Abstract
Background.
Outcomes with current chemotherapy in metastatic urothelial carcinoma (MUC) remain poor. Lenalidomide, an antiangiogenic and immunomodulatory agent, enhances the effects of chemotherapy in preclinical studies. In this phase Ib/II study, we sought to determine a tolerable dose of lenalidomide in combination with gemcitabine and cisplatin (GCL) in patients with MUC and to explore the safety and activity of this regimen.
Methods.
Patients with chemotherapy-naïve MUC received gemcitabine 1,000 mg/m2 on days 1 and 8 and cisplatin 70 mg/m2 on day 1 every 21 days. In phase Ib, there were four planned escalating dose levels of lenalidomide (10, 15, 20, and 25 mg) daily on days 1–14.
Results.
Seven patients received GCL in phase Ib. The dose of lenalidomide was not escalated beyond 10 mg because of cytopenias requiring repeated dose delays and reductions. Two additional patients were enrolled in phase II, but the study was ultimately terminated due to poor tolerability and slow accrual. The most frequent grade ≥3 adverse events were cytopenias and diarrhea. Three of the nine patients experienced an objective response (one complete response, two partial responses).
Conclusion.
Chronic administration of the GCL regimen was poorly tolerated because of additive and cumulative myelosuppression.
Author Summary
Discussion
Each year in the United States, more than 60,000 patients develop urothelial carcinoma (UC) and more than 12,000 die of the disease [1]. The combination of gemcitabine and cisplatin (GC) is a standard first-line therapy for metastatic UC (MUC) based on a randomized study demonstrating similar efficacy and less toxicity compared with a regimen of methotrexate, vinblastine, doxorubicin, and cisplatin [2]. Although the tolerability of chemotherapy for patients with MUC has improved, there have been no improvements in the efficacy of treatment for the past several decades, and novel approaches are clearly needed.
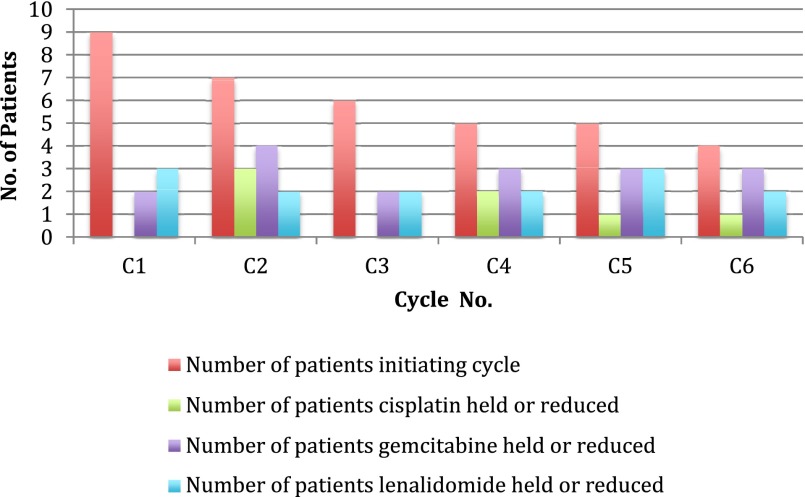
Lenalidomide, a potent thalidomide analog with antiangiogenic and immunomodulatory properties, has demonstrated antiproliferative and antiangiogenic effects in cell culture and xenograft models of UC and has been shown to enhance the antiproliferative properties of GC [3, 4]. Based on such findings, we initiated a phase Ib/II study exploring the combination of GC plus lenalidomide in chemo-naïve patients with MUC. Only one patient experienced a protocol-defined dose-limiting toxicity (grade 4 thrombocytopenia lasting >7 days) during phase Ib. However, a decision was made to expand the lenalidomide 10-mg dose-level cohort because of the need for frequent dose delays and dose reductions of gemcitabine, cisplatin, and lenalidomide, often occurring after cycle 1 (Fig. 1), to better characterize the safety and tolerability of the combination. There were no further dose-limiting toxicities, and phase II was opened at the lenalidomide 10-mg dose level. The trial was terminated after enrollment of an additional two patients because the regimen was deemed poorly tolerated for chronic administration because of the need for repeated dose delays and reductions coupled with slow accrual.
Figure 1.
Dose delays and dose reductions.
These findings highlight three critical points. First, conventional phase I designs aimed at defining recommended phase II dosing using only first-cycle toxicity data may not be optimal in the era of molecularly targeted therapies typically administered in a chronic fashion and often characterized by persistent and/or cumulative toxicities [5]. Second, despite promising preclinical data, there are practical challenges in combining targeted therapies with cytotoxic agents, sometimes related to off-target effects [6]. Third, poor accrual remains a critical barrier to progress in clinical drug development [7].
Supplementary Material
Footnotes
Access the full results at: Galsky-14-153.theoncologist.com
ClinicalTrials.gov Identifier: NCT01342172
Sponsor(s): Celgene
Principal Investigators: Neeraj Agarwal, Andrea B. Apolo, Matthew D. Galsky
IRB Approved: Yes
For Further Reading:David D. Chism, Michael E. Woods, Matthew I. Milowsky. Neoadjuvant Paradigm for Accelerated Drug Development: An Ideal Model in Bladder Cancer. The Oncologist 2013;18:933–940.
Implications for Practice:Recent recommendations to use the neoadjuvant setting in breast cancer as an accelerated drug development pathway make a similar approach in bladder cancer very appealing. The current article will review the rationale for consideration of bladder cancer as the ideal neoadjuvant model for accelerated drug development. Several factors including the ease of bladder tumor tissue collection performed as standard of care, the use of pathologic response as an intermediate marker for overall outcome, and a richer understanding of the important molecular pathways involved in bladder cancer development and progression make the neoadjuvant paradigm particularly relevant. The ability to conduct clinical trials that require fewer patients and efficiently explore disease biology will undoubtedly lead to the development of novel therapies and have a profound effect on every day medical practice.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.