The aim of this study was to determine everolimus tolerability and efficacy, in relation to previous treatments, in a compassionate use program for neuroendocrine tumors (NETs). Results showed that everolimus is safe and effective for the treatment of NETs of different origins. Higher severe toxicity occurred in patients previously treated with systemic chemotherapy and peptide receptor radionuclide therapy.
Keywords: Neuroendocrine tumors, Everolimus, Pancreatic endocrine tumors, Carcinoids, Compassionate use, Prognosis
Abstract
Everolimus is a valid therapeutic option for neuroendocrine tumors (NETs); however, data in a real-world setting outside regulatory trials are sparse. The aim of this study was to determine everolimus tolerability and efficacy, in relation to previous treatments, in a compassionate use program. A total of 169 patients with advanced progressive NETs treated with everolimus were enrolled, including 85 with pancreatic NETs (pNETs) and 84 with nonpancreatic NETs (non-pNETs). Previous treatments included somatostatin analogs (92.9%), peptide receptor radionuclide therapy (PRRT; 50.3%), chemotherapy (49.7%), and PRRT and chemotherapy (22.8%). Overall, 85.2% of patients experienced adverse events (AEs), which were severe (grade 3–4) in 46.1%. The most frequent severe AEs were pneumonitis (8.3%), thrombocytopenia (7.7%), anemia (5.3%), and renal failure (3.5%). In patients previously treated with PRRT and chemotherapy, a 12-fold increased risk for severe toxicity was observed, with grade 3–4 AEs reported in 86.8% (vs. 34.3% in other patients). In addition, 63.3% of patients required temporarily everolimus discontinuation due to toxicity. Overall, 27.8% of patients died during a median follow-up of 12 months. Median progression-free survival (PFS) and overall survival (OS) were 12 months and 32 months, respectively. Similar disease control rates, PFS, and OS were reported in pNETs and non-pNETs. In the real-world setting, everolimus is safe and effective for the treatment of NETs of different origins. Higher severe toxicity occurred in patients previously treated with systemic chemotherapy and PRRT. This finding prompts caution when using this drug in pretreated patients and raises the issue of planning for everolimus before PRRT and chemotherapy in the therapeutic algorithm for advanced NETs.
Implications for Practice:
Data reported outside regulatory trial settings are useful for physicians dealing with neuroendocrine tumors (NETs) and provide understanding of whether the findings obtained in those trials are consistent with clinical practice. In this real-world study of everolimus in advanced, progressive NETs, significantly higher severe toxicity was observed in patients with long-duration disease and in those previously treated with systemic chemotherapy and/or peptide receptor radionuclide therapy. These findings may help physicians to plan an optimal therapeutic strategy for these patients to avoid predictable severe toxicity that may also result in limitations for further treatments.
Introduction
In recent decades, several national cancer registries have reported significant increases in neuroendocrine tumor (NET) incidence [1]. Tumor behavior, and thus patient survival, depends on a number of factors, including primary site, tumor histology, proliferative index Ki-67, and staging [2–6].
The therapeutic approach of these diseases has changed dramatically due to the novel targeted therapies with the mammalian target of rapamycin inhibitor everolimus and the multitarget tyrosine kinase inhibitor sunitinib, which have been approved for advanced progressive pancreatic NETs (pNETs) [7, 8]. In randomized controlled trials, everolimus obtained a 65% decrease in the risk for tumor progression in pNETs [7] and a 23% decrease in patients with nonpancreatic NETs (non-pNETs) [9]. In addition, promising preclinical findings suggested that everolimus might be effective in aggressive endocrine neoplasms [10–13].
Data on NET therapeutic sequence and impact of previous treatments on everolimus tolerability and efficacy are lacking. Consequently, it is not clear where everolimus should be placed in the treatment algorithm for advanced NETs. Furthermore, despite promising findings from the cited trials [7, 9], very few data concern everolimus in NET patients in the real-world setting. The aim of this study was to determine everolimus tolerability and efficacy, in relation to previous treatments, in a real-world clinical setting of a compassionate use program (CUP).
Patients and Methods
Study Design
This study is a retrospective analysis of all consecutive patients with NETs included in a CUP organized by the drug manufacturer (Novartis International, Basel, Switzerland, http://www.novartis.com) that was open for participation from August 2008 to September 2012. The drug was given for free to the patients on appropriate request from each participant center. A total of 19 centers participated in this study.
Patients
Inclusion criteria were aged >18 years, histologically proven diagnosis of well or moderately differentiated NETs, disease progression (DP) documented by radiological examinations after failure of previous medical treatment, unresectable or metastatic disease, Eastern Cooperative Oncology Group performance status (PS) ≤2. Key exclusion criteria were impaired cardiac function, severe liver or renal disease, inadequate bone marrow reserve, uncontrolled diabetes, uncontrolled hypercholesterolemia or hypertriglyceridemia, presence of familial syndromes (multiple endocrine neoplasia type I, von Hippel-Lindau), or life expectancy <3 months. All patients provided full informed consent before starting everolimus treatment. The program was approved by the local ethics committee of each participating center.
Methods
Everolimus starting dose was 10 mg daily; the investigator had the option of starting at or reducing the dose to 5 mg daily depending on the patient’s baseline clinical status and tolerability. Treatment was continued until DP or intolerable toxicity occurred or if the patient withdrew informed consent. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events v. 3.0 [14].
Although this study had a retrospective design (i.e., the follow-up program was not fully standardized), computed tomography or magnetic resonance imaging assessments were performed at baseline and, usually, every 3 months after starting everolimus and were evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST 1.0) [15].
All data were prospectively collected at the center where the patient was treated. A unique computerized data sheet was created, and data were analyzed retrospectively.
Gastroenteropancreatic (GEP) NETs were classified accordingly to the World Health Organization 2010 classification [16].
Efficacy was analyzed by evaluating progression-free survival (PFS), overall survival (OS), and best overall response, which was defined as the best radiological tumor response from start of treatment until discontinuation. Patients were considered “responders” to treatment when disease control (DC) was achieved, in terms of stable disease, partial response, or complete response; otherwise, they were considered “nonresponders.” PFS was defined as the interval between beginning of treatment with everolimus and DP time or patient death from any cause if it occurred before documented DP. OS was defined as the interval between beginning of treatment and date of death from any cause. PFS and OS analysis were assessed using the Kaplan-Meier method, and the results were compared by log-rank test. Death was considered “during treatment” if it occurred while the patient was taking everolimus or in the 4-week period following the last dose administration if the drug had been already discontinued. Risk factors were expressed as hazard ratio (95% confidence interval). Logistic regression was used to identify possible predictors of severe toxicity. The multivariate model was constructed by enter method. Receiver operating characteristic (ROC) curve analysis was used to identify the cutoff level for Ki-67 as a predictor of response to everolimus (area under the curve: 0.624; p = .038 using nonresponders to treatment as a classification variable). The distribution of continuous variables was reported as median and interquartile range (IQR; 25th to 75th percentiles) or median and range, as specified. The comparison between subgroups was carried out using Fisher’s exact test, χ2 test, or Mann-Whitney U test for continuous variables. A p value <.05 was considered significant. Statistical analysis was performed using Medcalc v.12 (MedCalc Software, Ostend, Belgium, http://www.medcalc.be).
Results
A total of 169 patients were enrolled in this study, including 85 with pNETs (50.3%) and 84 with non-pNETs (49.7%) (Table 1). Most frequent non-pNETs were jejunum-ileum tumors (31 patients, 18.3%) and lung tumors (22 patients, 13%). Other primary sites were reported in 18 patients (10.7%), whereas 13 patients (7.7%) had metastases from an unknown primary tumor. A total of 122 patients (72.2%) had GEP NETs. Of these, 27 (22.1%) had NET-G1 (13 pNETs and 14 non-pNETs), 88 (72.1%) had NET-G2 (66 pNETs and 22 non-pNETs), and the remaining 7 patients (5.8%) had G3 neuroendocrine carcinomas (NECs) (6 pNETs and 1 non-pNET). Overall, 156 patients (92.3%) had distant metastatic disease, whereas the remaining 13 patients (7.7%) had locally advanced unresectable disease. Performance status at beginning of treatment (baseline PS) was 0 in 91 patients (53.9%), 1 in 68 patients (40.2%), and 2 in the remaining 10 patients (5.9%).
Table 1.
General features of 169 enrolled patients
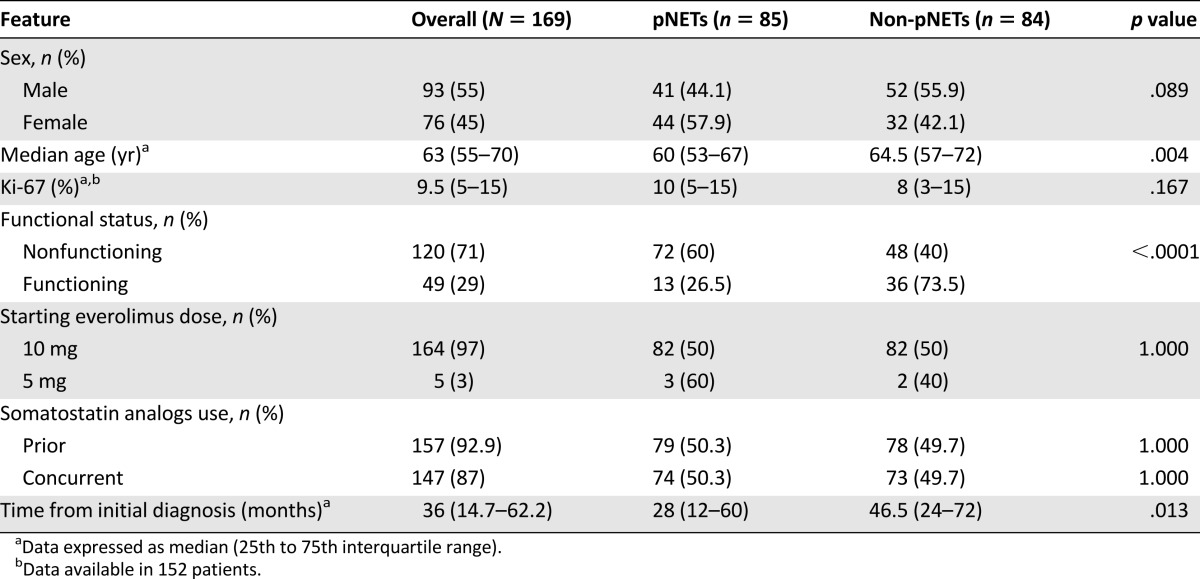
A total of 164 patients (97%) started everolimus treatment at a 10-mg daily dose, whereas the starting dose was 5 mg daily for the remaining 5 patients (3%). Everolimus treatment was associated with somatostatin analogs (SSAs) in 147 patients (87%; octreotide long-acting release in 125 patients, lanreotide autogel in 22 patients). Of these, 102 patients (60.3%) had nonfunctioning tumor. Conversely, in the remaining 22 patients (13%), it was given as a single therapy. Median duration of treatment was 6 months (range: 1–46 months). Specifically, 65 patients (38.5%) received everolimus for <6 months, whereas 54 patients (32%) were treated for 6–11 months, and the remaining 50 patients (29.5%) were treated for ≥12 months.
The most frequent treatments received before starting everolimus were SSAs (n = 157, 92.9%), peptide receptor radionuclide therapy (PRRT; n = 85, 50.3%), systemic chemotherapy (n = 84, 49.7%), and interferon (n = 18, 10.6%). With regard to patients pretreated with PRRT, 44 (51.8%) received therapy based on yttrium 90 (90Y), 22 patients (25.9%) received therapy based on lutetium 177 (177Lu), and 19 patients (22.3%) received a combination of both. Median cumulative dose was 524 mCi (25th–75th IQR: 337–700 mCi). Median interval of time between end of PRRT and everolimus-treatment initiation was 8 months (25th–75th IQR: 3–21 months). Among patients pretreated with systemic chemotherapy, the most frequent regimens were etoposide plus cisplatin in 29 patients (34.5%), 5-fluorouracil-based therapy in 29 patients (34.5%), and gemcitabine-based therapy in 12 patients (14.3%). The median interval between end of chemotherapy and everolimus initiation was 8 months (25th–75th IQR: 3–13 months). Fourteen patients (8.3%) received two or more therapeutic lines with systemic chemotherapy before starting everolimus. A total of 38 patients (22.5%) were previously treated with both chemotherapy (platinum-based in 20 patients) and PRRT (90Y based in 24 patients, 177Lu based in 11 patients, and a combination of both in the remaining 3 patients). In this group of patients, baseline PS was 0 in 20 patients (52.6%), 1 in 16 patients (42.1%), and 2 in the remaining 2 patients (5.3%).
Tolerability
Overall, 103 patients (60.9%) underwent definitive everolimus discontinuation. The primary reasons for discontinuation included DP (n = 85, 50.3%), toxicity (n = 15, 8.9%), and consent withdrawal (n = 3, 1.7%). All 15 patients who discontinued treatment for toxicity received everolimus for <12 months (of these, 12 patients received everolimus for <6 months).
A total of 107 patients (63.3%) required temporary treatment discontinuation due to toxicity. Of these, 42 patients (24.8%) required two or more discontinuations. Twenty-eight patients (16.6%) did not tolerate the standard dose of 10 mg/day and required everolimus-dose reduction to 5 mg/day.
Overall, 144 patients (85.2%) experienced AEs, which were severe (grade 3–4) in 78 subjects (46.1%). In this last group of patients, similar baseline PS was observed in comparison with those patients who did not have severe toxicity; PS was 0 in 50% versus 57.1%, 1 in 42.3% versus 38.5%, and 2 in 7.7% versus 4.4%, respectively (p = .515). Adverse event features and severity are detailed in Table 2.
Table 2.
Adverse events observed during everolimus treatment in ≥10% of patients

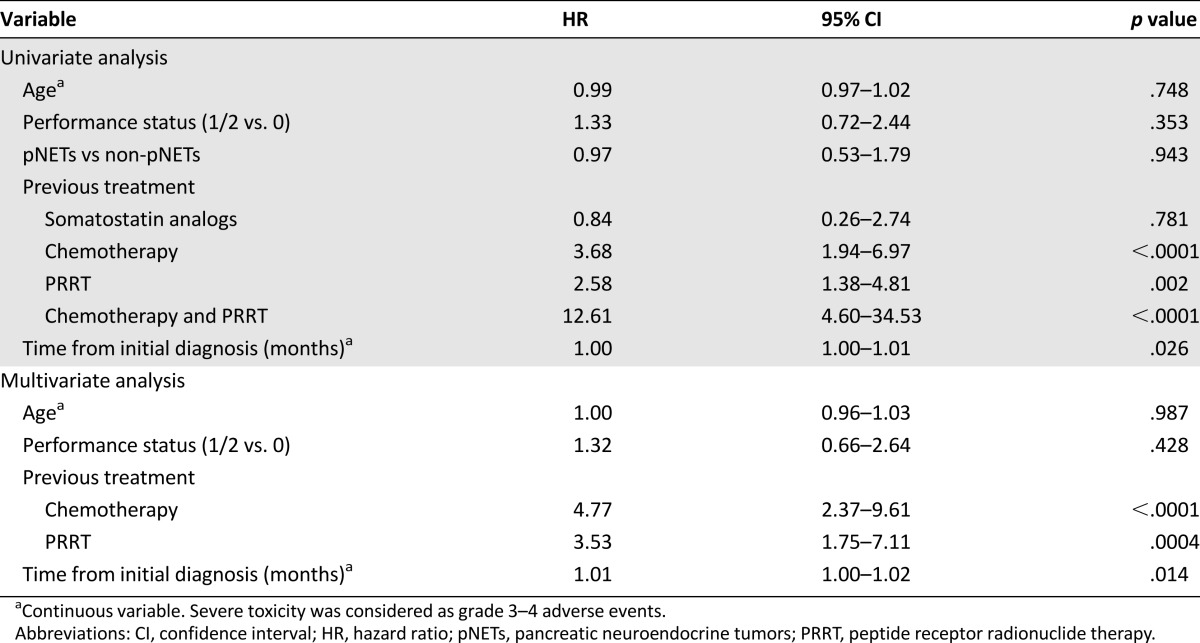
A significantly higher proportion of patients previously treated with both PRRT and chemotherapy before starting everolimus experienced severe toxicity during treatment; grade 3–4 AEs occurred in 86.8% (33 of 38 patients) versus 34.3% of the other patients (45 of 131) (p < .0001). The most frequent grade 3–4 AEs in this group of patients were hematological toxicity (11 of 38 patients, 28.9%), renal failure (5 of 38 patients, 13.2%), peripheral edema (5 of 38 patients, 13.2%), pneumonitis (3 of 38 patients, 7.9%), and mucositis (3 of 38 patients, 7.9%).
Previous treatment with either systemic chemotherapy or PRRT and interval of time between initial NET diagnosis and beginning of everolimus were independent significant predictors for grade 3–4 AEs. A further increased hazard ratio was observed when the combination of the two treatments was considered as a predictor for severe toxicity, with the hazard ratio being 12.61 (p < .0001) (Table 3).
Table 3.
Predictors for severe toxicity during everolimus treatment
Efficacy
A total of 128 patients (75.7%) demonstrated response to everolimus, with similar DC rates in pNETs and non-pNETs (77.6% vs. 73.8%, respectively; p = .422). Best overall response was stable disease in 114 patients (67.5%), partial response in 13 patients (7.7%), and complete response in 1 patient (0.5%). The remaining 41 patients (24.2%) did not respond to everolimus, and showed DP as best overall response. By ROC analysis, the Ki-67 value of 12% was identified as a better cutoff level to discriminate between responders and nonresponders. In fact, DC was observed in 57.4% and 84.6% of patients with Ki-67 >12% and ≤12%, respectively (p = .0007).
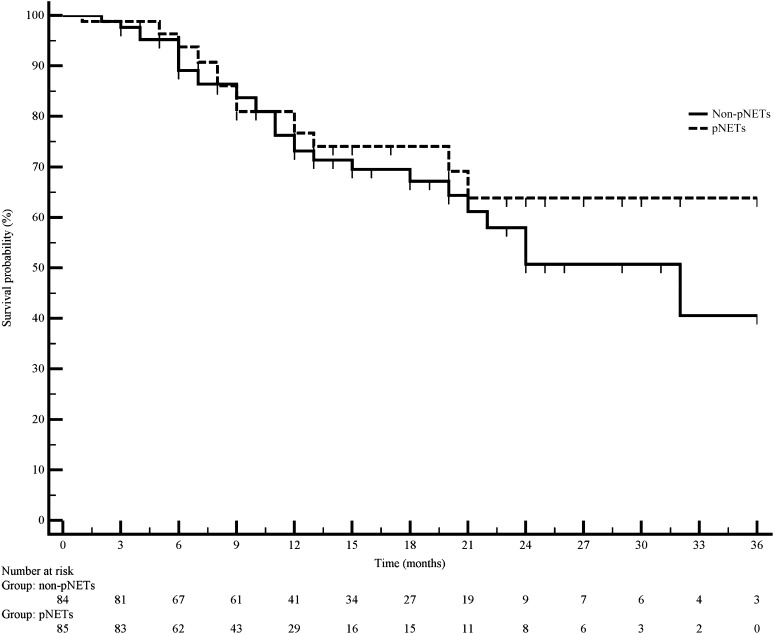
Median PFS was 12 months. Similar findings were observed in pNETs and non-pNETs, with median PFS being 11 months and 12 months, respectively (p = .789) (Fig. 1).
Figure 1.
Progression-free survival in pNETs and non-pNETs. p = .789.
Abbreviation: pNET, pancreatic neuroendocrine tumor.
During a median follow-up period of 12 months (25th–75th IQR: 7–18.2 months), a total of 47 patients died at a median interval of 9 months (25th–75th IQR: 6–12.7 months) from the beginning of treatment, resulting in a mortality rate of 27.8%. Seventeen deaths (10%) occurred during everolimus treatment (none directly related to the study drug). Of these, 15 deaths were attributable to DP, whereas the remaining 2 were a consequence of pneumonitis occurring during everolimus therapy. The first patient was a 55-year-old male who had metastatic pNET and had not responded previously to chemotherapy (etoposide plus cisplatin) or to PRRT (90Y based), which was discontinued 4 months before starting everolimus. This patient suffered from moderate chronic renal failure (glomerular filtration rate: 55 mL/min), and white blood cell count was at lower-normal limit. The second patient was an 80-year-old man with metastatic, small-bowel NET with carcinoid syndrome, again, pretreated with PRRT (90Y based).
Median OS was 32 months, with similar findings between pNETs and non-pNETs (median survival: not reached and 32 months, respectively) (Fig. 2). The 2-year specific survival rate was 55.5%.
Figure 2.
Overall survival in pNETs and non-pNETs. p = .381.
Abbreviation: pNET, pancreatic neuroendocrine tumor.
Significantly different OS was observed for patients who had or did not have DC during everolimus treatment; median specific survival was not reached and was 12 months in responders and nonresponders, respectively (2-yr survival rate was 63.9% and 26%, respectively; p < .0001) (Fig. 3). A significantly higher proportion of nonresponders died during follow-up in comparison to treatment responders (21 of 41, 51.2% vs. 26 of 128, 20.3%; p = .0002).
Figure 3.
Overall survival in patients who achieved disease control (responders) vs. patients who did not achieve disease control (nonresponders) during everolimus treatment. Disease control is defined as stable disease plus partial response plus complete response. p < .0001.
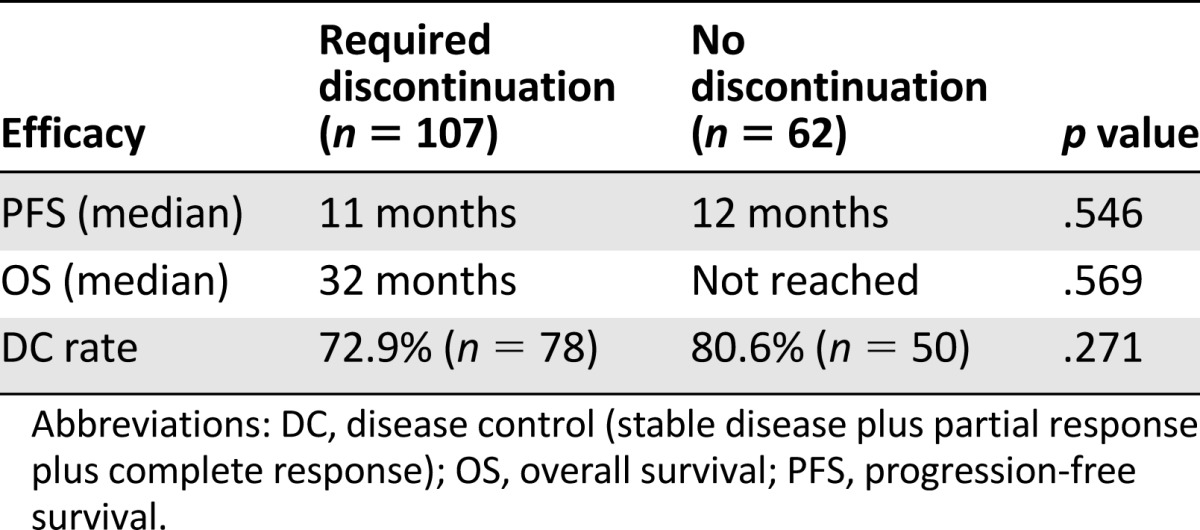
No differences were observed in terms of PFS, OS, and DC rates in patients who required everolimus temporary discontinuation due to toxicity compared with those who did not need drug discontinuation (Table 4).
Table 4.
Everolimus efficacy in patients who required temporary drug discontinuation due to toxicity
Discussion
Current data report tolerability and efficacy of everolimus for the treatment of advanced, progressive NETs in the real-world setting of a CUP. Data reported outside multicenter phase III trial settings are needed to understand whether the findings obtained in those trials are consistent with clinical practice, in which the experimental design criteria needed for registration purposes are not always used [17].
One of the findings of this study is the significant 12-fold increased risk for severe toxicity during everolimus treatment in patients who had been treated previously with both chemotherapy and PRRT. Data on everolimus tolerability in this group of pretreated patients are lacking. As far as patients previously treated with chemotherapy are concerned, data may be obtained from the phase II trial performed in advanced pNETs [18] and from the subanalysis of the group of patients enrolled in RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) [19]. In the last study, 50% of patients had been pretreated with chemotherapy with similar efficacy and a similar safety profile to those patients who had not received chemotherapy before beginning everolimus [19]. Similarly, 66% of patients treated with sunitinib in the phase III study [8] had been treated with chemotherapy. Conversely, a lower proportion of patients enrolled in either the RADIANT-3 trial or the sunitinib trial had received prior treatment with somatostatin analogs, in comparison with the present study (49% and 35%, respectively, vs. 92.9%).
With regard to patients previously treated with PRRT, a single retrospective analysis has been published recently on 24 progressive NETs after 177Lu-octreotate [20]. Although an acceptable safety profile is reported, higher proportions of severe thrombocytopenia, hyperglycemia, and fatigue were observed in comparison with the RADIANT trials [7, 9]. Furthermore, only 29.1% of those patients had been pretreated with chemotherapy in comparison to the RADIANT-3 trial (46.1%) [7] and to this study (49.7%). Finally, a longer interval of time between PRRT and everolimus treatment has been reported in comparison with the present study (18 months and 8 months, respectively). Consequently, a reliable comparison between the paper by Kamp et al. [20] and other studies is not feasible.
The tolerability reported in this study is similar to that observed in phase III RADIANT trials [7, 9], with severe toxicity occurring in up to 8% of patients; however, some differences need to be taken into account when comparing specific safety profiles observed in these studies. In fact, some very common AEs observed in patients enrolled in the RADIANT trials, usually as grade 1–2 toxicity (e.g., stomatitis, rash, diarrhea), were more rarely reported in this study. These differences may be related to several factors, including the different, lower awareness of physicians who participated in the CUP of reporting mild toxicities, as well as to the retrospective approach used in this study. On the contrary, the higher number of thrombocytopenia, renal failure, and severe pneumonitis events reported in this study are likely to be related to the pretreatment administered before everolimus (PRRT in 50.3% of patients, chemotherapy in 49.7%, both treatments in 22.8%).
The detailed analysis of patients previously treated with PRRT and chemotherapy showed that 86.8% experienced grade 3–4 AEs, which mainly affected bone marrow and kidney (28.9% and 13.2% of patients, respectively); as is well known, these are considered possible limiting organs for such systemic treatments [21]. Interestingly, renal toxicity was not recorded in previous trials [7, 9, 18, 22] but was observed as grade 3–4 toxicity in 4.2% of patients in the study by Kamp et al. [20]. This finding supports the hypothesis that a higher risk for AEs, also concerning renal function, exists in patients pretreated with PRRT.
Conversely, a very good safety profile was reported in a recent experience on everolimus in combination with long-acting octreotide as first-line therapy. Data on 50 naïve advanced NETs showed a single grade 4 AE in 1 patient and no report of grade 3–4 thrombocytopenia or pneumonitis [23].
Based on these considerations, we can assume that the risk of severe toxicity with everolimus varies in respect to previous treatments. This observation not only prompts the use of particular caution when planning this therapy in heavily pretreated patients but also suggests that everolimus might be better placed before chemotherapy and PRRT in the therapeutic sequence of advanced NETs.
As far as efficacy is concerned, in this study, similar PFS and DC rates were observed in pNETs and non-pNETs. Specifically, PFS values were consistent with those reported in the RADIANT trials, being 11 months in pNETs (equivalent to that reported in RADIANT-3 trial) [7] and 12 months in non-pNETs (16 months and 11 months by central and local review, respectively, in the RADIANT-2 trial) [9]. DC was achieved in 77.6% of pNETs (vs. 70.2% in the RADIANT-3 trial) [7] and 73.8% of non-pNETs (vs. 77% in the RADIANT-2 trial) [9]. These data confirm that everolimus treatment can improve the clinical outcome of advanced, progressive NETs with different primaries. As far as survival is concerned, similar rates were observed between pNETs and non-pNETs. This finding seems to disagree with a number of previous observations that have considered pNETs as more aggressive in comparison to other primary sites [2, 24]. This discrepancy may be due to different factors (e.g., higher median age, longer interval between initial NET diagnosis and everolimus treatment) in non-pNETs compared with pNETs (Table 1) and the heterogeneity of primary tumors in the group of non-pNETs. In fact, in the present study, there is a relatively high proportion of lung and unknown primary tumors, which, as is well known, are related to worse survival compared with small bowel primary tumors, which account for only 18.3% of patients in this series. This confirms that caution should be used when comparing different populations of NETs other than pancreatic because heterogeneous diseases with different prognosis may coexist in this subgroup. Furthermore, as recently shown, in NET patients with advanced, progressive disease, additional factors other than the primary site (e.g., Ki-67 and specific metastatic dissemination) affect patient survival [25].
As far as the relationship between response to treatment and survival is concerned, significantly better survival was observed in patients who achieved DC (responders) compared with those patients who did not reach DC (nonresponders) (Fig. 3). In fact, data on the possible effect of everolimus on survival in NETs are difficult to analyze. Due to the study design and the possibility of crossing over from the treatment arm to the placebo arm in both RADIANT trials [7, 9], the survival results could not be assessed [26, 27]. The better survival observed in responders to everolimus suggests a possible positive impact of treatment on patients’ survival. Further prospective studies with longer follow-up, specifically designed to investigate survival as a primary end point in NETs treated with everolimus, are necessary to verify this observation.
As an additional novel finding, we observed that a significantly higher proportion of patients with Ki-67 ≤12% responded to everolimus compared with patients with Ki-67 >12% (p = .0007). Data on the correlation between Ki-67 value and response to medical treatment are scarce. Other studies have reported better response to SSAs and PRRT in patients with Ki-67 <5% [28, 29], whereas in the Nordic study, a higher response rate to systemic chemotherapy in NEC-G3 when Ki-67 was >55% [30] was observed; however, no data have been reported in patients treated with everolimus to date.
When data on everolimus efficacy were analyzed in relationship to the need for temporary discontinuation due to toxicity, no differences were observed in PFS, OS, or DC rates in the different subgroups of patients (Table 4), showing that the treatment remains effective even in patients with lower drug tolerance.
Conclusion
This study suggests that in a real-world setting, everolimus is a safe and effective treatment for advanced, progressive NETs, with similar efficacy in pNETs and non-pNETs. Better response to therapy can be expected in tumors with Ki-67 ≤12%. Significantly higher severe toxicity was observed in patients with long-duration disease and in those previously treated with systemic chemotherapy and/or PRRT. These findings prompt caution when using this drug in such patients and raise the issue of planning for the use of everolimus before PRRT and chemotherapy in the therapeutic algorithm for advanced NETs to avoid predictable severe toxicity that may also result in limitations for further treatments.
Acknowledgments
Maria Rinzivillo and Nicola Fazio contributed equally. This work was supported by the Italian Association for Neuroendocrine Tumors (It.A.Net), Associazione Italiana per la Ricerca sul Cancro (AIRC), Sapienza University of Rome (Grant C26A12WBRB), and the Italian Ministry of Education, Research and University (FIRB RBAP11884M, RBAP1153LS, 2010TYCL9B_002) Sant'Andrea ONLUS.
Footnotes
For Further Reading:Daniel Castellano, Emilio Bajetta, Ashok Panneerselvam et al. Everolimus Plus Octreotide Long-Acting Repeatable in Patients With Colorectal Neuroendocrine Tumors: A Subgroup Analysis of the Phase III RADIANT-2 Study. The Oncologist 2013;18:46–53.
Implications for Practice:The incidence of neuroendocrine tumors (NETs) originating in the colon or rectum is increasing, and patients diagnosed with these tumors have a poor prognosis. The RADIANT-2 study explored the efficacy of the mammalian target of rapamycin inhibitor everolimus used in combination with the somatostatin analog octreotide long-acting repeatable (LAR) in patients with NETs and symptoms of carcinoid syndrome. The comparison population received placebo plus octreotide LAR. This article reports the results of subanalyses of a group of patients who had primary colorectal NETs. Patients with colorectal NETs who received everolimus plus octreotide LAR had a significantly longer median survival without disease progression (progression-free survival) of 29.9 months (n = 19) compared with those who received placebo plus octreotide LAR (6.6 months; n = 20). Although only a small subset of patients enrolled in the RADIANT-2 study had colorectal NETs, these findings support additional everolimus plus octreotide LAR studies in these patients.
Author Contributions
Conception/Design: Francesco Panzuto, Maria Rinzivillo, Gianfranco Delle Fave
Provision of study material or patients: Francesco Panzuto, Maria Rinzivillo, Nicola Fazio, Filippo de Braud, Gabriele Luppi, Maria Chiara Zatelli, Francesca Lugli, Paola Tomassetti, Ferdinando Riccardi, Carmen Nuzzo, Maria Pia Brizzi, Antongiulio Faggiano, Alberto Zaniboni, Elisabetta Nobili, Davide Pastorelli, Stefano Cascinu, Marco Merlano, Silvana Chiara, Lorenzo Antonuzzo, Chiara Funaioli, Francesca Spada, Sara Pusceddu, Annalisa Fontana, Maria Rosaria Ambrosio, Alessandra Cassano, Davide Campana, Giacomo Cartenì, Marialuisa Appetecchia, Alfredo Berruti, Annamaria Colao, Massimo Falconi, Gianfranco Delle Fave
Collection and/or assembly of data: Francesco Panzuto, Maria Rinzivillo, Nicola Fazio, Filippo de Braud, Gabriele Luppi, Maria Chiara Zatelli, Francesca Lugli, Paola Tomassetti, Ferdinando Riccardi, Carmen Nuzzo, Maria Pia Brizzi, Antongiulio Faggiano, Alberto Zaniboni, Elisabetta Nobili, Davide Pastorelli, Stefano Cascinu, Marco Merlano, Silvana Chiara, Lorenzo Antonuzzo, Chiara Funaioli, Francesca Spada, Sara Pusceddu, Annalisa Fontana, Maria Rosaria Ambrosio, Alessandra Cassano, Davide Campana, Giacomo Cartenì, Marialuisa Appetecchia, Alfredo Berruti, Annamaria Colao, Massimo Falconi, Gianfranco Delle Fave
Data analysis and interpretation: Francesco Panzuto, Maria Rinzivillo, Massimo Falconi, Gianfranco Delle Fave
Manuscript writing: Francesco Panzuto, Massimo Falconi, Gianfranco Delle Fave
Final approval of manuscript: Francesco Panzuto, Maria Rinzivillo, Nicola Fazio, Filippo de Braud, Gabriele Luppi, Maria Chiara Zatelli, Francesca Lugli, Paola Tomassetti, Ferdinando Riccardi, Carmen Nuzzo, Maria Pia Brizzi, Antongiulio Faggiano, Alberto Zaniboni, Elisabetta Nobili, Davide Pastorelli, Stefano Cascinu, Marco Merlano, Silvana Chiara, Lorenzo Antonuzzo, Chiara Funaioli, Francesca Spada, Sara Pusceddu, Annalisa Fontana, Maria Rosaria Ambrosio, Alessandra Cassano, Davide Campana, Giacomo Cartenì, Marialuisa Appetecchia, Alfredo Berruti, Annamaria Colao, Massimo Falconi, Gianfranco Delle Fave
Disclosures
Gianfranco Delle Fave: Novartis (RF, paid to institution); Francesco Panzuto: Novartis (RF, paid to institution); Maria Rinzivillo: Novartis (RF, paid to institution); Nicola Fazio: Novartis, Ipsen (C/A); Filippo de Braud: Novartis (C/A, RF); Antongiulio Faggiano: Ipsen, Italfarmaco (C/A, RF); Novartis (RF, paid to institution); Stefano Cascinu: Celgene, Novartis (H); Celgene (ET); Francesca Spada: Novartis, Ipsen (C/A); Annamaria Colao: Novartis (RF, paid to institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Fraenkel M, Kim MK, Faggiano A, et al. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 3.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: Tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–3341. doi: 10.1002/cncr.25855. [DOI] [PubMed] [Google Scholar]
- 4.Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: Analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–2377. doi: 10.1200/JCO.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- 5.Panzuto F, Campana D, Fazio N, et al. Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology. 2012;96:32–40. doi: 10.1159/000334038. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 9.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 10.Zatelli MC, Minoia M, Filieri C, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2010;95:968–976. doi: 10.1210/jc.2009-1641. [DOI] [PubMed] [Google Scholar]
- 11.Zatelli MC, Minoia M, Martini C, et al. Everolimus as a new potential antiproliferative agent in aggressive human bronchial carcinoids. Endocr Relat Cancer. 2010;17:719–729. doi: 10.1677/ERC-10-0097. [DOI] [PubMed] [Google Scholar]
- 12.Bollard J, Couderc C, Blanc M, et al. Antitumor effect of everolimus in preclinical models of high-grade gastroenteropancreatic neuroendocrine carcinomas. Neuroendocrinology. 2013;97:331–340. doi: 10.1159/000347063. [DOI] [PubMed] [Google Scholar]
- 13.Gagliano T, Bellio M, Gentilin E, et al. mTOR, p70S6K, AKT, and ERK1/2 levels predict sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial carcinoids. Endocr Relat Cancer. 2013;20:463–475. doi: 10.1530/ERC-13-0042. [DOI] [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed December 31, 2013.
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 17.Lauer MS, D’Agostino RB., Sr The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–1581. doi: 10.1056/NEJMp1310102. [DOI] [PubMed] [Google Scholar]
- 18.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombard-Bohas C, Yao JC, Hobday TJ, et al. Efficacy and safety of everolimus in patients with advanced low- or intermediate-grade pancreatic neuroendocrine tumors previously treated with chemotherapy: Radiant-3 subgroup analysis. J Clin Oncol. 2012;30(suppl 34):224a. [Google Scholar]
- 20.Kamp K, Gumz B, Feelders RA, et al. Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after (177)Lu-octreotate. Endocr Relat Cancer. 2013;20:825–831. doi: 10.1530/ERC-13-0254. [DOI] [PubMed] [Google Scholar]
- 21.Bergsma H, van Vliet EI, Teunissen JJ, et al. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26:867–881. doi: 10.1016/j.bpg.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajetta E, Catena L, Fazio N, et al. doi: 10.1002/cncr.28726. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: An ITMO group study. Cancer 2014 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 25.Panzuto F, Merola E, Rinzivillo M, et al. Advanced digestive neuroendocrine tumors: Metastatic pattern is an independent factor affecting clinical outcome. Pancreas. 2014;43:212–218. doi: 10.1097/MPA.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RT, Delle Fave G. Promising advances in the treatment of malignant pancreatic endocrine tumors. N Engl J Med. 2011;364:564–565. doi: 10.1056/NEJMe1013903. [DOI] [PubMed] [Google Scholar]
- 27.Yao JC, Lagunes DR, Kulke MH. Targeted therapies in neuroendocrine tumors (NET): Clinical trial challenges and lessons learned. The Oncologist. 2013;18:525–532. doi: 10.1634/theoncologist.2012-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezziddin S, Opitz M, Attassi M, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38:459–466. doi: 10.1007/s00259-010-1610-2. [DOI] [PubMed] [Google Scholar]
- 29.Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol. 2013;25:232–238. doi: 10.1097/MEG.0b013e328359d1a6. [DOI] [PubMed] [Google Scholar]
- 30.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]