negative for carcinoembyonic antigen or thyroid transcription factor-1 [26]. To date, there has been no single IHC marker identified with both high sensitivity and specificity for screening or diagnosis. Soluble mesothelin-related proteins might be useful in the diagnosis, treatment, and monitoring of MPM, although they have not been proven to be prognostic [27–29]. Recent studies suggested high sensitivity and specificity of fibulin-3 (plasma and effusion levels) in MPM diagnosis, but further validation is needed [30].
The most widely used staging system for MPM is the TNM system adopted by the American Joint Committee on Cancer (AJCC). Clinical staging of mesothelioma is often based on radiographic findings. Compared with traditional computed tomography (CT) scanning, positron emission tomography/CT (PET/CT) imaging appears to be more accurate in preoperative assessment of potentially resectable tumors [31], and higher standardized uptake value (>4) appears to be a poor risk factor [32]. Tumor upstaging through detection of T4 disease or nodal/distant metastases was frequent with PET/CT compared with CT alone, avoiding surgery in up to 30%–40% of MPM patients felt to have potentially resectable tumors [33, 34]. Although useful, the current AJCC system is inadequate to accurately define surgical candidacy, and it provides no clear prognostic insights [35]. The International Association for the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group have created an MPM patient database and are incorporating this information as the basis for the planned 8th edition of the TNM system, expected in late 2015 [36].
Current Surgical Management
The role of surgery in the management of MPM remains controversial [37]. Four therapeutic surgical procedures have been defined: extrapleural pneumonectomy (EPP), extended pleurectomy/decortication (e-P/D) or radical P/D, P/D, and partial pleurectomy (Table 1).
Table 1.
International Association for the Study of Lung Cancer surgical definitions
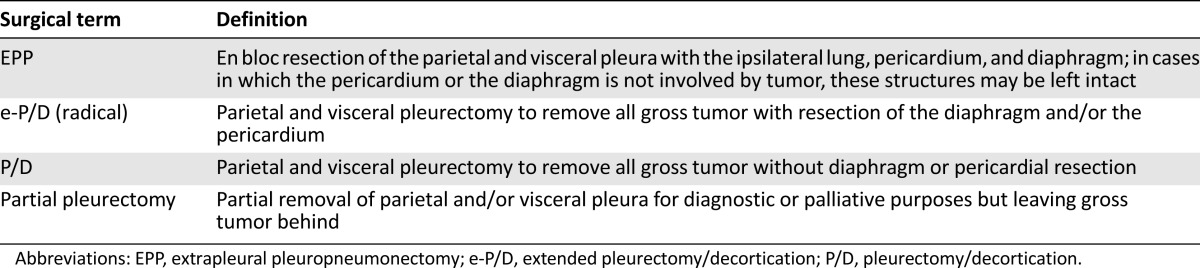
To evaluate the effectiveness of EPP to extend quality-adjusted survival within multimodality therapy, the Mesothelioma and Radical Surgery (MARS) trial group first performed a phase II feasibility study [38]. A total of 112 eligible patients recruited from 11 collaborating centers in the U.K. entered the first registration to receive platinum-based chemotherapy. Fifty patients (45%) were eventually randomized to EPP (24 of 50) or best nonsurgical care (26 of 50). A total of 67% (16 of 24) in the surgery arm completed EPP satisfactorily [39]. Median survival (after induction chemotherapy) was 14.4 months for the EPP group and 19.5 months for the non-EPP group. Median quality-of-life scores were lower in the EPP group, although not statistically significant [39]. The sample size was insufficient to analyze outcome as the primary endpoint, but the results have prompted debate that EPP offers no survival benefit and possibly harms patients within the multimodality treatment setting.
The morbidity associated with EPP has led to the development of alternative lung-sparing procedures such as P/D and e-P/D. In a systemic review of 11 retrospective studies, Zahid et al. concluded that these procedures may lead to superior survival rates but at the cost of higher morbidity rates with palliative treatment [40]. Radical P/D achieved a higher median survival than best supportive care (14.5 versus 4.5 months) and nonradical decortication (15.3 versus 7.1 months, p < .001) but had a complication rate of 30% and an operative mortality rate of 9.1% [40]. In another systemic review of 1,270 patients, Teh et al. reported a 1-year postoperative survival rate of 51%, but it dropped to 9% at 5 years [41].
To date, there are no randomized comparisons of these two surgical approaches (Table 2). The choice of procedure can be influenced by multiple factors, including patient age and comorbidity, clinical stage, patient wishes, and expertise at specific surgical centers. Based on a Web-based survey from 62 mesothelioma surgeons at 39 centers in 14 countries [42], most surgeons (88%) agreed that the goal of cytoreductive surgery should be macroscopic complete resection of tumor, which could most often be achieved by EPP (90%) or e-P/D (68%) but less so by P/D (23%).
Table 2.
Multimodality data comparing EPP and P/D
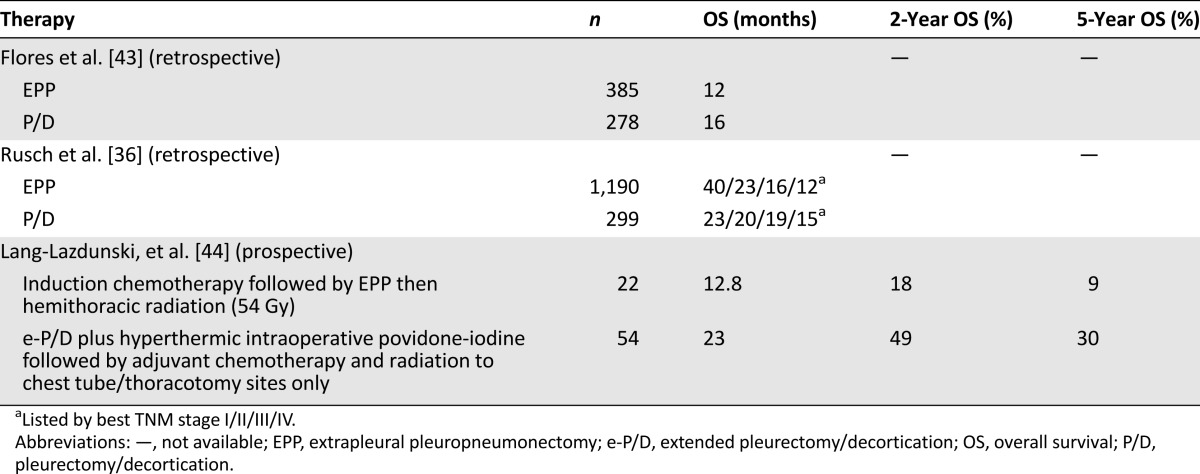
In an extensive retrospective case series including 663 consecutive patients undergoing EPP or P/D at three U.S. mesothelioma surgical centers, Flores et al. reported better median survival for P/D versus EPP (16 versus 12 months) [43]. This was statistically significant (p < .001) after controlling for sex, histology, stage, and receipt of multimodality therapy. Compared with EPP, P/D was associated with lower operative mortality (3% versus 7%) and lower distant recurrence rate (35% versus 66%) but not local recurrence rate (65% versus 33%) [43]. The IASLC also analyzed its database of 3,101 patients from 15 centers on 4 continents and showed a survival benefit of EPP only for stage I patients (40 versus 23 months) [36]; however, this analysis could be subject to selection bias.
Lang-Lazdunski et al. compared two trimodality regimens involving EPP or e-P/D in a prospective series involving 36 patients [44]. Compared with EPP, all patients in the e-P/D group were able to complete trimodality therapy (100% versus 68%) and with significantly better median survival (23 versus 12.8 months) and 5-year survival (30.1% versus 9%) [44].
Two randomized trials are set to open soon: the MARS-2 trial, to compare e-P/D with platinum/pemetrexed chemotherapy versus chemotherapy alone, and a European Organization for Research and Treatment of Cancer (EORTC) trial, to compare e-P/D either preceded or followed by chemotherapy in early stage MPM [37]. These will hopefully clarify the role of e-P/D as part of a multimodality treatment approach.
Multimodality Therapy
At diagnosis, only a minority of MPM patients are candidates for definitive surgery. These patients have significantly better outcomes when managed with a multimodal approach rather than by surgery alone, and this finding has been confirmed in both retrospective and prospective studies [36, 45–47]. Consequently, an upfront multidisciplinary evaluation is essential.
At diagnosis, only a minority of MPM patients are candidates for definitive surgery. These patients have significantly better outcomes when managed with a multimodal approach rather than by surgery alone, and this finding has been confirmed in both retrospective and prospective studies.
Radiation therapy is conventionally delivered after surgery for local control and is conventionally performed after EPP. In a phase II trial conducted by Rusch et al. [48], adjuvant radiation following EPP at a median dose of 54 Gy was well tolerated and was associated with prolonged survival for early stage (I/II) tumors (median survival: 33.8 months). To further improve local control and to minimize toxicity, Rice et al. explored the use of intensity-modulated radiation therapy (IMRT) after EPP without routine chemotherapy [49]. Median OS and 3-year survival was 14.2 months and 20% (n = 63), and further improvement in survival was associated with early stage (node-negative) and epithelioid histology. Only three patients had recurrence within the irradiated field, but distant metastases (54%) remained significant, indicating the need for combined systemic therapy [49]. Cho and colleagues recently published their phase I/II experience with neoadjuvant IMRT [50]. Twenty-five eligible patients (18% of a total of 138 patients screened) all completed IMRT (25 Gy to the entire ipsilateral hemithorax with concomitant 5-Gy boost to areas at risk) within 1 week prior to EPP. Adjuvant chemotherapy was offered to ypN2 patients (5 of 13 of these patients actually received chemotherapy). No perioperative mortality was observed, although 13 patients (52%) developed grade ≥3 surgical complications. Cumulative 3-year survival reached 84% in epithelial subtypes compared with 13% in biphasic subtypes (p = .0002), suggesting this novel approach may be preferred for selected patient subgroups. Larger numbers of patients and longer follow-up are also necessary.
Adjuvant chemotherapy and intraoperative therapies also lead to improved survival after EPP or e-P/D. In a retrospective analysis, adjuvant systemic chemotherapy significantly improved survival compared with surgery alone (35 versus 13 months) [51]. Hyperthermic intraoperative intracavitary cisplatin perfusion immediately after EPP can be performed with acceptable morbidity and mortality [52]. Sugarbaker et al. investigated the addition of this approach among patients with favorable prognostic factors (i.e., epithelial histology, low tumor burden, female sex, or male with normal hemoglobin) [53]. Of the 103 identified patients, 72 received hyperthermic intraoperative cisplatin chemotherapy. This group exhibited a significantly longer interval to recurrence (27.1 vs 12.8 months) and OS (35.3 vs 22.8 months) compared with patients without treatment. The benefits were particularly evident among the subgroups of patients who had not received hemithoracic radiotherapy and who had pathologic stage N1 or N2 disease.
Friedberg et al. evaluated intraoperative photodynamic therapy (PDT) in patients who underwent macroscopic complete resection (14 with modified EPP, 14 with e-P/D) [54]. The pleurectomy plus PDT group had significantly better survival at a median follow-up of 2.1 years (median OS not yet reached versus 8.4 months), which was also superior to previously reported results for e-PD alone. The authors extended their cohort to include 38 patients treated with e-PD plus PDT, most with stage III/IV disease and epithelial histology, and 35 patients received chemotherapy [55]. At a median follow-up of 34.4 months, the median OS and progression-free survival (PFS) were 31.7 and 9.6 months, respectively. In another prospective study, Lang-Lazdunski et al. assessed e-P/D and hyperthermic pleural lavage with povidone-iodine followed by prophylactic radiation (to thoracotomy and chest tube sites) and adjuvant chemotherapy in comparison to neoadjuvant chemotherapy followed by EPP and adjuvant radiation [44]. Survival was significantly better in the e-P/D group compared with EPP (23 versus 12.8 months). Although inconclusive, this result suggested that povidone-iodine lavage is safe and may be an effective intraoperative adjunct to pleurectomy.
Systemic Therapy
Despite these varied surgical approaches and controversies, the majority of MPM patients present with unresectable disease or are deemed inoperable due to age or medical comorbidities and are primarily treated with systemic therapies with the goals of disease palliation and survival prolongation [56].
Cytotoxic Therapy
Meta-analyses have shown that most single-agent chemotherapies exhibit low activity, with the exception of cisplatin [57, 58]. Response rates are higher with combination therapy compared with single agents, and platinum-based regimens are superior to non-platinum-based regimens [56].
Vogelzang et al. were the first to test the efficacy of cisplatin plus pemetrexed in a phase III clinical trial (Evaluation of Mesothelioma in a Phase III Trial of Pemetrexed With Cisplatin [EMPHACIS]) [59]. A total of 456 patients were randomized to receive either pemetrexed plus cisplatin or cisplatin alone. Compared with single-agent cisplatin, patients in the combination chemotherapy arm had improved response rate (RR; 41.3% versus 16.7%, p < .0001), time to progression (5.7 versus 3.9 months, p = .001), and OS (12.1 versus 9.3 months, p = .020). After 117 patients had enrolled, folic acid and vitamin B12 were added, resulting in a significant reduction in toxicities in the pemetrexed plus cisplatin arm without adversely affecting survival. The EORTC conducted a similar phase III trial comparing the combination of raltitrexed plus cisplatin with cisplatin alone and confirmed that combination therapy is superior [60]. Consequently, the cisplatin-antifolate combination is currently considered standard of care as first-line treatment.
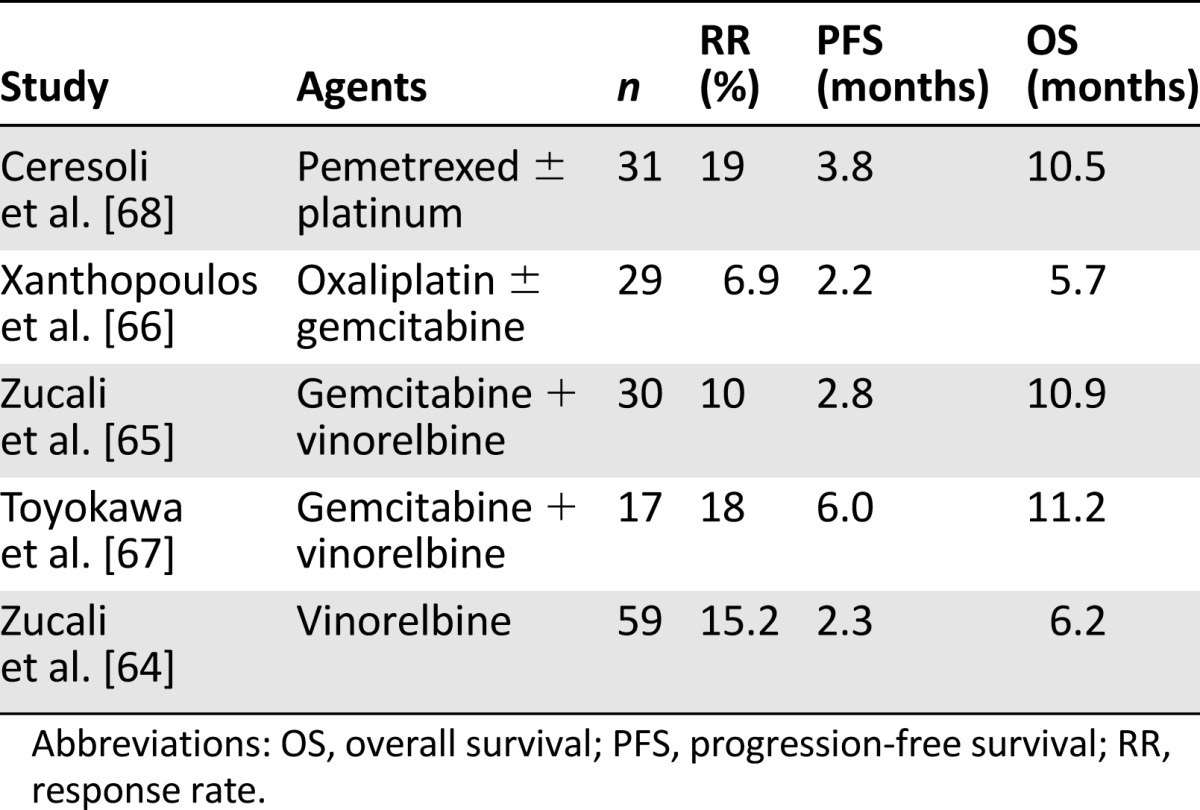
Chemotherapy beyond first-line treatment has been less well studied, and the optimal regimen is not known [61]. Poststudy chemotherapy (PSC; most commonly single agent gemcitabine or vinorelbine) in the EMPHACIS trial was associated with prolonged survival; but it was not clear whether this was associated with PSC or whether patients who had prolonged survival tended to receive more chemotherapy [62]. A multicenter phase III study compared second-line pemetrexed plus best supportive care (BSC) and BSC alone in pemetrexed-naïve patients with relapsed MPM [63]. Second-line pemetrexed significantly increased median PFS, time to progression, and time to treatment failure but provided no OS benefit (8.4 versus 9.7 months); however, 52% of patients in the BSC arm received chemotherapy at time of progression. Cancer and Leukemia Group B is conducting a randomized phase II trial (CALGB 30901) of pemetrexed versus observation for MPM patients without progression after first-line pemetrexed plus platinum chemotherapy and hopefully will clarify the role of maintenance pemetrexed. Table 3 summarizes the current evidence for second-line (and beyond) chemotherapy [64–68].
Table 3.
Activity of second-line regimens after pemetrexed-based chemotherapy
Targeted Therapy
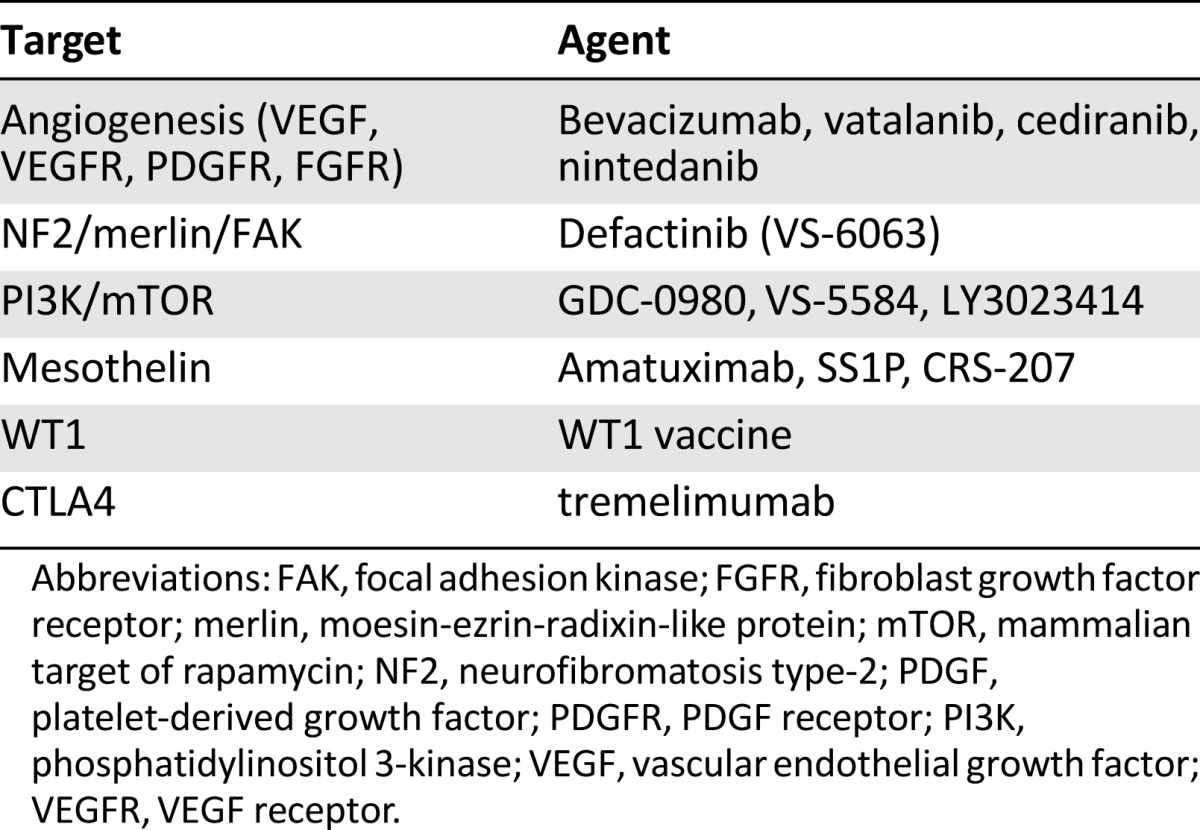
The need for more effective therapies for MPM has prompted basic research to identify novel therapeutic targets (Table 4).
Table 4.
Targets of interest and corresponding agents in mesothelioma
Epigenetic regulation of tumor suppressor genes has emerged as an important mechanism that leads to tumorigenesis. The histone deacetylase family proteins (HDACs) inhibit DNA transcription through histone modifications, and its overexpression and/or aberrant function have been found in many cancers, including mesothelioma [69, 70]. Vorinostat is one of the best-studied HDAC inhibitors and currently is approved by the U.S. Food and Drug Administration for cutaneous T-cell lymphoma treatment. The original phase I trial of vorinostat included 13 patients with MPM, and 2 of them had a partial response (15.4%) [71]. This led to a multicenter phase III study (VANTAGE 014) of vorinostat in patients who progressed after first-line pemetrexed-based chemotherapy. Despite the huge collaborative efforts, this largest-ever randomized trial in mesothelioma (660 patients) failed to show a benefit in OS (30.7 versus 27.1 weeks, p = .858) and only a marginal improvement in PFS (6.3 versus 6.1 weeks, p < .001) [72].
Mesothelioma cells secrete and express several angiogenic factors such as vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR), platelet-derived growth factor (PDGF) and PDGF receptor (PDGFR), and fibroblast growth factor receptor (FGFR) [73]. Bevacizumab, an anti-VEGF monoclonal antibody, did not significantly improve either PFS or OS in patients with advanced MPM when added to first-line gemcitabine-cisplatin chemotherapy [74]. Another phase II trial evaluated the addition of bevacizumab to first-line pemetrexed and cisplatin but failed to achieve its primary endpoint (33% improvement in PFS at 6 months compared with historical controls) [75]. Interim analysis from a French multicenter randomized phase II/III trial of pemetrexed and cisplatin with or without bevacizumab (MAPS) was recently reported [76]. Compared with chemotherapy alone, patients in the bevacizumab arm had a RR of 14% and a better disease control (73.5% versus 43.2%; p = .01) at 6 months. This trial will hopefully complete recruitment soon [77], and its final results are expected to clarify the role (if any) of bevacizumab in MPM treatment.
Nintedanib (BIBF 1120; Boehringer Ingelheim GmbH, Ingelheim, Germany, http://www.boehringer-ingelheim.com) is a potent oral triple angiokinase inhibitor that targets all three major angiogenic pathways [78]. In phase I/II clinical trials, nintedanib showed an acceptable safety profile and antitumor activities [79]. In the phase III LUME-Lung 1 study for patients with non-small cell lung cancer, second-line nintedanib plus docetaxel significantly improved PFS compared with docetaxel alone (3.4 versus 2.7 months; p = .0019) and improved OS in patients with adenocarcinoma histology (12.6 versus 10.3 months; p = .0359) [80]. An ongoing randomized multicenter phase II trial will evaluate nintedanib in combination with pemetrexed and cisplatin followed by maintenance nintedanib compared with chemotherapy alone in patients with unresectable MPM. SWOG is also studying the addition of the oral anti-VEGFR tyrosine kinase inhibitor cediranib versus placebo to pemetrexed-cisplatin in a randomized phase II trial.
Loss of the tumor suppressor protein moesin-ezrin-radixin-like protein (merlin) causes activation of multiple mitogenic signaling pathways, including the mammalian target of rapamycin (mTOR) and focal adhesion kinase (FAK) pathways [81]. About 40% of MPM patients carry inactivating mutations in the neurofibromin 2 (NF2) gene, which encodes for merlin [82, 83], and overexpression of FAK has been implicated in increased invasiveness of mesothelioma cell lines [84]. A recently reported phase I study of GSK2256098 (an oral FAK inhibitor; GlaxoSmithKline, Brentford, U.K., http://www.gsk.com) that included 23 patients with recurrent MPM suggested that merlin loss may result in improved PFS response to FAK inhibition [85]. Defactinib (VS-6063; Verastem, Cambridge, MA, http://www.verastem.com) is a highly potent, selective FAK inhibitor. A phase II randomized multicenter study of defactinib maintenance in MPM patients who have not progressed after first-line pemetrexed-platinum chemotherapy is actively recruiting.
The phosphatidylinositol 3-kinase (PI3K), AKT, and mTOR (PI3K/AKT/mTOR) pathway is one of the key regulators in cell survival, proliferation, and apoptosis [86]. Aberrant signaling cascade has been demonstrated in several cancer types, including mesothelioma [87, 88]. Because merlin is a negative regulator of the mTOR pathway, mTOR and merlin loss has become a target of interest in MPM [89]. The mTOR inhibitor rapamycin showed a much enhanced growth-inhibitory effect on merlin-negative mesothelioma cells compared with merlin-positive cells [90]. A SWOG phase II study of post-front-line mTOR inhibitor everolimus (RAD001) failed to show activity in unselected patients [91]. GDC-0980 (Genentech, South San Francisco, CA, http://www.gene.com) is a potent, selective oral PI3K/mTOR dual inhibitor that has demonstrated broad activity in various xenograft cancer models [92]. In a recently reported phase I study by Dolly et al., this drug showed noticeable antitumor activity in MPM patients at a generally well-tolerated dose [93]. Two additional early stage studies on dual PI3K/mTOR inhibitors LY3023414 (Eli Lilly and Company, Indianapolis, IN, http://www.lilly.com) and VS-5584 (Verastem) are currently recruiting patients with advanced cancers, including MPM.
Immunotherapy
The immune system plays a fundamental role in tumor surveillance and tumor growth control. Although highly infiltrated by a population of immune cells, mesothelioma appears to enjoy an “immune tolerance” state [94] . Decrease in cytotoxic T cells and natural killer lymphocytes and antigen-presenting cells, increase in regulatory T cells, and production of immunoregulatory cytokines may all contribute to the suppression of immune response [95, 96]. Consequently, reconstitution of the immune system to target tumor cells has become an attractive approach and one of the most active areas in mesothelioma research [97].
Although highly infiltrated by a population of immune cells, mesothelioma appears to enjoy an “immune tolerance” state. Decrease in cytotoxic T cells and natural killer lymphocytes and antigen-presenting cells, increase in regulatory T cells, and production of immunoregulatory cytokines may all contribute to the suppression of immune response.
Mesothelin is a cell-surface glycoprotein widely expressed in normal and malignant mesothelial cells and in other solid tumors [98]. It may be a useful biomarker and an important target for mesothelin-expressing tumors [99] and may promote tumor invasion and matrix metalloproteinase 9 expression in MPM [100]. Amatuximab (MORAb-009; Morphotek, Exton, PA, http://www.morphotek.com), a high-affinity monoclonal antibody toward mesothelin, has been evaluated in a phase I trial [101]. In 24 previously treated patients (including 13 with MPM), amatuximab was well tolerated, and 11 patients had stable disease after receiving at least one cycle. In a single-arm phase II study of amatuximab plus pemetrexed and cisplatin, Hassan et al. reported a partial RR of 39% (n = 30), and 51% (n = 39) had stable disease [102]. The same group has also investigated SS1P, a recombinant immunotoxin consisting of an antimesothelin antibody linked to a Pseudomonas exotoxin [103]. In a phase I trial, SS1P was well tolerated and showed activity in heavily pretreated patients with mesothelin-expressing cancers [104]. In a recently published phase II study, major antitumor response was observed in 3 of 10 patients with advanced chemorefractory mesothelioma when SS1P was given together with immunosuppression [105]. CRS-207 (Aduro BioTech, Berkeley, CA, http://www.adurobiotech.com) is a live-attenuated Listeria monocytogene vaccine designed to express mesothelin that was shown to be safe and to produce mesothelin-specific T-cell responses in a phase I trial that included five patients with MPM [106]. A phase IB trial of CRS-207 in combination with pemetrexed and cisplatin as front-line therapy is currently accruing MPM patients (ClinicalTrials.gov identifier NCT01675765).
WT1 protein is an oncogenic transcription factor commonly overexpressed in MPM. Processed WT1 peptides can be presented to the immune system, making it an attractive target for T-cell-based immunotherapy [107]. Krug et al. designed a WT1 vaccine and found it to be safe and effective in a pilot study [108]. The group is currently testing the vaccine in a randomized phase II trial in MPM patients with minimal disease burden after multimodality therapy [109]. Dao et al. engineered a fully human “T cell receptor–like” monoclonal antibody, ESK1 [110]. They found that ESK1 bound to several cancer cell lines (including mesothelioma) and primary leukemia cells with high avidity and nearly cleared all leukemia in two mouse models without toxicity. These exciting preclinical data have positioned ESK1 to be tested further in clinical trials.
In normal epithelial cells, transforming growth factor β (TGF-β) is a potent growth inhibitor and promoter of cellular differentiation [111]. However, tumor cells are often insensitive to this cytokine and can “utilize” TGF-β to promote tumor angiogenesis and host immunosuppression [112]. Significant levels of TGF-β are produced in MPM cells lines and in primary MPM tissues and pleural effusions [113, 114]. GC1008 (fresolimumab; Genzyme, Cambridge, MA, http://www.genzyme.com) is a human monoclonal antibody capable of neutralizing all mammalian isoforms of TGF-β with high affinity [115]. The first phase II trial of GC1008 in pretreated progressive MPM was terminated, unfortunately, after only 13 enrollments when the manufacturer discontinued development of the antibody for oncology indications [116]. Although partial or complete radiographic responses were not observed, 3 patients showed stable disease at 3 months. Serum from 5 patients showed new or enhanced levels of antitumor antibodies, and these patients had increased median OS compared with those who did not show new or enhanced antitumor antibody levels (15 versus 7.5 months; p < .03).
Sterman et al. evaluated locally administered immunotherapy using two intrapleural doses of an adenoviral vector encoding human interferon-α (Ad.IFN-α2b), and five of nine patients showed evidence of disease stability or tumor regression in the pilot study [117]. The investigators then conducted a phase I/II trial involving repeated intrapleural “vaccination” with Ad.IFN-α2b concomitant with high-dose cyclooxygenase-2 inhibitor celecoxib, followed by standard first-line (pemetrexed-based) or second-line (gemcitabine-based) chemotherapy [118]. The overall RR was 31%, and the disease control rate was 78%. Patients who received first-line chemotherapy (n = 14) had median survival of 10.5 months, whereas second-line patients (n = 21) had median survival of 15.0 months. Randomized multicenter trials are awaited to confirm these promising results.
The antitumor activity of T cells can be inhibited by negative regulatory “checkpoint” proteins on the cell surface, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and programmed cell death 1 (PD1) [119]. Preclinical studies have demonstrated that CTLA4 blockage could augment endogenous responses to tumor cells, leading to tumor cell death [120]. In a recently published single-arm phase II study, Calabrò et al. evaluated tremelimumab (MedImmune, Gaithersburg, MD, https://www.medimmune.com), a human IgG2 monoclonal antibody to CTLA4, in patients with chemotherapy-resistant MPM [121]. Although the study did not reach its primary endpoint of a 17% target RR, the disease control rate was 31% (9 of 29 patients). The median PFS and OS were 6.2 and 10.7 months, respectively. A larger multicenter randomized phase II trial comparing tremelimumab to placebo in the second- or third-line setting is currently recruiting. Trials of PD1 and ligand PD-L1 monoclonal antibodies are awaited.
Conclusion
Despite the advancements in surgical approaches, radiation techniques, and modern chemotherapeutics, MPM remains a highly lethal disease that is rarely cured. Only a small percentage of fit patients with good prognostic factors may benefit from multimodality therapy, underscoring the importance of surgical candidate selection. MPM inevitably progresses after standard antifolate-platinum chemotherapy and is resistant to other cytotoxic agents; no trials in the second- or third-line setting have shown a survival benefit. Clinical trial accruals of MPM have been hampered by the rarity of the disease; therefore, international collaborations are essential. Basic researchers have identified new biomarkers, explored novel antitumor mechanisms, and successfully translated several findings into exciting targeted agents that are actively being tested in the clinic (Table 5). We remain confident that, in the near future, effective therapies for MPM will result from these investigations and give patients realistic hope for meaningful prolongation of survival with this disease.
Table 5.
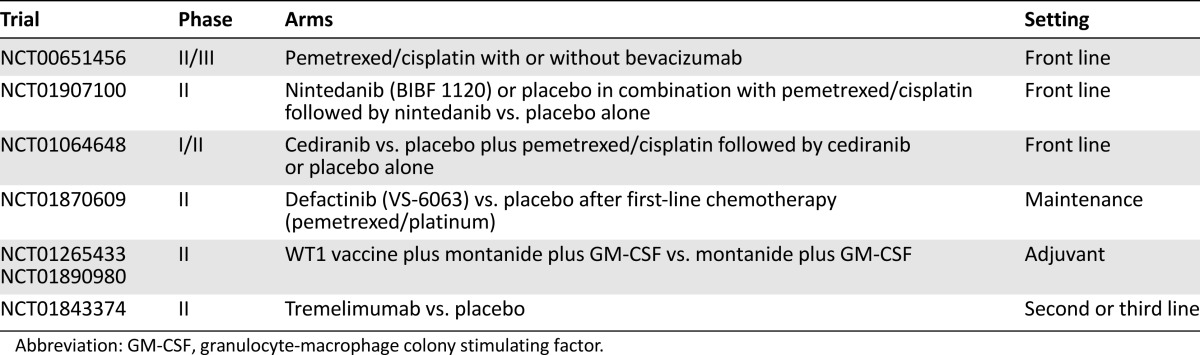
Ongoing randomized trials of targeted agents and immunotherapies in mesothelioma
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
For Further Reading:Kyle W. Robinson, Alan B. Sandler. The Role of MET Receptor Tyrosine Kinase in Non-Small Cell Lung Cancer and Clinical Development of Targeted Anti-MET Agents. The Oncologist 2013;18:115–122.
Implications for Practice:Identification of the role of the HGF–MET pathway in cancer, and specifically in non-small cell lung cancer (NSCLC) has led to the development of pharmaceutical agents targeting this pathway. In particular, MET's role in secondary resistance to EGFR-directed therapies has led to the investigation of combining MET-directed agents with erlotinib in patients with metastatic NSCLC. This article reviews the early development of MET-directed therapies as well as currently ongoing Phase III studies. We await the results of these studies, which will determine whether targeting MET in combination with EGFR is a valid clinical option in patients whose cancers progress following treatment with EGFR inhibitors.
Author Contributions
Conception/Design: Jing Ai, James P. Stevenson
Collection and/or assembly of data: Jing Ai, James P. Stevenson
Data analysis and interpretation: Jing Ai, James P. Stevenson
Manuscript writing: Jing Ai, James P. Stevenson
Final approval of manuscript: Jing Ai, James P. Stevenson
Disclosures
James P. Stevenson: Verastem, Boehringer Ingelheim, Medimmune (RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Previous version: SEER Cancer Statistics Review, 1975-2010. Available at http://seer.cancer.gov/csr/1975_2010/ Updated June 14, 2013.
- 2.Robinson BM. Malignant pleural mesothelioma: An epidemiological perspective. Ann Cardiothorac Surg. 2012;1:491–496. doi: 10.3978/j.issn.2225-319X.2012.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103(suppl):373S–376S. doi: 10.1378/chest.103.4_supplement.373s. [DOI] [PubMed] [Google Scholar]
- 4.Tan E, Warren N, Darnton AJ, et al. Projection of mesothelioma mortality in Britain using bayesian methods. Br J Cancer. 2010;103:430–436. doi: 10.1038/sj.bjc.6605781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer. 1980;46:2736–2740. doi: 10.1002/1097-0142(19801215)46:12<2736::aid-cncr2820461233>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34:718–721. [PubMed] [Google Scholar]
- 7.Hansen J, de Klerk NH, Musk AW, et al. Environmental exposure to crocidolite and mesothelioma: Exposure-response relationships. Am J Respir Crit Care Med. 1998;157:69–75. doi: 10.1164/ajrccm.157.1.96-11086. [DOI] [PubMed] [Google Scholar]
- 8.Metintas S, Metintas M, Ucgun I, et al. Malignant mesothelioma due to environmental exposure to asbestos: Follow-up of a Turkish cohort living in a rural area. Chest. 2002;122:2224–2229. doi: 10.1378/chest.122.6.2224. [DOI] [PubMed] [Google Scholar]
- 9.Pan XL, Day HW, Wang W, et al. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am J Respir Crit Care Med. 2005;172:1019–1025. doi: 10.1164/rccm.200412-1731OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulmez I, Kart L, Buyukoglan H, et al. Evaluation of malignant mesothelioma in central Anatolia: A study of 67 cases. Can Respir J. 2004;11:287–290. doi: 10.1155/2004/204793. [DOI] [PubMed] [Google Scholar]
- 11.Tward JD, Wendland MM, Shrieve DC, et al. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 12.Teta MJ, Lau E, Sceurman BK, et al. Therapeutic radiation for lymphoma: Risk of malignant mesothelioma. Cancer. 2007;109:1432–1438. doi: 10.1002/cncr.22526. [DOI] [PubMed] [Google Scholar]
- 13.Gibb H, Fulcher K, Nagarajan S, et al. Analyses of radiation and mesothelioma in the US Transuranium and Uranium Registries. Am J Public Health. 2013;103:710–716. doi: 10.2105/AJPH.2012.300928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comar M, Zanotta N, Pesel G, et al. Asbestos and SV40 in malignant pleural mesothelioma from a hyperendemic area of north-eastern Italy. Tumori. 2012;98:210–214. doi: 10.1177/030089161209800205. [DOI] [PubMed] [Google Scholar]
- 15.Cristaudo A, Foddis R, Vivaldi A, et al. SV40 enhances the risk of malignant mesothelioma among people exposed to asbestos: A molecular epidemiologic case-control study. Cancer Res. 2005;65:3049–3052. doi: 10.1158/0008-5472.CAN-04-2219. [DOI] [PubMed] [Google Scholar]
- 16.Lundstig A, Dejmek A, Eklund C, et al. No detection of SV40 DNA in mesothelioma tissues from a high incidence area in Sweden. Anticancer Res. 2007;27:4159–4161. [PubMed] [Google Scholar]
- 17.Manfredi JJ, Dong J, Liu WJ, et al. Evidence against a role for SV40 in human mesothelioma. Cancer Res. 2005;65:2602–2609. doi: 10.1158/0008-5472.CAN-04-2461. [DOI] [PubMed] [Google Scholar]
- 18.Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone M, Yang H, Pass HI, et al. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer. 1993;72:394–404. doi: 10.1002/1097-0142(19930715)72:2<394::aid-cncr2820720214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: Validation of CALGB and EORTC prognostic scoring systems. Thorax. 2000;55:731–735. doi: 10.1136/thorax.55.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadota K, Suzuki K, Sima CS, et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: A clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol. 2011;6:896–904. doi: 10.1097/JTO.0b013e318211127a. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother. 2011;60:1721–1728. doi: 10.1007/s00262-011-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen M, Limon J, Niezabitowski A, et al. Calretinin and other mesothelioma markers in synovial sarcoma: Analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol. 2001;25:610–617. doi: 10.1097/00000478-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Creaney J, Christansen H, Lake R, et al. Soluble mesothelin related protein in mesothelioma. J Thorac Oncol. 2006;1:172–174. [PubMed] [Google Scholar]
- 28.Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol. 2008;3:1317–1324. doi: 10.1097/JTO.0b013e318187491c. [DOI] [PubMed] [Google Scholar]
- 29.Rai AJ, Flores RM, Mathew A, et al. Soluble mesothelin related peptides (SMRP) and osteopontin as protein biomarkers for malignant mesothelioma: Analytical validation of ELISA based assays and characterization at mRNA and protein levels. Clin Chem Lab Med. 2010;48:271–278. doi: 10.1515/CCLM.2010.066. [DOI] [PubMed] [Google Scholar]
- 30.Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012;367:1417–1427. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plathow C, Staab A, Schmaehl A, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: Initial results. Invest Radiol. 2008;43:737–744. doi: 10.1097/RLI.0b013e3181817b3d. [DOI] [PubMed] [Google Scholar]
- 32.Flores RM. The role of PET in the surgical management of malignant pleural mesothelioma. Lung Cancer. 2005;49(suppl 1):S27–S32. doi: 10.1016/j.lungcan.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen JB, Ravn J, Loft A, et al. Preoperative staging of mesothelioma by 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography fused imaging and mediastinoscopy compared to pathological findings after extrapleural pneumonectomy. Eur J Cardiothorac Surg. 2008;34:1090–1096. doi: 10.1016/j.ejcts.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox BE, Subramaniam RM, Peller PJ, et al. Utility of integrated computed tomography-positron emission tomography for selection of operable malignant pleural mesothelioma. Clin Lung Cancer. 2009;10:244–248. doi: 10.3816/CLC.2009.n.033. [DOI] [PubMed] [Google Scholar]
- 35.Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg. 2012;1:438–448. doi: 10.3978/j.issn.2225-319X.2012.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association for the Study of Lung Cancer mesothelioma database. J Thorac Oncol. 2012;7:1631–1639. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 37.Hiddinga BI, van Meerbeeck JP. Surgery in mesothelioma—where do we go after MARS? J Thorac Oncol. 2013;8:525–529. doi: 10.1097/JTO.0b013e31828353d7. [DOI] [PubMed] [Google Scholar]
- 38.Treasure T, Waller D, Tan C, et al. The Mesothelioma and Radical Surgery randomized controlled trial: The MARS feasibility study. J Thorac Oncol. 2009;4:1254–1258. doi: 10.1097/JTO.0b013e3181ae26ae. [DOI] [PubMed] [Google Scholar]
- 39.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zahid I, Sharif S, Routledge T, et al. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812–817. doi: 10.1510/icvts.2010.256271. [DOI] [PubMed] [Google Scholar]
- 41.Teh E, Fiorentino F, Tan C, et al. A systematic review of lung-sparing extirpative surgery for pleural mesothelioma. J R Soc Med. 2011;104:69–80. doi: 10.1258/jrsm.2010.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: A consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol. 2011;6:1304–1312. doi: 10.1097/JTO.0b013e3182208e3f. [DOI] [PubMed] [Google Scholar]
- 43.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626, 626.e1–626.e3. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 44.Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:737–743. doi: 10.1097/JTO.0b013e31824ab6c5. [DOI] [PubMed] [Google Scholar]
- 45.Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: A phase II trial. J Thorac Oncol. 2006;1:289–295. [PubMed] [Google Scholar]
- 46.Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007–3013. doi: 10.1200/JCO.2008.20.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196–1202. doi: 10.1093/annonc/mdm093. [DOI] [PubMed] [Google Scholar]
- 48.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;122:788–795. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 49.Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685–1692; discussion 1692–1693. doi: 10.1016/j.athoracsur.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 50.Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: The “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol. 2014;9:397–402. doi: 10.1097/JTO.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 51.Aziz T, Jilaihawi A, Prakash D. The management of malignant pleural mesothelioma; single centre experience in 10 years. Eur J Cardiothorac Surg. 2002;22:298–305. doi: 10.1016/s1010-7940(02)00273-7. [DOI] [PubMed] [Google Scholar]
- 52.Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J Thorac Cardiovasc Surg. 2009;138:405–411. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 53.Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg. 2013;145:955–963. doi: 10.1016/j.jtcvs.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 54.Friedberg JS, Mick R, Culligan M, et al. Photodynamic therapy and the evolution of a lung-sparing surgical treatment for mesothelioma. Ann Thorac Surg. 2011;91:1738–1745. doi: 10.1016/j.athoracsur.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 55.Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012;93:1658–1665; discussion 1665–1667. doi: 10.1016/j.athoracsur.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fennell DA, Gaudino G, O’Byrne KJ, et al. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol. 2008;5:136–147. doi: 10.1038/ncponc1039. [DOI] [PubMed] [Google Scholar]
- 57.Berghmans T, Paesmans M, Lalami Y, et al. Activity of chemotherapy and immunotherapy on malignant mesothelioma: A systematic review of the literature with meta-analysis. Lung Cancer. 2002;38:111–121. doi: 10.1016/s0169-5002(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 58.Ellis P, Davies AM, Evans WK, et al. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: A systematic review and practice guideline. J Thorac Oncol. 2006;1:591–601. [PubMed] [Google Scholar]
- 59.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 60.van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–6889. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 61.Ceresoli GL, Zucali PA, Gianoncelli L, et al. Second-line treatment for malignant pleural mesothelioma. Cancer Treat Rev. 2010;36:24–32. doi: 10.1016/j.ctrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Manegold C, Symanowski J, Gatzemeier U, et al. Second-line (post-study) chemotherapy received by patients treated in the phase III trial of pemetrexed plus cisplatin versus cisplatin alone in malignant pleural mesothelioma. Ann Oncol. 2005;16:923–927. doi: 10.1093/annonc/mdi187. [DOI] [PubMed] [Google Scholar]
- 63.Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol. 2008;26:1698–1704. doi: 10.1200/JCO.2006.09.9887. [DOI] [PubMed] [Google Scholar]
- 64.Zucali PA, Perrino M, Lorenzi E, et al. Vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Lung Cancer. 2014;84:265–270. doi: 10.1016/j.lungcan.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer. 2008;112:1555–1561. doi: 10.1002/cncr.23337. [DOI] [PubMed] [Google Scholar]
- 66.Xanthopoulos A, Bauer TT, Blum TG, et al. Gemcitabine combined with oxaliplatin in pretreated patients with malignant pleural mesothelioma: An observational study. J Occup Med Toxicol. 2008;3:34. doi: 10.1186/1745-6673-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toyokawa G, Takenoyama M, Hirai F, et al. Gemcitabine and vinorelbine as second-line or beyond treatment in patients with malignant pleural mesothelioma pretreated with platinum plus pemetrexed chemotherapy. Int J Clin Oncol. 2013 doi: 10.1007/s10147-013-0619-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Ceresoli GL, Zucali PA, De Vincenzo F, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer. 2011;72:73–77. doi: 10.1016/j.lungcan.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: Targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–1420. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 70.Paik PK, Krug LM. Histone deacetylase inhibitors in malignant pleural mesothelioma: Preclinical rationale and clinical trials. J Thorac Oncol. 2010;5:275–279. doi: 10.1097/JTO.0b013e3181c5e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: Clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7:257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 72.Krug LM, Kindler H, Calvert H, et al. VANTAGE 014: Vorinostat (V) in patients with advanced malignant pleural mesothelioma (MPM) who have failed prior pemetrexed and either cisplatin or carboplatin therapy: A phase III, randomized, double-blind, placebo-controlled trial. Eur J Cancer. 2011;47:2–3. [Google Scholar]
- 73.Remon J, Lianes P, Martínez S, et al. Malignant mesothelioma: New insights into a rare disease. Cancer Treat Rev. 2013;39:584–591. doi: 10.1016/j.ctrv.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol. 2012;30:2509–2515. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dowell JE, Dunphy FR, Taub RN, et al. A multicenter phase II study of cisplatin, pemetrexed, and bevacizumab in patients with advanced malignant mesothelioma. Lung Cancer. 2012;77:567–571. doi: 10.1016/j.lungcan.2012.05.111. [DOI] [PubMed] [Google Scholar]
- 76.Zalcman G, Margery J, Scherpereel A, et al. IFCT-GFPC-0701 MAPS trial, a multicenter randomized phase II/III trial of pemetrexed-cisplatin with or without bevacizumab in patients with malignant pleural mesothelioma. J Clin Oncol. 2010;28(suppl):7020a. [Google Scholar]
- 77.Zalcman G, Mazieres J, Scherpereel A, et al. IFCT-GFPC-0701 MAPS trial, a multicenter randomized phase III trial of pemetrexed-cisplatin with or without bevacizumab in patients with malignant pleural mesothelioma (MPM) J Clin Oncol. 2012;30(suppl):TPS7112a. [Google Scholar]
- 78.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 79.Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res. 2010;16:311–319. doi: 10.1158/1078-0432.CCR-09-0694. [DOI] [PubMed] [Google Scholar]
- 80.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 81.McClatchey AI, Fehon RG. Merlin and the ERM proteins—regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianchi AB, Mitsunaga SI, Cheng JQ, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci USA. 1995;92:10854–10858. doi: 10.1073/pnas.92.24.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sekido Y, Pass HI, Bader S, et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995;55:1227–1231. [PubMed] [Google Scholar]
- 84.Poulikakos PI, Xiao GH, Gallagher R, et al. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 85.Soria J-C, Gan HK, Arkenau H-T, et al. Phase I clinical and pharmacologic study of the focal adhesion kinase (FAK) inhibitor GSK2256098 in pts with advanced solid tumors. J Clin Oncol. 2012;30(suppl):3000a. [Google Scholar]
- 86.Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 87.Ramos-Nino ME, Vianale G, Sabo-Attwood T, et al. Human mesothelioma cells exhibit tumor cell-specific differences in phosphatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther. 2005;4:835–842. doi: 10.1158/1535-7163.MCT-04-0243. [DOI] [PubMed] [Google Scholar]
- 88.de Assis LV, Locatelli J, Isoldi MC. The role of key genes and pathways involved in the tumorigenesis of Malignant Mesothelioma. Biochim Biophys Acta. 2014;1845:232–247. doi: 10.1016/j.bbcan.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 89.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.López-Lago MA, Okada T, Murillo MM, et al. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29:4235–4249. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garland LL, Ou S-H, Moon J, et al. SWOG 0722: A phase II study of mTOR inhibitor everolimus (RAD001) in malignant pleural mesothelioma (MPM) J Clin Oncol. 2012;30(suppl):7083a. doi: 10.1097/JTO.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wallin JJ, Edgar KA, Guan J, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 93.Dolly S, Krug LM, Wagner AJ et al. Evaluation of tolerability and anti-tumor activity of GDC-0980, an oral PI3K/mTOR inhibitor, administrated to patients with advanced malignant pleural mesothelioma (MPM). Presented at: International Association for the Study of Lung Cancer 15th World Conference on Lung Cancer; October 27–31, 2013; Sydney, Australia. [Google Scholar]
- 94.Grégoire M. What’s the place of immunotherapy in malignant mesothelioma treatments? Cell Adhes Migr. 2010;4:153–161. doi: 10.4161/cam.4.1.11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 97.Bograd AJ, Suzuki K, Vertes E, et al. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother. 2011;60:1509–1527. doi: 10.1007/s00262-011-1103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18:2478–2489. doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hassan R, Jahan TM, Kindler HL, et al. Amatuximab, a chimeric monoclonal antibody to mesothelin, in combination with pemetrexed and cisplatin in patients with unresectable pleural mesothelioma: Results of a multicenter phase II clinical trial. J Clin Oncol. 2012;30(suppl):7030a. [Google Scholar]
- 103.Li Q, Verschraegen CF, Mendoza J, et al. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–1335. [PubMed] [Google Scholar]
- 104.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 105.Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13:e301–e310. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krug LM, Dao T, Brown AB, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1467–1479. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krug L, Tsao AS, Kass S, et al. Randomized, double-blinded, phase II trial of a WT1 peptide vaccine as adjuvant therapy in patients with malignant pleural mesothelioma (MPM) J Clin Oncol. 2011;29(suppl):TPS139a. [Google Scholar]
- 110.Dao T, Yan S, Veomett N, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med. 2013;5:176ra33. doi: 10.1126/scitranslmed.3005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar-Singh S, Weyler J, Martin MJ, et al. Angiogenic cytokines in mesothelioma: A study of VEGF, FGF-1 and -2, and TGF beta expression. J Pathol. 1999;189:72–78. doi: 10.1002/(SICI)1096-9896(199909)189:1<72::AID-PATH401>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 114.DeLong P, Carroll RG, Henry AC, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4:342–346. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 115.Lonning S, Mannick J, McPherson JM. Antibody targeting of TGF-β in cancer patients. Curr Pharm Biotechnol. 2011;12:2176–2189. doi: 10.2174/138920111798808392. [DOI] [PubMed] [Google Scholar]
- 116.Stevenson JP, Kindler HL, Papasavvas E, et al. Immunological effects of the TGFβ-blocking antibody GC1008 in malignant pleural mesothelioma patients. OncoImmunology. 2013;2:e26218. doi: 10.4161/onci.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sterman DH, Haas A, Moon E, et al. A trial of intrapleural adenoviral-mediated Interferon-α2b gene transfer for malignant pleural mesothelioma. Am J Respir Crit Care Med. 2011;184:1395–1399. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sterman D, Alley E, Recio A et al. A pilot and feasibility trial evaluating two different chemotherapy regimens in combination with intrapleural adenoviral-mediated interferon-alpha (SCH 721015, Ad.hIFN-alpha2b) gene transfer for malignant pleural mesothelioma [abstract]. Presented at: International Association for the Study of Lung Cancer 15th World Conference on Lung Cancer; October 27–31, 2013; Sydney, Australia. [Google Scholar]
- 119.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 120.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: An overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 121.Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]


