The purpose of this study was to investigate the extent of pre-exercise participation (“preparticipation”) cardiovascular screening in a heterogeneous cohort of adult cancer patients. The patient risk-stratification profile strongly suggests that the use of formalized preparticipation cardiovascular screening is required in all oncology scenarios, but risk of an exercise-induced event is low, suggesting that the use of exercise testing is not required for pre-exercise clearance in the majority of patients.
Keywords: Exercise tolerance testing, Safety, Pre-exercise clearance, Cancer, Oncology, Adverse events
Abstract
Background.
The purpose of this study was to investigate the extent of pre-exercise participation (“preparticipation”) health screening in a heterogeneous cohort of adult cancer patients.
Methods.
Patients (n = 413) with histologically confirmed solid or hematologic malignancy were categorized into preparticipation health screening risk stratification based on American College Sports Medicine (ACSM) recommendations. Risk of an exercise-related event was evaluated during a symptom-limited cardiopulmonary exercise test (CPET) with 12-lead electrocardiography (ECG).
Results.
Participant risk was categorized as low risk (n = 59, 14%), moderate risk (n = 217, 53%), and high risk (n = 137, 33%). Mean peak oxygen consumption was 21.7 ± 6.7 mL/kg−1 per minute−1 or 19.5 ± 21.7% below age- and sex-predicted sedentary values. No major serious adverse events or fatal events were observed during CPET procedures. A total of 31 positive ECG tests were observed, for an event rate of 8%. ACSM risk stratification did not predict the risk of a positive test. Age, statin use, antiplatelet therapy use, cardiovascular disease, prior treatment with anthracycline or radiation therapy, and being sedentary were predictors of a positive test (all p < .10).
Conclusion.
The patient risk-stratification profile strongly suggests that the use of formalized preparticipation health screening is required in all oncology scenarios; however, risk of an exercise-induced event is low, suggesting that the use of exercise testing is not required for pre-exercise clearance in the majority of patients.
Implications for Practice:
We studied the use of pre-exercise clearance to optimize the safety and efficacy of exercise training in patients with cancer. The majority of patients with solid or hematologic malignancies were classified as moderate or high risk for an exercise-related event, creating a strong rationale for mandatory formalized cardiovascular screening for all oncology patients before exercise participation. Based on the low absolute incidence of events, exercise testing in pre-exercise screening clearance is not required for the majority of cancer patients. We found that widely utilized screening recommendations have suboptimal utility in cancer patients, highlighting the urgent need for the development of oncology-specific criteria.
Introduction
Meta-analyses and systematic reviews conclude that physical activity and structured exercise training can prevent or mitigate a number of physiological and psychosocial sequelae associated with cancer and cancer therapy [1–3]. Based on this evidence, several international agencies have published cancer-specific exercise guidelines for cancer patients both during and following the completion of primary therapy [4–7]. In light of these guidelines, together with strong interest from patients [8], an increasing clinical conundrum facing oncology professionals is how to appropriately screen patients prior to exercise participation.
The risk of an adverse cardiovascular event during light- to moderate-intensity exercise in healthy individuals is low; therefore, the benefits of regular exercise far outweigh the potential risks [9, 10]. Nevertheless, the incidence of serious adverse events (SAEs; life threatening; e.g., myocardial infarction, sudden cardiac death) during structured exercise training in cardiac patients is 10 times that of healthy individuals [11]. Hence, the risk of an exercise-related event is dependent on the extent of underlying concomitant comorbid disease.
Cancer patients are often older, and 30%–80% will either have overt cardiovascular disease (CVD) or be at risk of developing of CVD at the time of cancer diagnosis [12]. In addition, normal age-related pathologies are also compounded by the direct and indirect effects of anticancer therapy [13] Consequently, cancer patients may be at heightened risk of an exercise-related event. Pre-exercise participation (“preparticipation”) health screening guidelines are established for noncancer clinical populations [14]. Most guidelines stratify individuals into low-, moderate-, and high-risk categories based on demographic and medical variables. However, no study to date has formally evaluated the appropriateness of existing preparticipation health screening guidelines in the oncology setting.
Accordingly, the purpose of this study was to evaluate the need and extent of preparticipation health screening in a heterogeneous cohort of adult cancer patients. To inform the development of oncology-specific screening guidelines, a secondary purpose was to explore the demographic and medical predictors of exercise-related events.
Methods
Study Population and Preparticipation Risk Stratification
Patients (n = 413) with histologically confirmed diagnosis of a solid or hematologic malignancy potentially eligible for research studies in the Cardio-Oncology Research Laboratory at Duke University Medical Center (Durham, NC) were studied. Data were retrospectively evaluated in the following research studies: cross-sectional studies evaluating exercise capacity following the completion of primary adjuvant therapy or as a baseline screening tool to assess study eligibility for randomized controlled trials investigating the efficacy of supervised exercise training interventions. Additional inclusion criteria were >18 years or age, no contraindications to a symptom-limited cardiopulmonary exercise test (CPET) [15, 16], and primary oncologist or cardiologist approval.
On the basis of medical chart review, all participants were categorized into preparticipation risk stratification categories based on American College Sports Medicine (ACSM) guidelines (supplemental online Table 1) [17]. Institutional review board approval was received and written informed consent was obtained from all patients prior to the commencement of any study-related procedures.
Clinical Parameters and Performance Status
Demographic and medical characteristics were abstracted from electronic medical chart review. Performance status was evaluated by the attending oncologist using the Karnofsky performance scale. Exercise behavior was assessed by self-report using the Godin Leisure Time Exercise Questionnaire [18]. Blood levels of fasting glucose and low-density lipoprotein were assessed at study entry.
Cardiopulmonary Exercise Testing
All participants performed a symptom-limited CPET on a motor-driven treadmill (T-2100; GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com) or an electronically braked cycle ergometer (Lode Inc., Groningen, Netherlands, http://www.lode.nl/en/) with breath-by-breath expired gas analysis (TrueOne 2400; Parvo Medics, Sandy, UT, http://www.parvo.com) to assess peak oxygen consumption (VO2peak). All tests were conducted by two certified exercise physiologists, according to standard guidelines [15, 16]. In brief, to stabilize gas measurement, 3 minutes of resting metabolic data were collected prior to exercise initiation. Workload increments (every 2 minutes for treadmill and every 1 minute for bike) were determined by the clinical condition of the participant and the metabolic response to exercise. All participants were monitored continuously with 12-lead electrocardiography (ECG) during exercise and 5 minutes of recovery. During exercise, oxyhemoglobin saturation was monitored continuously using finger pulse oximetry, whereas blood pressure was measured manually by auscultatory sphygmomanometer every 2 minutes. All data were recorded as the highest 30-second value elicited during exercise testing. Mean percentage of age- and sex-predicted peak heart rate and VO2peak was calculated from the equation provided by Jones et al. [19] and Fitzgerald et al. [20] for women and by Wilson and Tanaka [21] for men.
Exercise-Related SAEs and Positive Test Criteria
Exercise-related events were operationalized as any event occurring during CPET procedures. SAEs were defined as the occurrence of any of the following: significant angina, sustained ventricular tachycardia, myocardial infarction, external defibrillation or implantable cardioverter-defibrillator discharge, syncope, provision of cardiac life support medications, direct admission to the emergency room, or death. A positive test was defined as identification of any of the following ECG changes: significant ischemic changes in ECG during exercise or recovery or development of exercise-induced bundle-branch block [22]. Criteria for ischemic changes in ECG included 0.1 mV deviation of the ST segment horizontal to or away from the baseline isoelectric line at 0.08 second after the J-point in the absence of significant resting ST-T abnormalities or left bundle-branch block. ST-segment changes toward the isoelectric line were not considered positive, regardless of the magnitude of change. If the baseline ECG revealed a J-ST-segment depression >0.05 mV, “double criteria” (an additional 0.2 mV) of ST depression was required with the appropriate horizontal or downsloping morphology to qualify as a positive test [23]. A single interpreter (A.A.K.) performed all ECG interpretation in a blinded fashion. Interobserver reproducibility for positive tests was assessed by repeating measurements in 20 randomly selected subjects (M.G.K.; no discrepancies were observed).
Statistical Analysis
Descriptive statistics were used to assess demographic and medical characteristics of the participants. For categorical parameters, Cochran-Armitage trend tests were used to examine trends across ACSM risk classifications (low, moderate, high risk) and Fisher’s exact tests were used to examine differences in patients based on exercise test results (positive versus negative), whereas analysis of variance was used to test for overall differences in continuous variables; post hoc (Tukey) analysis was used for pairwise comparisons, when appropriate. To examine demographic and medical predictors of a positive exercise test, we first examined the univariate associations using logistic regression models for each possible predictor individually; all predictors with p < .10 were included as candidate predictors in the multivariate model. Logistic regression using backward selection was used to develop the best model. All p values for between-group comparisons were performed with and without Bonferroni adjustment to account for multiple comparisons. Results are presented with adjusted analyses. A two-sided significance level of p = .05 was used for all statistical tests. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, http://www.sas.com).
Results
Clinical Characteristics and Preparticipation Risk Stratification
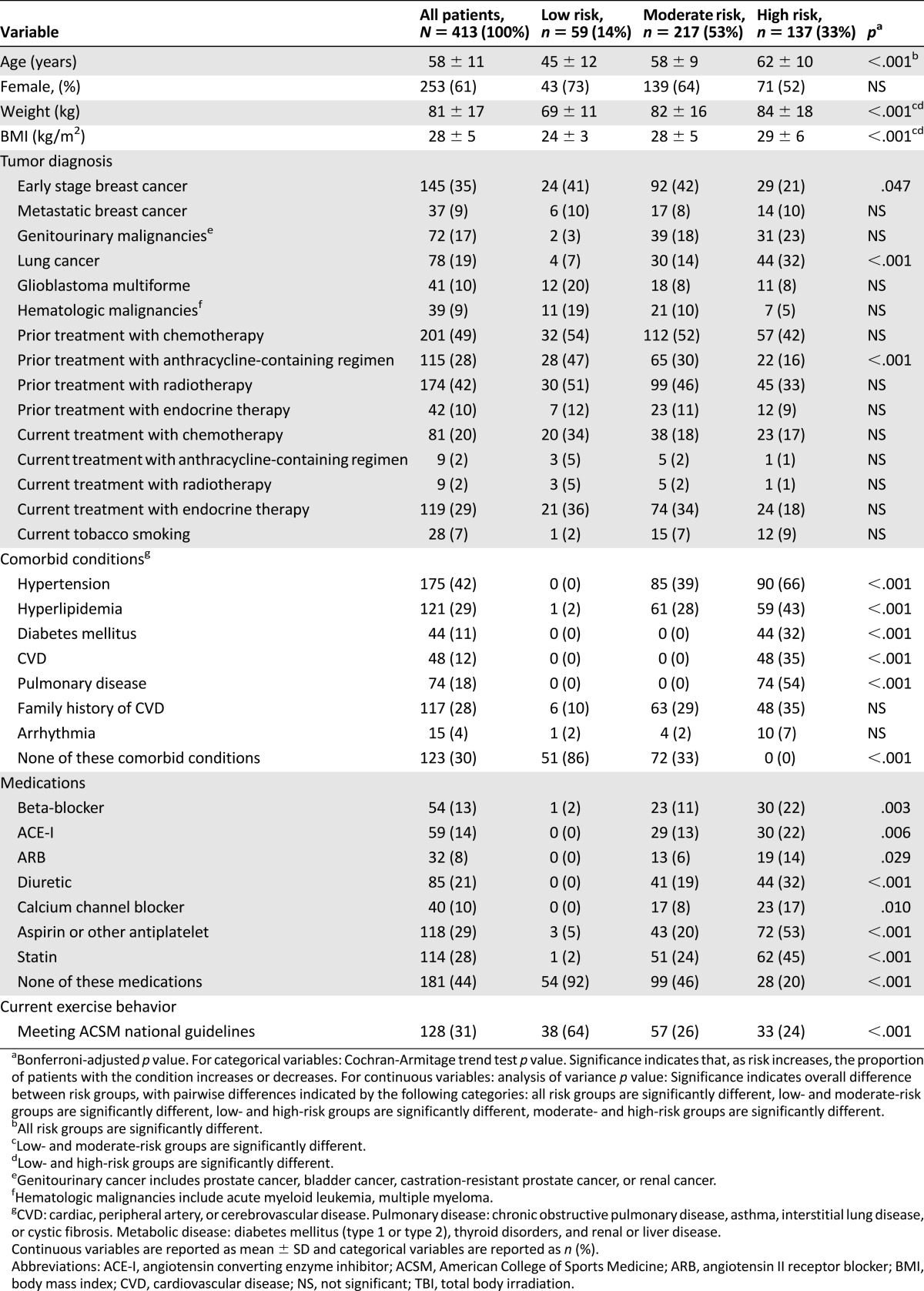
Details regarding the profiles of the participants are provided in Table 1. Participants were categorized according to ACSM recommendations as low risk (n = 59, 14%), moderate risk (n = 217, 53%), and high risk (n = 137, 33%). Patients classified as low risk were younger, reported higher exercise behavior, and had fewer comorbid diseases and/or lower disease medication use in comparison with patients classified as moderate and high risk (all p < .05).
Table 1.
Demographic and medical characteristics of participants
Exercise Testing Data
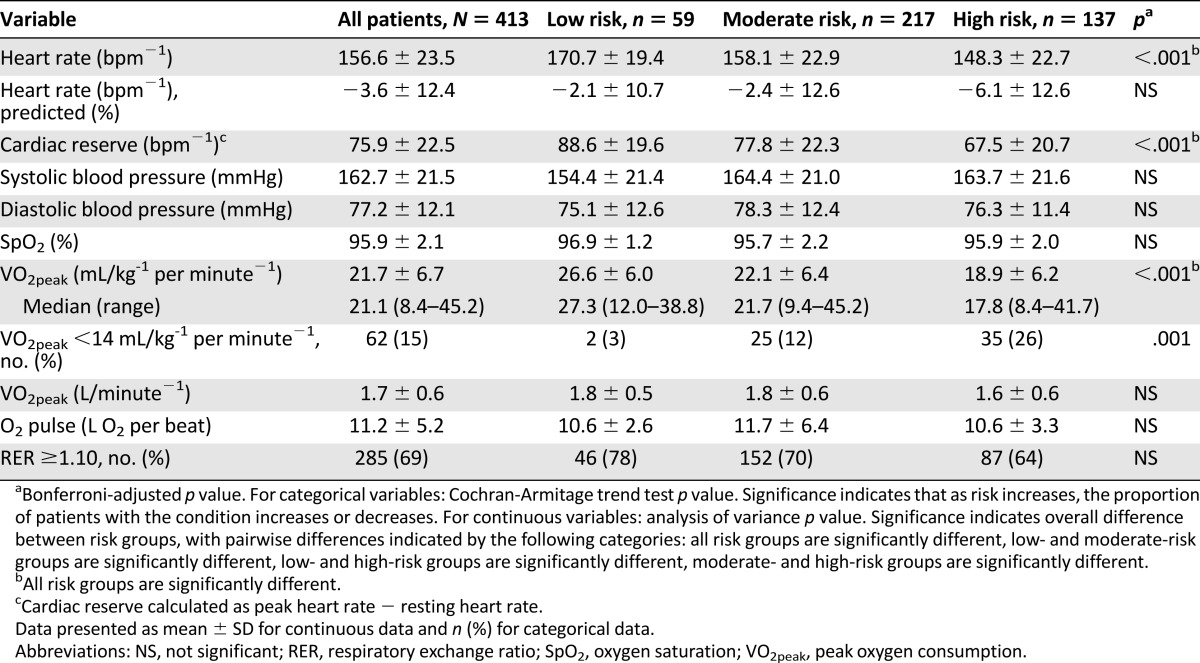
For the overall sample, mean VO2peak was 21.7 ± 6.7 mL/kg−1 per minute−1 (range: 8.4 mL/kg−1 per minute−1 to 45.2 mL/kg−1 per minute−1), the equivalent of 19.5 ± 21.7% below age- and sex-predicted sedentary values (or 5.1 ± 1.2 metabolic equivalents). VO2peak declined across increasing risk classification (from low to high risk, all p < .05) (Table 2). Sixty-two patients (15%) had severely marked impairments in VO2peak (<14 mL/kg−1 per minute−1).
Table 2.
Peak exercise testing data
Preparticipation Risk Stratification and Exercise-Related SAEs
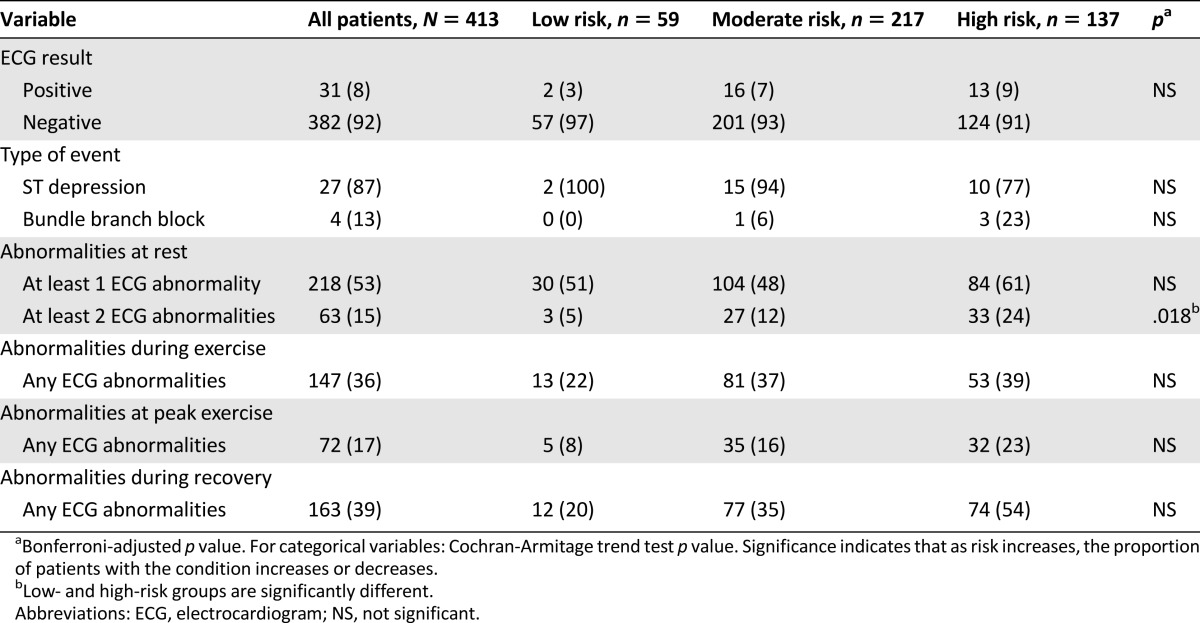
For the overall sample, no major SAEs were observed during CPET procedures. A total of 31 positive tests were observed, for an event rate of 8% (Table 3). The incidence of positive tests increased across ASCM risk stratification: 3.4% (2 of 59) for low-risk patients, 7.4% (16 of 217) for moderate-risk patients, and 9.5% (13 of 137) for high-risk patients; the differences between groups was not significant (p > .05). Of the positive tests, 27 (87%) were ischemic ECG changes reflected by significant ST-segment depression and 4 (13%) were exercise-induced bundle-branch blocks. All patients with a positive test were referred for further evaluation to their primary care physician and/or cardiologist, as appropriate (supplemental online Table 2). Of these, four were diagnosed with nonocclusive coronary artery disease, left anterior descending ischemia, or unchanged aortic stenosis; two were started on beta-blocker medication, and one underwent vagal maneuver. The type and extent of evaluation was at the discretion of the attending physician and did not necessarily include a full clinical stress test; 7 of the 31 patients with a positive test (under our laboratory conditions) subsequently underwent a full clinical stress test, with clinically significant disease confirmed in 4 (57%).
Table 3.
Abnormalities and complications at rest and during exercise testing
Predictors of an Exercise-Induced Positive Test
Age, statin use, antiplatelet therapy use, history of CVD (cardiac, peripheral artery, or cerebrovascular disease), history of anthracycline therapy or radiation therapy, diagnosis of glioblastoma multiforme, and not meeting national exercise guidelines were significant predictors of a positive test (all p < .10). Exploratory logistic regression analyses indicated that history of cardiovascular disease was the only independent predictor of an exercise-related positive test (odds ratio: 4.311; 95% confidence interval: 1.891–9.829; p = .001).
Discussion
The principal findings of this study were as follows. First, in this heterogeneous cohort, the majority of cancer patients were classified as either moderate or high risk for having an exercise-related event according to established preparticipation health screening guidelines [17]. Second, the risk of major SAEs during maximal exercise testing was very low (there were no major SAEs); the positive test event rate was 8%. Third, widely utilized preparticipation screening recommendations developed in noncancer clinical populations may be suboptimal for use in the oncology setting. To our knowledge, this study is the first to formally evaluate the appropriateness (and the need for) preparticipation health guidelines in patients with cancer.
Cancer patients represent a population at potentially higher risk for exercise-related events due to normal age-related pathologies in conjunction with the compounding effects of anticancer therapy [13]. The initial objective of our study was to evaluate the preparticipation risk-stratification distribution in our cohort [14]. More than 85% of patients were classified as moderate or high risk, with only ∼15% classified as low risk. Indeed, 30%–40% of patients had at least one documented CVD risk factor (e.g., hypertension, hyperlipidemia), whereas 15%–18% had documented overt CVD or pulmonary disease. Finally, exercise capacity (VO2peak) was, on average, ∼20% below that of age-matched sedentary but otherwise healthy individuals; 15% had a VO2peak <14 mL/kg−1 per minute−1, the threshold criteria for referral for heart transplantation in patients with heart failure. On this basis, we contend that formalized preparticipation health screening using either the ACSM risk stratification guidelines or other available tools (e.g., Physical Activity Readiness Questionnaire) should be required for all oncology patients prior to the initiation of any exercise program.
There is currently a lack of consensus regarding the level of preparticipation health screening required in clinical populations, particularly incorporation of exercise testing [17, 24, 25]. Some organizations advocate for exercise testing of patients at high risk of CVD prior to engaging in moderate-intensity exercise [25], whereas others recommend that exercise testing is not required [24]. We observed no SAEs (life threatening), indicating that the risk of a cardiovascular event during maximal exercise (i.e., CPET) is very low in a heterogeneous population of cancer patients. The low incidence rate is consistent with prior work in noncancer clinical populations, for which SAE rates during maximal exercise testing are reported as 0.5 per 100,000 tests in healthy individuals and 2–5 per 100,000 tests in patients with CVD [11, 26, 27]. Despite the lack of major SAEs, we observed a positive-test event rate of 8%, which is lower than that reported in comparable studies in other noncancer cohorts of a similar age range. In 825 healthy volunteers (aged 22–89 years) enrolled in the Baltimore Longitudinal Study of Aging, for example, Rywik et al. [28] observed ischemic ST-segment changes in 25.9% of subjects either during or after maximal exercise testing. Similarly, Hirotani et al. [29] reported a positive stress incidence rate of 16.9% in patients with chronic respiratory diseases without a history of CVD.
It is noteworthy that our positive test incidence rate was observed in a cohort that had undergone multigated health screening to determine research study eligibility. Consequently, our findings may underestimate the actual incidence of positive tests in the broader oncology population. Large prospective studies are required to adequately address this question. In the interim, we contend our findings support the conclusion that maximal CPET is a relatively safe and tolerable procedure for patients with cancer, but exercise testing does not need to be incorporated as part of preparticipation health screening clearance for the vast majority of patients.
Despite this broad recommendation, a relatively small subset of patients (<10%) may be at higher risk for an exercise-related event, and exercise testing may be beneficial for this group. To this end, an important practical goal is to accurately identify those patients truly at high risk of having an exercise-related event. Given the lack of oncology-specific preparticipation recommendations, we tested the utility of the widely utilized ACSM preparticipation health screening guidelines. In support of these guidelines, the incidence of positive tests increased across ASCM risk stratification; however, stratification did not significantly discriminate between low-, moderate-, and high-risk groups. Furthermore, the proportion of positive tests in patients classified as moderate or high risk was comparable (7% versus 9%). Consequently, ACSM risk stratification may have suboptimal appropriateness in the oncology setting, at least for predicting the risk of a positive test. This underscores the need for the development of evidence-based, oncology-specific preparticipation screening guidelines [30]. In exploratory analyses, we identified several candidate factors associated with the risk of a positive test, including noncancer factors (CVD or CVD medication) and cancer-specific factors (e.g., treatment anthracycline or radiation). Validation in an independent data set is required.
This study has important limitations. Foremost, important selection biases exist because all patients underwent research study eligibility screening, received oncologist approval for exercise-testing procedures, and volunteered to participate in a research study. Consequently, our results are not generalizable to an unscreened adult cancer cohort outside the context of a research study. In addition, the sample sizes in some cancer diagnoses and treatment categories were small, providing limited statistical power to appropriately test the utility of cardiovascular and pre-exercise screening guidelines. Finally, the number of events was low, providing limited power to detect group differences.
Conclusion
The use of formalized preparticipation health screening is required in all oncology patients prior to the initiation of exercise. However, the risk of an exercise-induced event is very low, suggesting that exercise testing is not required as part of screening procedures in the majority of patients. Widely utilized screening recommendations have suboptimal validity in cancer patients, highlighting the urgent need for the development of oncology-specific criteria. Our findings provide important and timely information for exercise and oncology professionals to facilitate effective preparticipation health clearance to optimize the safety and benefits of exercise for patients with cancer.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgment
Dr. Lee W. Jones is funded, in part, by grants from the National Cancer Institute.
Author Contributions
Conception/Design: Lee W. Jones, Aarti A. Kenjale
Provision of study material or patients: Lee W. Jones, Aarti A. Kenjale, Jeffrey Peppercorn
Collection and/or assembly of data: Lee W. Jones, Aarti A. Kenjale, Whitney E. Hornsby, Theresa Crowgey, Michel G. Khouri, Amy R. Lane, Caroline E. Bishop
Data analysis and interpretation: Lee W. Jones, Aarti A. Kenjale, Samantha Thomas, James E. Herndon II, Neil D. Eves, Pamela S. Douglas
Manuscript writing: Lee W. Jones, Aarti A. Kenjale, Whitney E. Hornsby, Theresa Crowgey, Samantha Thomas, James E. Herndon II, Michel G. Khouri, Amy R. Lane, Caroline E. Bishop, Neil D. Eves, Jeffrey Peppercorn, Pamela S. Douglas
Final approval of manuscript: Lee W. Jones, Aarti A. Kenjale, Whitney E. Hornsby, Theresa Crowgey, Samantha Thomas, James E. Herndon II, Michel G. Khouri, Amy R. Lane, Caroline E. Bishop, Neil D. Eves, Jeffrey Peppercorn, Pamela S. Douglas
Disclosures
The authors indicated no financial relationships.
References
- 1.Knols R, Aaronson NK, Uebelhart D, et al. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 5.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SC, Spence RR, Galvao DA, et al. Australian Association for Exercise and Sport Science position stand: Optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg JP, Velthuis MJ, Gijsen BC, et al. Guideline “cancer rehabilitation”. Ned Tijdschr Geneeskd. 2011;155:A4104. in Dutch. [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Peterson B, McBride C, et al. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 9.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 10.Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher GF, Balady G, Froelicher VF, et al. Exercise standards. A statement for healthcare professionals from the American Heart Association. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 12.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 13.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 14.Balady GJ, Chaitman B, Driscoll D, et al. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Circulation. 1998;97:2283–2293. doi: 10.1161/01.cir.97.22.2283. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 18.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 19.Jones NL, Summers E, Killian KJ. Influence of age and stature on exercise capacity during incremental cycle ergometry in men and women. Am Rev Respir Dis. 1989;140:1373–1380. doi: 10.1164/ajrccm/140.5.1373. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald MD, Tanaka H, Tran ZV, et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: Relation to training status. Am J Physiol Heart Circ Physiol. 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- 22.Grady TA, Chiu AC, Snader CE, et al. Prognostic significance of exercise-induced left bundle-branch block. JAMA. 1998;279:153–156. doi: 10.1001/jama.279.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Sanchis J, Bodí V, Núñez J, et al. Usefulness of early exercise testing and clinical risk score for prognostic evaluation in chest pain units without preexisting evidence of myocardial ischemia. Am J Cardiol. 2006;97:633–635. doi: 10.1016/j.amjcard.2005.09.107. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Preventive Services Task Force Screening for coronary heart disease: Recommendation statement. Ann Intern Med. 2004;140:569–572. doi: 10.7326/0003-4819-140-7-200404060-00001. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: Summary article: S report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 26.Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation. 2012;126:2465–2472. doi: 10.1161/CIRCULATIONAHA.112.110460. [DOI] [PubMed] [Google Scholar]
- 27.Scardovi AB, Coletta C, De Maria R, et al. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2007;8:608–612. doi: 10.2459/01.JCM.0000281698.53983.4e. [DOI] [PubMed] [Google Scholar]
- 28.Rywik TM, Zink RC, Gittings NS, et al. Independent prognostic significance of ischemic ST-segment response limited to recovery from treadmill exercise in asymptomatic subjects. Circulation. 1998;97:2117–2122. doi: 10.1161/01.cir.97.21.2117. [DOI] [PubMed] [Google Scholar]
- 29.Hirotani A, Maekura R, Okuda Y, et al. Exercise-induced electrocardiographic changes in patients with chronic respiratory diseases: Differential diagnosis by 99mTc-tetrofosmin SPECT. J Nucl Med. 2003;44:325–330. [PubMed] [Google Scholar]
- 30.Jones LW. Evidence-based risk assessment and recommendations for physical activity clearance: Cancer. Appl Physiol Nutr Metab. 2011;36(suppl 1):S101–S112. doi: 10.1139/h11-043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


