Abstract
Background.
The combination of gemcitabine plus capecitabine and sunitinib (GCS) shows activity in metastatic renal cell carcinoma (mRCC). We tested the multitargeted “chemo-switch” regimen as first-line treatment in patients with mRCC.
Methods.
We assessed the maximum tolerated dose and antitumor activity of GCS in treatment-naïve, advanced mRCC patients. Treatment consisted of intravenous gemcitabine on days 1 and 8, oral capecitabine twice daily on days 1–14, and oral sunitinib daily for six 21-day cycles, followed by sunitinib monotherapy at the investigator’s discretion. Dose level 0 (DL0) was gemcitabine 1,000 mg/m2 per day plus capecitabine 650 mg/m2 per 12 hours plus sunitinib 37.5 mg/day; DL1 was gemcitabine 1,000 mg/m2 per day plus capecitabine 850 mg/m2 per 12 hours plus sunitinib 37.5 mg/day.
Results.
Sixteen patients were enrolled. At DL1, two of four patients had dose-limiting toxicity (DLT; grade 3 diarrhea and grade 4 thrombocytopenia). The dose was reduced to DL0 when only 1 of 12 patients experienced DLT (grade 3 diarrhea, grade 3 mucositis, and grade 3 thrombocytopenia). Dose reductions were frequent (58% of patients), and only seven patients were able to receive the three drugs for more than three cycles. One patient achieved a complete response, three had partial responses, and the best response for four was stable disease.
Conclusion.
The safety profile of the combination does not seem manageable in this patient population. No further development of the combination is recommended.
Author Summary
Discussion
Treatment with agents targeting vascular endothelial growth factor (VEGF) receptors and mammalian target of rapamycin have reached a plateau in terms of median progression-free and overall survival, and several strategies have attempted to improve the outcome.
Pietras et al. developed the “chemo-switch” concept, applying the combination of targeted agents with chemotherapy using the standard maximum tolerated doses (MTDs) in combination with low doses of chemotherapeutic drugs given continuously on a daily basis (metronomic chemotherapy). A synergistic effect has been demonstrated in xenograft mouse models, and we saw promising data in a prior clinical trial [1].
The tyrosine kinase inhibitor sunitinib inhibits several receptor tyrosine kinases, including VEGF and platelet-derived growth factor 2 (PDGF2) [2], and has shown efficacy in patients with metastatic renal cell carcinoma (mRCC), as first- and as second-line treatment [3, 4]. It is believed that the anti-VEGF and the anti-PDFG effects act in a synergistic way with metronomic chemotherapy. Consequently, the triple combination of gemcitabine plus capecitabine and sunitinib was tested in our phase I study as first-line therapy in advanced RCC or mRCC patients to determine the MTD, to assess safety, and to observe preliminary efficacy results [5].
Three patients showed dose-limiting toxicity (DLT): two at dose level 1 (DL1; grade 4 thrombocytopenia in one patient and grade 3 diarrhea and thrombocytopenia in the other) and one at DL0 (grade 3 mucositis and thrombocytopenia). One grade 5 cardiovascular toxicity unrelated to trial medication was seen. Eight patients suffered serious adverse events (SAEs) (Table 1). The study treatment-related causes for hospitalization in the other SAE-presenting patients were thrombocytopenia, neutropenia, mucositis, hypertensive crisis, asthenia, and anorexia. In addition, other AEs not related to the study medication caused hospitalization or prolonged hospitalization. Disease progression was reported as the cause of hospitalization in three patients, and it was not considered related to the study medication in any case.
Table 1.
Patients with severe adverse events
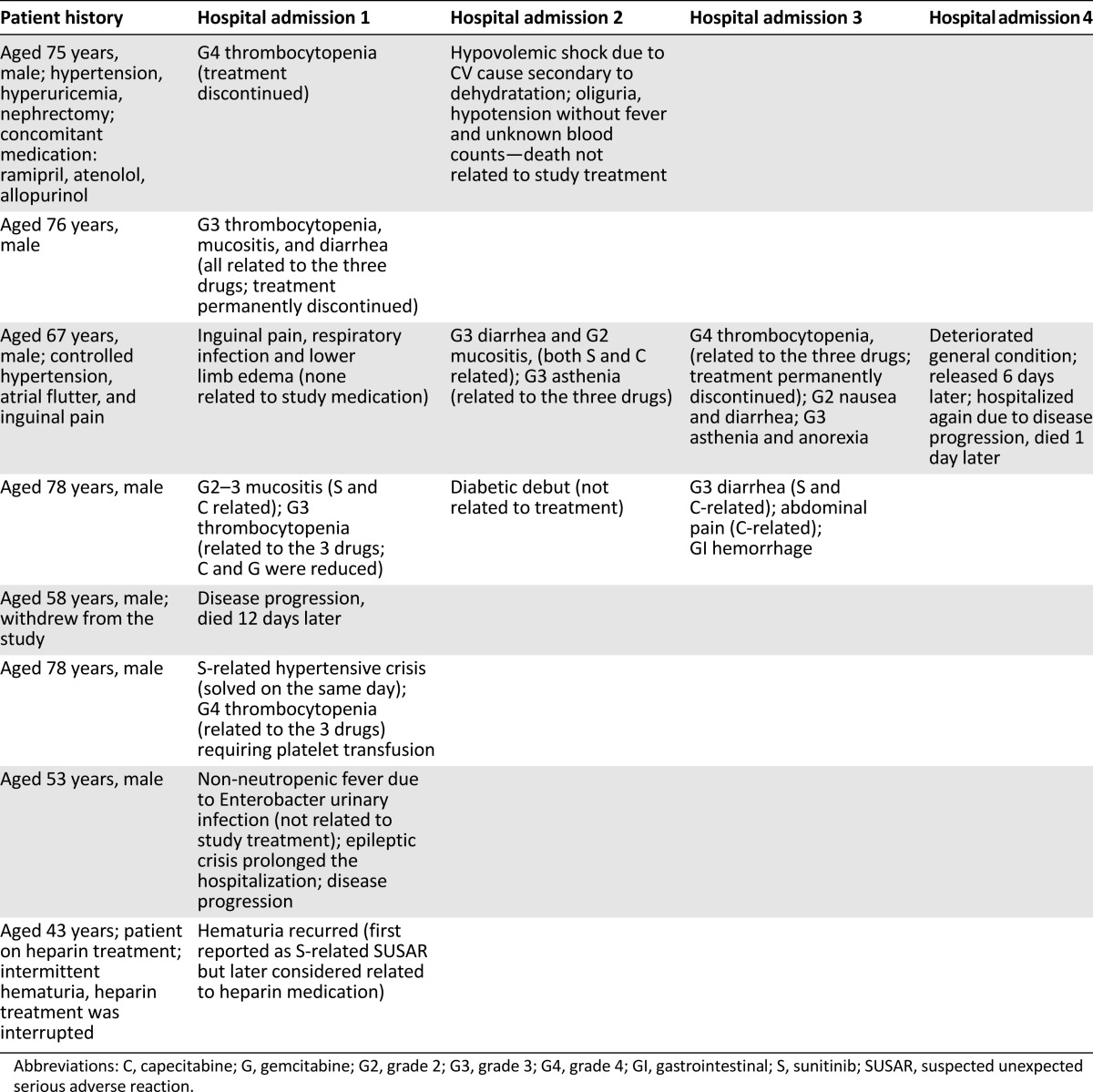
The regimen was poorly tolerated by patients, and more than half experienced asthenia, neutropenia, thrombocytopenia, mucositis, diarrhea, or nausea. The combination of gemcitabine plus capecitabine alone in RCC patients showed a high incidence of grade 3–4 neutropenia and persistent grade 1–2 fatigue and gastrointestinal toxicities in addition to hand-foot syndrome events that would generally be unacceptable by a large percentage of patients [6]. Although hand-foot syndrome is also a common adverse event of sunitinib [7, 8], the incidence of this event in our study was low (four events in three patients) compared with that observed in the study using gemcitabine plus capecitabine alone [6], probably due to the lower dose of capecitabine in the current study.
Although data on efficacy are limited, the triple combination does not seem to be better than sunitinib alone as first-line treatment [4, 8] and as second-line treatment [3, 9].
The use of sunitinib in combination with gemcitabine at MTDs followed by capecitabine administered in a metronomic way, exploring the chemo-switch concept, did not prove feasible because of toxicity and premature treatment discontinuation. This triple combination cannot be recommended for further study.
Supplementary Material
Acknowledgment
J.B. is currently affiliated with the Dana-Farber Cancer Institute/Harvard Medical School, Boston, MA.
Footnotes
Access the full results at: Bellmunt-14-72.theoncologist.com
European Clinical Trial Registry Identifier: EudraCT: 2007-001021-10
Sponsor(s): SOGUG (Spanish Oncology Genito Urinary Group) 07-02
Principal Investigator: Joaquim Bellmunt
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


