A paucity of data exists on pharmacokinetically guided 5 fluorouracil (5-FU) dosing in the community setting. This multicenter study demonstrated the practicality of using a simple methodology to guide 5-FU dosing in the community setting and resulted in significantly fewer underdosed patients and less gastrointestinal toxicity.
Keywords: Fluorouracil, Pharmacokinetic, Dosing, Colorectal, Community, Personalized
Abstract
Background.
Pharmacokinetically guided (PK-guided) versus body surface area-based 5-fluorouracil (5-FU) dosing results in higher response rates and better tolerability. A paucity of data exists on PK-guided 5-FU dosing in the community setting.
Patients and Methods.
Seventy colorectal cancer patients, from one academic and five community cancer centers, received the mFOLFOX6 regimen (5-FU 2,400 mg/m2 over 46 hours every 2 weeks) with or without bevacizumab at cycle 1. The 5-FU continuous-infusion dose was adjusted for cycles 2–4 using a PK-guided algorithm to achieve a literature-based target area under the concentration-time curve (AUC). The primary objective was to demonstrate that PK-guided 5-FU dosing improves the ability to achieve a target AUC within four cycles of therapy. The secondary objective was to demonstrate reduced incidence of 5-FU-related toxicities.
Results.
At cycles 1 and 4, 27.7% and 46.8% of patients achieved the target AUC (20–25 mg × hour/L), respectively (odds ratio [OR]: 2.20; p = .046). Significantly more patients were within range at cycle 4 compared with a literature rate of 20% (p < .0001). Patients had significantly higher odds of not being underdosed at cycle 4 versus cycle 1 (OR: 2.29; p = .037). The odds of a patient being within range increased by 30% at each subsequent cycle (OR: 1.30; p = .03). Less grade 3/4 mucositis and diarrhea were observed compared with historical data (1.9% vs 16% and 5.6% vs 12%, respectively); however, rates of grade 3/4 neutropenia were similar (33% vs 25%–50%).
Conclusion.
PK-guided 5-FU dosing resulted in significantly fewer underdosed patients and less gastrointestinal toxicity and allows for the application of personalized colorectal cancer therapy in the community setting.
Implications for Practice:
There is consistent support in the literature for a clinically important relationship between 5-fluorouracil (5-FU) exposure and clinical outcomes. Clinical trials reflect the advantage of pharmacokinetically guided 5-FU dosing to enhance therapeutic outcomes; however, the paucity of interventional studies performed in the community setting partially explains the lack of assimilation into clinical practice. Our multicenter study demonstrated the practicality of using a simple methodology to guide 5-FU dosing in the community setting and resulted in significantly fewer underdosed patients and less gastrointestinal toxicity. These results establish a level of feasibility, and we believe the data accumulated to date are sufficient to consider further assessment and use of pharmacokinetically guided 5-FU dosing in clinical practice.
Introduction
5-Fluorouracil (5-FU) has remained the backbone of colorectal cancer (CRC) therapy for more than 50 years [1–5]. Compared with bolus dosing, continuous intravenous infusion of 5-FU has allowed for higher tolerable doses to be administered to patients, thereby enhancing clinical effectiveness [6–9]. However, dosing of 5-FU is currently based on body surface area (BSA), with a resulting 100-fold interindividual pharmacokinetic (PK) variability [9]. This variation is partially explained by genetic differences in dihydropyrimidine dehydrogenase (DPD), the metabolizing enzyme of 5-FU, and differences in distribution and clearance based on age, weight, sex, disease state, organ function, and diet [10, 11]. When dosed according to BSA, 5-FU administration results in up to one-third of patients developing severe dose-limiting toxicities and 50% of patients failing to respond to therapy [9, 12]. Previous studies report that 20%–30% of patients treated with conventional dosing have 5-FU exposure levels within a previously established therapeutic range (area under the concentration-time curve [AUC] 20–25 mg × hours/L [h/L]), whereas approximately 40%–60% and 10%–20% are under and over this therapeutic AUC threshold, respectively [13].
The suggested AUC target of 20–25 mg × h/L and the clinical validity of PK-guided 5-FU dosing have been demonstrated in several studies [9, 14–17]. Gamelin et al. initially observed a close relationship between acute toxicity and AUC0–8 hours >24 mg × h/L in 40 CRC patients [18]. Subsequently, a phase II PK-guided trial in 158 CRC patients targeting an AUC0–8 hours 16–24 mg × h/L demonstrated median overall survival (OS) of 19 months and an objective response rate (ORR) of 43.4% [9], approximately double the expected response rate of 5-FU and leucovorin administered by standard dosing. A randomized trial of 208 stage IV CRC patients receiving either conventionally dosed 5-FU or PK-guided 5-FU targeting an AUC 20–24 mg × h/mL demonstrated ORRs of 18.3% and 33.7% (p = .004), respectively, and median OS of 16 and 22 months (p = .08), respectively [16]. A similar trial comparing conventionally dosed 5-FU and PK-guided 5-FU in patients receiving the FOLFOX regimen (5-FU, oxaliplatin, leucovorin) identified ORRs of 46% and 69.5%, respectively, and median OS of 22 and 28 months, respectively, along with decreased incidence of grade 3/4 diarrhea and mucositis in the PK-guided dosing group [15].
To date, PK-guided 5-FU dosing has not been widely integrated into clinical practice, in part because of the paucity of data that demonstrates the ability to personalize 5-FU dosing in the community setting [13]. Given the evidence for PK-guided therapy, we conducted a multicenter study to investigate the application of PK-guided 5-FU dosing in a predominantly community setting, using a commercially available single-sample immunoassay to measure drug exposure. The primary objective was to demonstrate the ability to achieve a target AUC (20–25 mg × h /L) within four cycles of therapy in CRC patients receiving modified FOLFOX6 with or without bevacizumab. The secondary objective was to demonstrate that PK-guided 5-FU dosing decreases the incidence of 5-FU-related toxicities, including neutropenia, mucositis, and diarrhea, when compared with historical controls.
Patients and Methods
Patients
Patients from one academic and five community cancer centers scheduled to receive planned treatment with mFOLFOX6 with or without bevacizumab for histologically confirmed CRC (adjuvant or metastatic) were eligible to participate in the study. All patients were required to have an Eastern Cooperative Oncology Group performance status ≤2; have life expectancy of >6 months; provide written informed consent; and have adequate bone marrow and organ function, defined as absolute neutrophil count ≥1,500/mm3, platelets ≥100,000/mm3, serum creatinine ≤1.5 mg/dL, total bilirubin ≤1.5 times upper limit of normal (ULN), and aspartate and alanine aminotransferase ≤2.5 × ULN (or ≤5 × ULN in the presence of liver metastases).
Patients were not eligible if they were pregnant, had serious concomitant systemic disorders, or were unable to refrain from eating chocolate from any source beginning 12 hours prior to day 1 of each cycle and throughout the 46-hour 5-FU continuous infusion (CIV; theobromine, a compound found in chocolate, interferes with the assay and results in falsely elevated AUCs). Patients taking warfarin, enoxaparin, aminophylline, or theophylline were excluded from the study.
Patients were considered eligible for analysis if they completed four cycles of planned mFOLFOX6 with PK samples obtained at each cycle. In addition, patients removed from PK-guided therapy secondary to toxicity and those with only one missing PK sample (not due to a sampling error) were included in the analysis. Patients were excluded from analysis if 5-FU was dosed by any other means not including PK or toxicity or if there were concerns about 5-FU test-result validity due to sampling and/or processing errors.
The trial protocol was approved by the institutional review boards of all trial sites, and informed consent was obtained from each participant prior to enrollment.
Treatment and Dose Adjustment
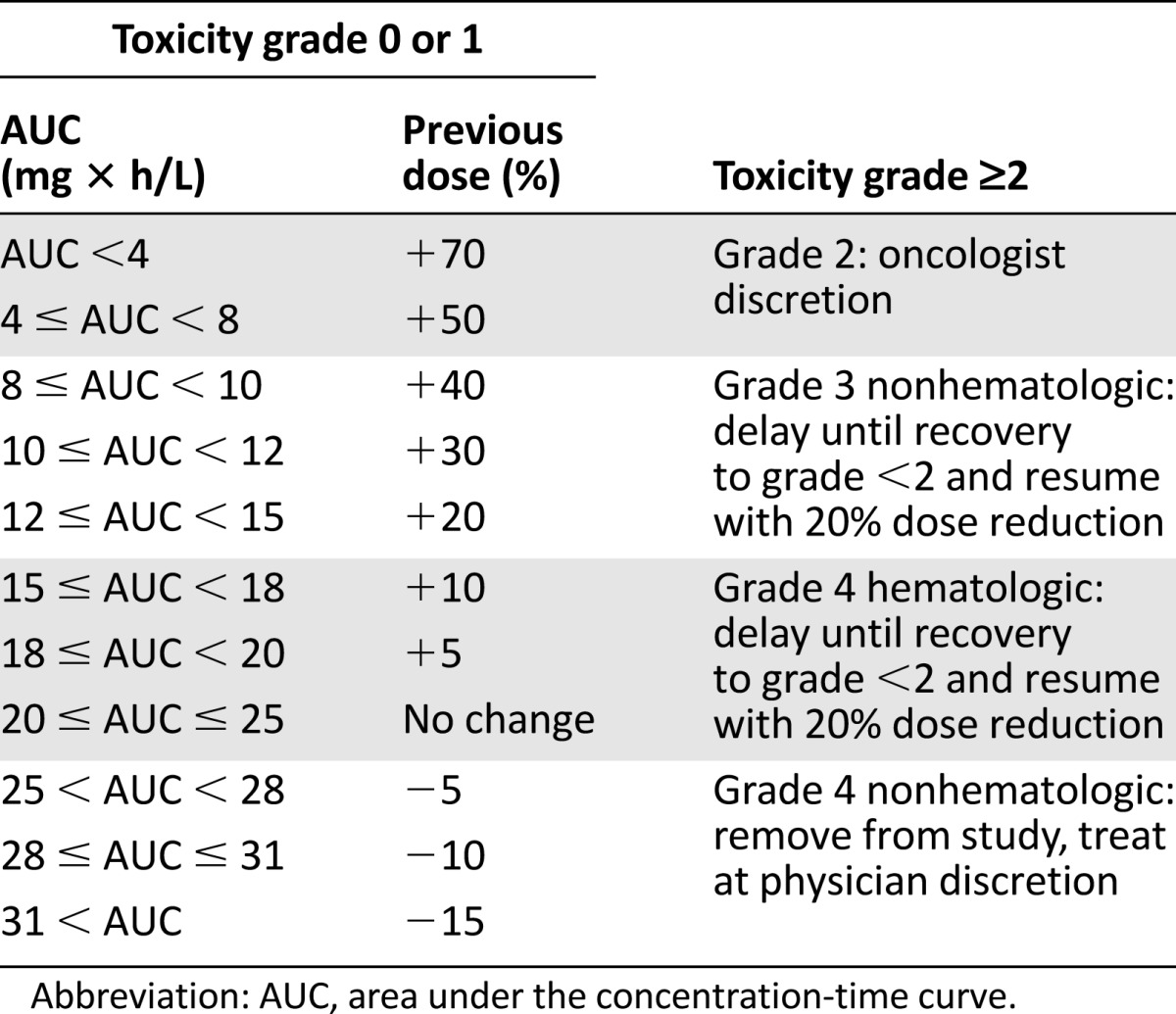
All patients received mFOLFOX6 at cycle 1: oxaliplatin 85 mg/m2 over 2 hours, leucovorin 200–400 mg/m2 over 2 hours, 5-FU 400 mg/m2 bolus, 5-FU 2,400 mg/m2 CIV over 46 hours (via a battery-operated pump) with or without bevacizumab, as determined by the primary treating physician. The 5-FU pumps were prefilled at central infusion pharmacies, primed with saline, and estimated to deliver drug within 30 minutes. Subsequent cycles were repeated every 2 weeks. Oxaliplatin, leucovorin, and 5-FU bolus dosage remained the same throughout treatment (unless adjusted for toxicity), whereas the 5-FU CIV dose was adjusted for cycles 2–4 using a PK-guided dose-adjustment algorithm targeting an AUC range of 20–25 mg × h/L (Table 1).
Table 1.
5-Fluorouracil dose adjustment algorithm
During cycles 1–4, a peripheral blood sample was obtained 2–44 hours after the start of the continuous infusion into an EDTA tube. Given that the half-life of 5-FU is approximately 10 minutes (±2 minutes) [6], steady state should theoretically be achieved within 1 hour. A stabilizing agent (derivative of uracil with properties that irreversibly inhibit DPD) was added to the sample immediately. The EDTA tube was inverted at least 3 times and centrifuged at 2,000g for 10–15 minutes. The plasma was pipetted from the sample into a 3-mL cryovial tube labeled with a bar code. A test request form was completed, including the infusion start time, draw time, infusion duration, patient identification, diagnosis, and dose. The sample was mailed to Myriad Genetics, Inc. (Salt Lake City, UT, https://www.myriad.com) for AUC analysis using a nanoparticle-based immunoassay. AUC is determined by multiplying the steady-state concentration by infusion duration.
Beginning with cycle 2, the 5-FU CIV dose was adjusted before each cycle according to an algorithm based on the results of the AUC from the preceding cycle, with a target AUC of 20–25 mg × h/L (Table 1). The dose adjustment algorithm was based on a linear relationship observed between plasma levels and 5-FU dose and has been published previously [9, 16]. In the event the AUC dropped out of the target AUC range in a subsequent cycle, the 5-FU CIV dose was readjusted based on the algorithm.
Follow-Up and Patient Monitoring
Patients underwent biweekly physical examinations and toxicity assessment before administration of each of the first five cycles. In addition, documentation of concomitant medications and foods were recorded at each cycle. The last toxicity assessment was made prior to receiving cycle 5.
Assessment of Toxicity
Patients underwent biweekly physical examinations and toxicity assessment using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 before administration of each of the first five cycles. Dose reductions for clinical toxicity are described in Table 1. For patients experiencing no toxicity or grade ≤1, the dose adjustment was made according to the algorithm. In the event that a patient experienced a 5-FU-related toxicity of grade ≥2 but had an AUC value that was below the target, the toxicity and subsequent dose reduction took precedence over the PK-guided dose escalation. In the event of a grade 2 nonhematologic toxicity associated with 5-FU (diarrhea, hand-foot syndrome, or mucositis), the dose was reduced at the discretion of the primary treating oncologist. In the event of grade 3 nonhematologic toxicity or grade 4 hematologic toxicity, 5-FU treatment was withheld until recovery of the toxicity to grade ≤2 and restarted with a 20% dose reduction of bolus and CIV 5-FU. Patients were removed from the study in cases of grade 4 nonhematologic toxicity. Toxicity data were collected through a secure Web-based clinical research platform, OnCore (Forte Research Systems, Madison, WI, https://forteresearch.com/enterprise-research-oncore/).
Statistical Analyses
For the primary objective of demonstrating an increased percentage of patients in range by cycle 4 using PK-guided dose adjustments versus non-PK-guided adjustments, a null hypothesis of 20% was used based on historical data [15, 16]. Using a 1-sided significance level of .05 and a 1-sample exact binomial test, a sample size of 50 had 72% power to detect a difference of 15% (alternative hypothesis of 35%) and 90% power to detect a difference of 20%. Comparisons between toxicity rates of patients receiving and not receiving bevacizumab and those with and without dose escalations were made using Fisher’s exact tests. Generalized linear models were used to perform logistic regression while accounting for multiple cycles per patient. In addition to estimating odds ratios (ORs) for being in range at cycle 4 versus cycle 1, not being underdosed, and being in range at each subsequent cycle, this method was used to evaluate the association between AUC range and grade 3/4 toxicities over all cycles.
Results
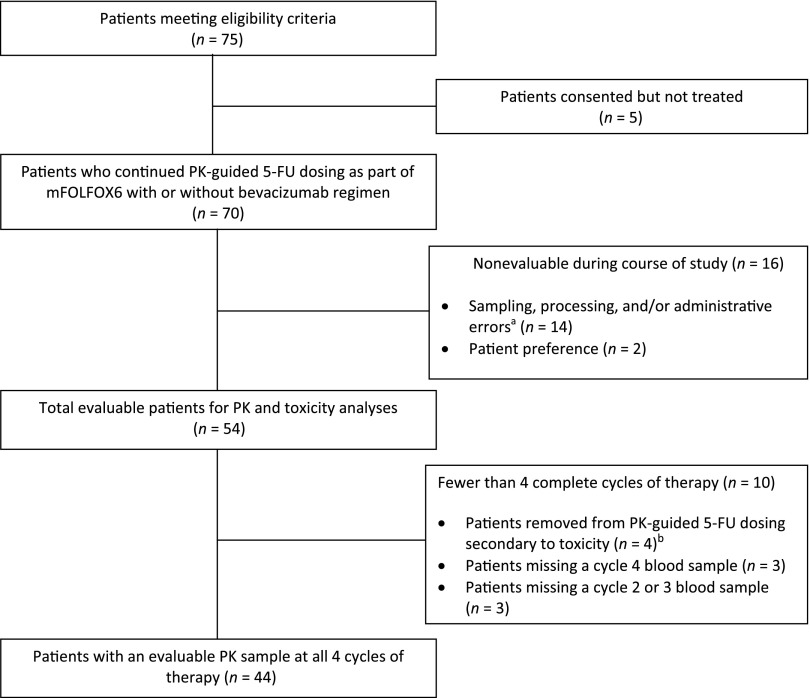
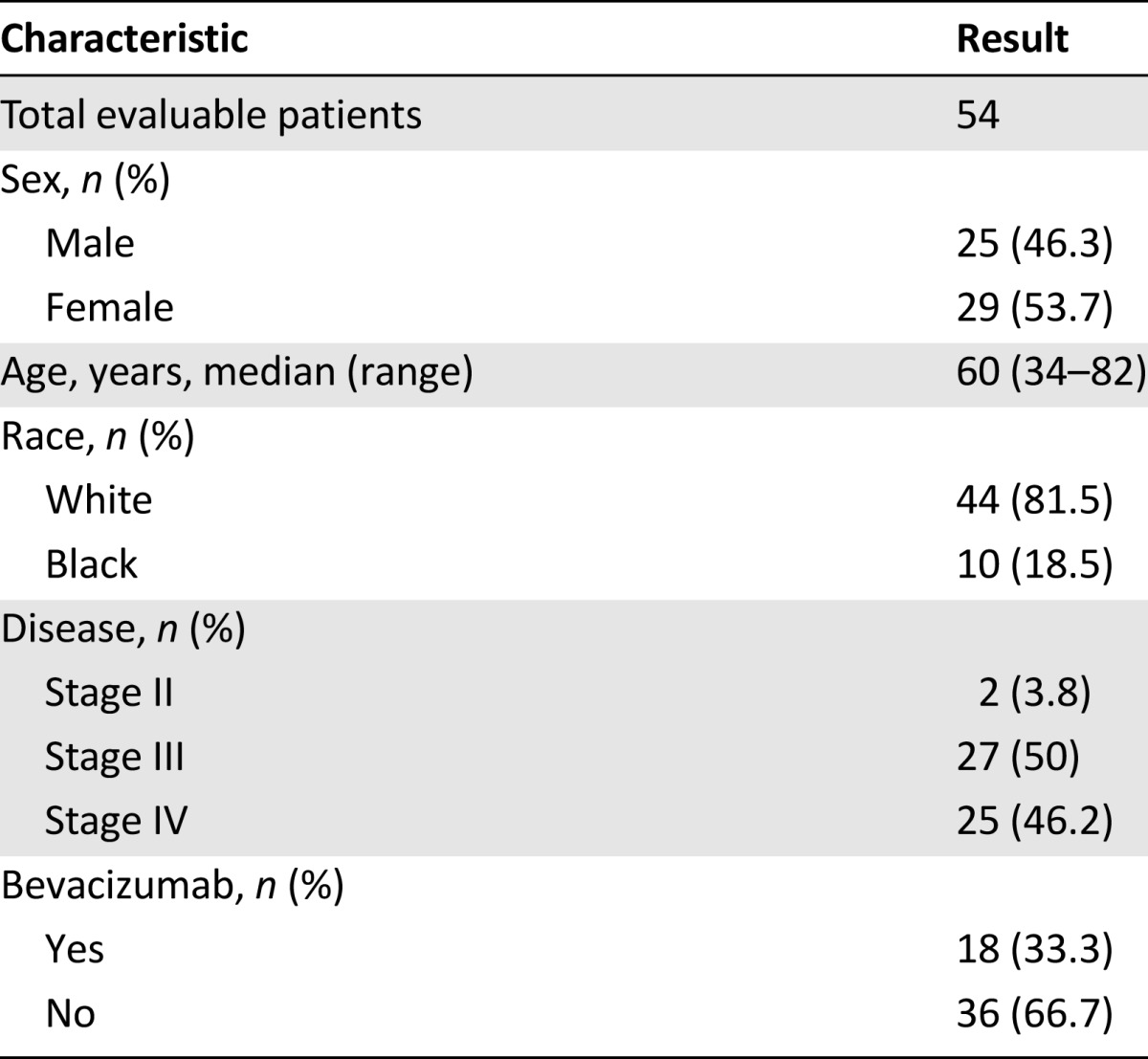
Seventy-five patients gave informed consent for the study between May 2010 and October 2012, with five patients subsequently not receiving treatment. Of the remaining 70 patients receiving mFOLFOX6 with or without bevacizumab, 16 patients were removed from the study secondary to protocol violations (n = 14) and patient preference (n = 2). The remaining 54 patients were deemed evaluable for “any-cycle” analyses, including PK and/or toxicity (Fig. 1). Baseline characteristics are summarized in Table 2. Of the 54 patients evaluable for any-cycle analyses, 25 (46.2%) had metastatic disease and 18 (33.3%) received bevacizumab in addition to chemotherapy. Four patients discontinued the study secondary to toxicity, and 6 patients had 1 missing blood sample for PK analysis (3 at cycle 2 or 3 and 3 at cycle 4), resulting in 44 patients with an evaluable PK sample at all 4 cycles and 47 patients with an evaluable PK sample at cycles 1 and 4. Sixteen of the 54 evaluable patients were treated and enrolled at the University of North Carolina (UNC) Cancer Hospital, whereas the remaining 38 were treated and enrolled at community-based hospitals within the UNC Cancer Network.
Figure 1.
CONSORT diagram. aSample drawn after pump finished infusing, sample not analyzed secondary to labeling issues; patient given hydration via peripheral line and sample drawn without wasting, thus diluted; omission of stabilizing agent and/or blood sample hemolyzed. In addition, three of these patients had protocol violations such as per-protocol dose adjustment not made. bAll four patients completed two cycles of PK-guided 5-FU prior to being removed.
Abbreviations: 5-FU, 5-fluorouracil; mFOLFOX6, modified FOLFOX regimen (5-FU, leucovorin, oxaliplatin); PK, pharmacokinetic.
Table 2.
Baseline patient characteristics
5-FU Exposure as Measured by AUC
The odds of a patient achieving the target AUC increased from cycle 1 (27.7%; 13 of 47) to cycle 4 (46.8%; 22 of 47) (OR: 2.20; 95% confidence interval [CI]: 1.01–4.76; p = .046). With an exact binomial CI of (32.1%–61.9%) for the percent of patients in range by cycle 4 (46.8%), PK-guided 5-FU dosing resulted in significantly more patients within the prespecified AUC range compared with a historical rate of 20% (p < .0001), satisfying the primary objective. In addition, patients had significantly higher odds of not being underdosed (i.e., not having an AUC <20 mg × h/L) at cycle 4 versus cycle 1 (OR: 2.29; 95% CI: 1.05–4.96; p = .037). The odds of a patient being within range increased by 30% at each subsequent cycle (OR: 1.30; 95% CI: 1.03–1.66; p = .03) using the defined algorithm.
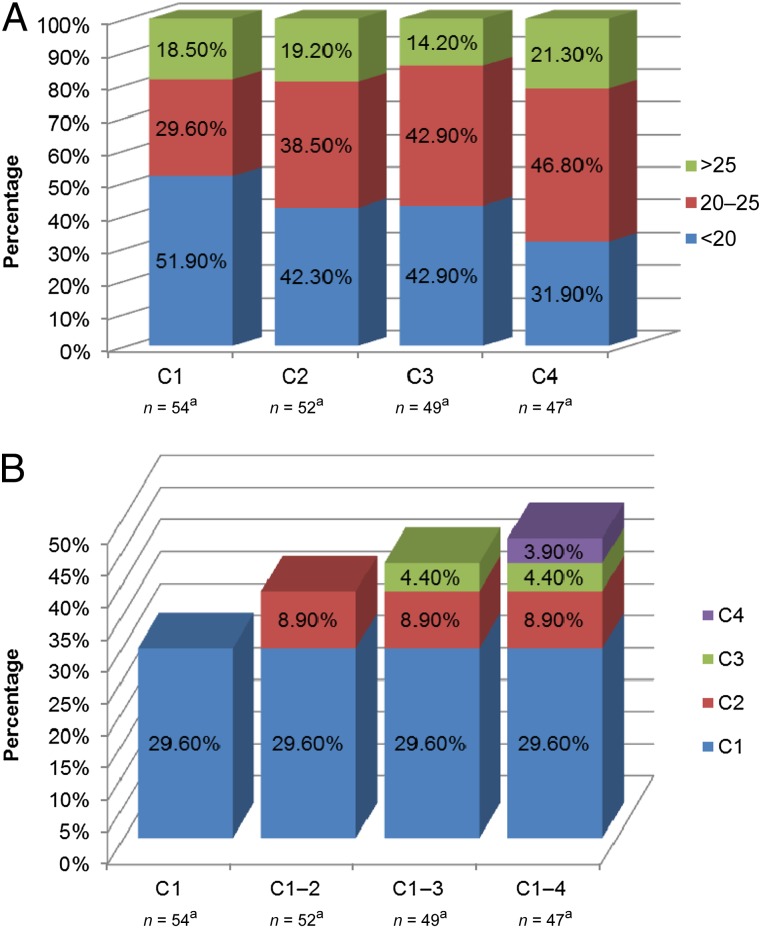
Figure 2A demonstrates the percentage of patients below, within, and above the prespecified AUC threshold for all evaluable patients in the any-cycle analysis (n = 54) at each independent cycle. At cycle 1, 29.6% of patients (16 of 54) were within range, compared with 46.8% (22 of 47) at cycle 4. Figure 2B demonstrates the cumulative percentage of patients achieving the target AUC from cycle 1 to cycle 4. The median AUCs at cycle 1 for men and women were 19 and 21 mg × h/L (not statistically significant), respectively, consistent with previous literature reporting lower DPD enzyme activity and higher 5-FU exposure in female patients compared with male patients [19]. No significant differences were noted in median AUC between bevacizumab- and non-bevacizumab-treated patients (p = .76).
Figure 2.
Percentage of patients below, within and above the prespecified area under the concentration-time curve (AUC) threshold at each cycle. (A): Significantly more patients were within range and significantly less were underdosed at cycle 4 compared with cycle 1 (p < .05). The percentages are reflective of the total number of independent patient samples at each cycle (i.e., patients with an AUC >25 mg × h/L at cycle 1 are not necessarily the same patients with an AUC >25 mg × h/L at cycle 4). aNumber of patients with an evaluable blood sample for AUC analysis at each cycle. (B): Cumulative percentage of patients achieving the target AUC at each cycle. aNumber of patients with an evaluable blood sample for AUC analysis at each cycle.
Abbreviation: C, cycle.
There was evidence of intrapatient variability in assay results, with 80% of all patients (43 of 54) achieving the target AUC during at least 1 of the 4 cycles and no patients within range at every cycle. The coefficient of variation in AUC decreased from 30.6% to 24.9% for cycles 1 and 4, respectively. The trend toward decreased PK variability and fewer patients underdosed at cycle 4 is reflected in an increase of the median AUC from 19 to 21 mg × h/L.
Moreover, 76% of patients (41 of 54) required a protocol-guided dose increase at any point during the 4 cycles of PK-guided therapy. Of these 41 patients, 27 (66%) required their first dose increase prior to the administration of cycle 2. The median 5-FU dose necessary for patients successfully achieving the targeted AUC at cycle 4 was 2,580 ± 377 mg/m2 (range: 1,925–3,484 mg/m2; supplemental online Fig. 1 shows a bar graph of patients’ cycle 4 dose).
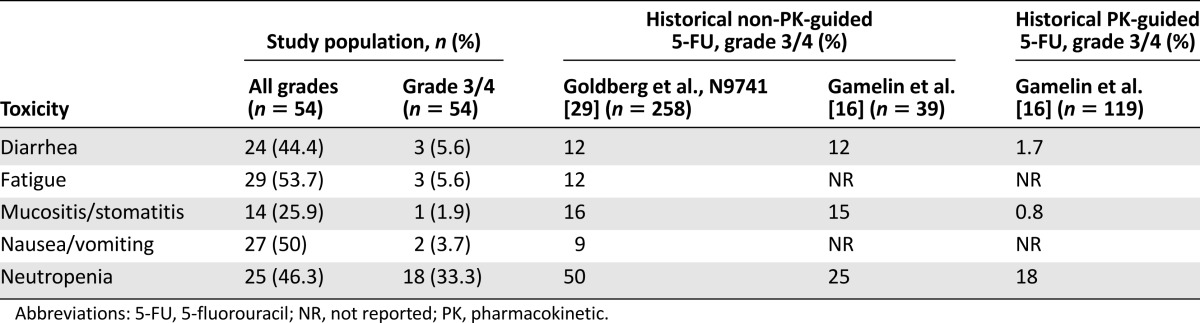
Toxicity
As indicated in Table 3, the incidence of grade 3/4 neutropenia was similar in patients treated with PK-guided mFOLFOX6 compared with historical non-PK-guided mFOLFOX6 (33% vs. 25%–50%); however, a decrease in the incidence of grade 3/4 diarrhea and mucositis was noted (5.6% vs. 12% and 1.9% vs. 15%, respectively) (Table 3).
Table 3.
Toxicity assessment
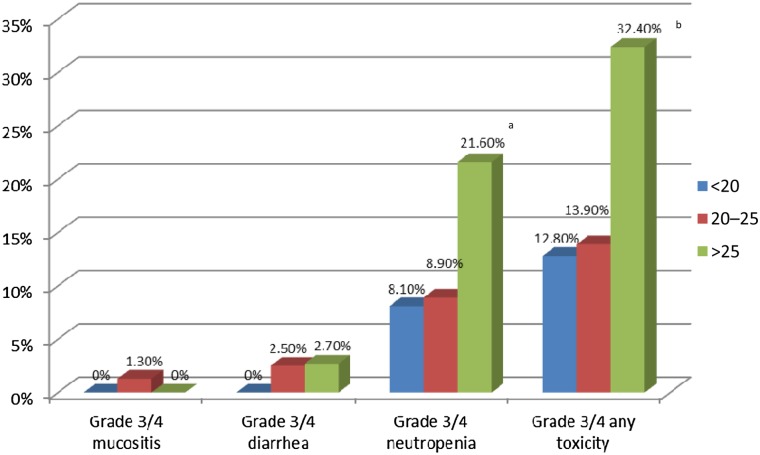
The incidence of grade 3/4 neutropenia was similar for cycles during which the target AUC was achieved (8.9%) compared with cycles during which the AUC was <20 mg × h/L (8.1%). However, a higher incidence of grade 3/4 neutropenia was observed in cycles during which AUC was >25 mg × h/L compared with <20 mg × h/L (21.6% vs. 8.1%, p = .05) (Fig. 3). Significantly higher rates of any grade 3/4 toxicity (e.g., neutropenia, diarrhea, mucositis, fatigue, nausea or vomiting) were noted in patients with AUC >25 mg × h/L compared with AUC <20 mg × h/L (32.4% vs. 12.8%, respectively; p = .007) (Fig. 3). Importantly, of the patients having a PK-guided dose escalation at any cycle with no toxicities prior to the increase, 40.5% (15 of 37) had any grade 3/4 toxicity at any cycle after dose escalation compared with 69.2% (9 of 13) of patients who did not have a PK-guided dose escalation (p = .1). This suggests that PK-guided dose escalations did not result in significantly higher rates of toxicity compared with patients who did not require a dose escalation; however, a larger sample size would be needed to confirm this observation.
Figure 3.
Rates of toxicity at each area under the concentration-time curve (AUC) range during the cycle at which toxicity occurred. aA significantly higher incidence of grade 3/4 neutropenia was observed in cycles during which AUC was >25 mg × h/L compared with cycles during which AUC was <20 mg × hour/L (21.6% vs. 8.1%; p = .05). bSignificantly higher rates of any grade 3/4 toxicity (e.g., neutropenia, diarrhea, mucositis, fatigue, nausea or vomiting) were noted in patients with an AUC >25 mg × h/L compared with AUC <20 mg × h/L (32.4% vs. 12.8%, respectively; p = .007).
Discussion
There is consistent literature of support for a clinically important relationship between 5-FU exposure and both toxicity and efficacy [14, 17, 18, 20, 21]. These studies reflect the advantage in utilizing PK-guided 5-FU dosing to enhance therapeutic outcomes. The paucity of prospective intervention studies and few data from the community setting partially explain the lack of assimilation into clinical practice. Given the current evidence for PK-guided therapy and considering that 85% of U.S. cancer patients are diagnosed and treated in the community setting [22], our multicenter study investigated the application of PK-guided 5-FU dosing across one academic and five community cancer centers in North Carolina.
PK-guided 5-FU dosing resulted in significantly more patients achieving a prespecified target AUC and significantly greater odds of a patient not being underdosed and reduced PK variability. The concept of underdosing of chemotherapy is a rarely discussed aspect of current dosage-calculation strategies [12]. Consistent with previous literature, more than half of all patients (52%) in our study were underdosed at cycle 1, as indicated by an AUC <20 mg × h/L. The median 5-FU dose necessary for patients successfully achieving the target AUC at cycle 4 was 108% (range: 80%–145%) of the recommended 2,400-mg/m2 dose (similar to the 110% found by Capitain et al. in PK-guided FOLFOX [15] but less than the 119% found by Gamelin et al. [16] in PK-guided 5-FU alone). However, a nearly twofold range in dosing was required to achieve the targeted AUC range. Among the 15 patients who were still under the AUC threshold at cycle 4, 9 had toxicity-based dose reductions at cycle 4 that took precedence over a PK-guided dose adjustment and likely resulted in an AUC <20 mg × h/L. Considering that 28 of 54 patients were underdosed at cycle 1 and only 6 of those patients who received a PK-guided dose adjustment for cycle 4 remained under the AUC threshold, this suggests that close to 80% of eligible patients can avoid underdosing by using PK-guided 5-FU dosing.
Large intrapatient variations in 5-FU exposure were evident as 80% of patients achieved the target AUC at any cycle while only 47% remained within range at cycle 4. Variations may be due to administration time (circadian rhythm), PK sampling time, diet, concomitant medications, and other environmental factors. Targeting a limited therapeutic AUC range of 20–25 mg × h/L provides a challenge in applying PK-guided 5-FU dosing in clinical practice. Consequently, recent data suggest that a wider range of 20–30 mg × h/L results in greater success of achieving the target AUC and increased response without compromising safety [23]. However, this range has not been evaluated in a randomized clinical trial.
The 5-FU-associated toxicities observed in our study were consistent with the literature and included neutropenia, diarrhea, mucositis, stomatitis, nausea, vomiting, and fatigue. Despite 76% of patients receiving protocol-specified dose escalations, our study demonstrated increased tolerability and decreased rates of grade 3/4 diarrhea and mucositis and stomatitis compared with historical controls, consistent with previously published PK-guided 5-FU literature [9, 15, 16, 24]. Although this finding goes against the basic principles of pharmacology, the fact that 4 separate PK-guided 5-FU trials [9, 15, 16], totaling 420 colorectal cancer patients (including this study), resulted in fewer underdosed patients and lower rates of toxicity is of tremendous importance when considering its clinical applicability. Interestingly, there was no difference in the percentage of patients with an AUC >25 mg × h/L throughout all 4 cycles; however, higher rates of any grade 3/4 toxicity were observed in cycles with an AUC >25 mg × h/L (largely driven by grade 3/4 neutropenia).
The number of patients deemed “nonevaluable” (n = 16) throughout the course of the study was unexpectedly high, demonstrating the challenges of conducting a multicenter study in the outpatient setting when specialized testing is required. This also potentially speaks to the generalizability of this approach in the community setting and suggests that experience and training would be necessary to correctly implement this dosing approach. Fourteen patients had protocol violations that resulted in ineligibility, including samples being drawn after completion of infusion, sample dilution, omission of sample stabilizing agent, inadequate labeling of tubes, and per-protocol dose adjustment not made. Although factors such as patient compliance with returning to the clinic for PK sampling were beyond the control of the investigators, human errors involving sample handling were more frequent reasons for patient withdrawal after enrollment. This underscores the need for more attention to appropriate logistical training and coordination with patients about the reason for specific timing of office visits when personalized medicine strategies are being implemented in clinical practice. This is particularly critical for community-based practices with little experience in sample handling and clinical research. As a major barrier to widespread adoption, logistical issues must be addressed before implementation of PK-guided 5-FU dosing can be successful and effective.
The principal limitation of our study was the single-arm design, which did not allow for direct comparison of differing dosing strategies regarding toxicity. Inclusion of patients receiving both metastatic and adjuvant therapy also prevents the ability to draw conclusions about efficacy. However, to our knowledge, our study is the only prospective multicenter study utilizing a simplified, clinically applicable methodology for assessing 5-FU exposure in the outpatient setting, potentially allowing greater generalizability of results to the oncology community.
Proof-of-principle personalized chemotherapy dosing is noted in prospective studies of drugs including carboplatin [25, 26], high-dose methotrexate (HDMTX) [27], busulfan [28], and 5-FU [9, 15, 16] that confirm exposure-response relationships and decreased PK variability. In all of these cases, PK-guided dosing resulted in fewer patients underdosed, fewer toxicities, and improved outcomes [29]; however, in contrast to carboplatin, HDMTX, and busulfan, PK-guided 5-FU dosing has not been applied as a standard in clinical practice. Patients receiving PK-guided dosing may experience better outcomes than those observed for patients receiving newer but very costly targeted therapies or those from PK-guided monotherapy (i.e., 5-FU) versus conventionally dosed combination therapy (i.e., FOLFOX). In addition, PK-guided therapy may be more applicable to certain populations such as the elderly, for whom toxicity from “standard” therapy is often a practical concern.
Conclusion
The use of BSA-guided 5-FU dosing has resulted in significant underdosing of the majority of colorectal cancer patients. It is imperative to increase the general awareness of the beneficial effects and practicality of PK-guided 5-FU dosing. We identified multiple barriers to the successful implementation of PK-guided 5-FU dosing in clinical practice, including sampling times and coordination. Our study demonstrated that use of a simple methodology, along with appropriate logistical training, allows for the application of personalized 5-FU dosing in colorectal cancer patients treated in the community setting.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was funded by an investigator-initiated research grant to Howard L. McLeod from Myriad Genetics, Inc. Jai N. Patel and Christine M. Walko received salary support from this grant. The contents of this manuscript have been published and presented in part at the 2013 American Society of Clinical Oncology annual meeting. The study is registered on ClinicalTrials.gov (identifier: NCT01164215).
Author Contributions
Conception/Design: Jai N. Patel, Bert H. O’Neil, Joseph G. Ibrahim, Howard L. McLeod, Christine M. Walko
Provision of study material or patients: Jai N. Patel, Bert H. O’Neil, Gary B. Sherrill, Oludamilola A. Olajide, Prashanti M. Atluri, John J. Inzerillo, Christopher H. Chay, Howard L. McLeod, Christine M. Walko
Collection and/or assembly of data: Jai N. Patel, Bert H. O’Neil, Allison M. Deal, Howard L. McLeod, Christine M. Walko
Data analysis and interpretation: Jai N. Patel, Bert H. O’Neil, Allison M. Deal, Joseph G. Ibrahim, Howard L. McLeod, Christine M. Walko
Manuscript writing: Jai N. Patel, Bert H. O’Neil, Allison M. Deal, Joseph G. Ibrahim, Howard L. McLeod, Christine M. Walko
Final approval of manuscript: Jai N. Patel, Bert H. O’Neil, Allison M. Deal, Joseph G. Ibrahim, Gary B. Sherrill, Oludamilola A. Olajide, Prashanti M. Atluri, John J. Inzerillo, Christopher H. Chay, Howard L. McLeod, Christine M. Walko
Disclosures
Jai N. Patel: Myriad Genetics, Inc. (RF); John J. Inzerillo: Teva (H, speaker program, OI); Lilly (H, speaker program); Howard L. McLeod: Gentris Corporation (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Poon MA, O’Connell MJ, Wieand HS, et al. Biochemical modulation of fluorouracil with leucovorin: Confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol. 1991;9:1967–1972. doi: 10.1200/JCO.1991.9.11.1967. [DOI] [PubMed] [Google Scholar]
- 2.Pinedo HM, Peters GF. Fluorouracil: Biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French intergroup study. J Clin Oncol. 1997;15:808–815. doi: 10.1200/JCO.1997.15.2.808. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 6.Lokich JJ, Ahlgren JD, Gullo JJ, et al. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program Study. J Clin Oncol. 1989;7:425–432. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Rubio E, Aranda E, Martin M, et al. Weekly high-dose infusion of 5-fluorouracil in advanced colorectal cancer. Eur J Cancer. 1990;26:727–729. doi: 10.1016/0277-5379(90)90128-g. [DOI] [PubMed] [Google Scholar]
- 8.Ardalan B, Chua L, Tian EM, et al. A phase II study of weekly 24-hour infusion with high-dose fluorouracil with leucovorin in colorectal carcinoma. J Clin Oncol. 1991;9:625–630. doi: 10.1200/JCO.1991.9.4.625. [DOI] [PubMed] [Google Scholar]
- 9.Gamelin E, Boisdron-Celle M, Delva R, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: Results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol. 1998;16:1470–1478. doi: 10.1200/JCO.1998.16.4.1470. [DOI] [PubMed] [Google Scholar]
- 10.Diasio RB, Johnson MR. Dihydropyrimidine dehydrogenase: Its role in 5-fluorouracil clinical toxicity and tumor resistance. Clin Cancer Res. 1999;5:2672–2673. [PubMed] [Google Scholar]
- 11.Milano G, Etienne MC, Cassuto-Viguier E, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol. 1992;10:1171–1175. doi: 10.1200/JCO.1992.10.7.1171. [DOI] [PubMed] [Google Scholar]
- 12.Walko CM, McLeod HL. Will we ever be ready for blood level-guided therapy? J Clin Oncol. 2008;26:2078–2079. doi: 10.1200/JCO.2007.14.9609. [DOI] [PubMed] [Google Scholar]
- 13.Saif MW, Choma A, Salamone SJ, et al. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101:1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 14.Milano G, Roman P, Khater R, et al. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer. 1988;41:537–541. doi: 10.1002/ijc.2910410411. [DOI] [PubMed] [Google Scholar]
- 15.Capitain O, Asevoaia A, Boisdron-Celle M, et al. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: A phase II, proof-of-concept study. Clin Colorectal Cancer. 2012;11:263–267. doi: 10.1016/j.clcc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 17.Milano G, Etienne MC, Renée N, et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol. 1994;12:1291–1295. doi: 10.1200/JCO.1994.12.6.1291. [DOI] [PubMed] [Google Scholar]
- 18.Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996;77:441–451. doi: 10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Mattison LK, Fourie J, Desmond RA, et al. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians. Clin Cancer Res. 2006;12:5491–5495. doi: 10.1158/1078-0432.CCR-06-0747. [DOI] [PubMed] [Google Scholar]
- 20.Schneider M, Etienne MC, Milano G, et al. Phase II trial of cisplatin, fluorouracil, and pure folinic acid for locally advanced head and neck cancer: A pharmacokinetic and clinical survey. J Clin Oncol. 1995;13:1656–1662. doi: 10.1200/JCO.1995.13.7.1656. [DOI] [PubMed] [Google Scholar]
- 21.van Kuilenburg AB, Maring JG. Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients. Pharmacogenomics. 2013;14:799–811. doi: 10.2217/pgs.13.54. [DOI] [PubMed] [Google Scholar]
- 22.Zaren HA, Nair S, Go RS, et al. Early-phase clinical trials in the community: Results from the National Cancer Institute Community Cancer Centers Program Early-Phase Working Group baseline assessment. J Oncol Pract. 2013;9:e55–e61. doi: 10.1200/JOP.2012.000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaldate RR, Haregewoin A, Grier CE, et al. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. The Oncologist. 2012;17:296–302. doi: 10.1634/theoncologist.2011-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffour J, Roca L, Bressolle F, et al. Clinical impact of intesified 5-fluorouracil-based chemotherapy using a prospective pharmacokinetically-guided dosing approach: Comparative study in elderly and non-elderly patients with metastatic colorectal cancer. J Chemother. 2010;22:179–185. doi: 10.1179/joc.2010.22.3.179. [DOI] [PubMed] [Google Scholar]
- 25.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 26.Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol. 1992;10:520–528. doi: 10.1200/JCO.1992.10.4.520. [DOI] [PubMed] [Google Scholar]
- 27.Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 28.Kangarloo SB, Naveed F, Ng ES, et al. Development and validation of a test dose strategy for once-daily i.v. busulfan: Importance of fixed infusion rate dosing. Biol Blood Marrow Transplant. 2012;18:295–301. doi: 10.1016/j.bbmt.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Beumer JH. Without therapeutic drug monitoring, there is no personalized cancer care. Clin Pharmacol Ther. 2013;93:228–230. doi: 10.1038/clpt.2012.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.