Abstract
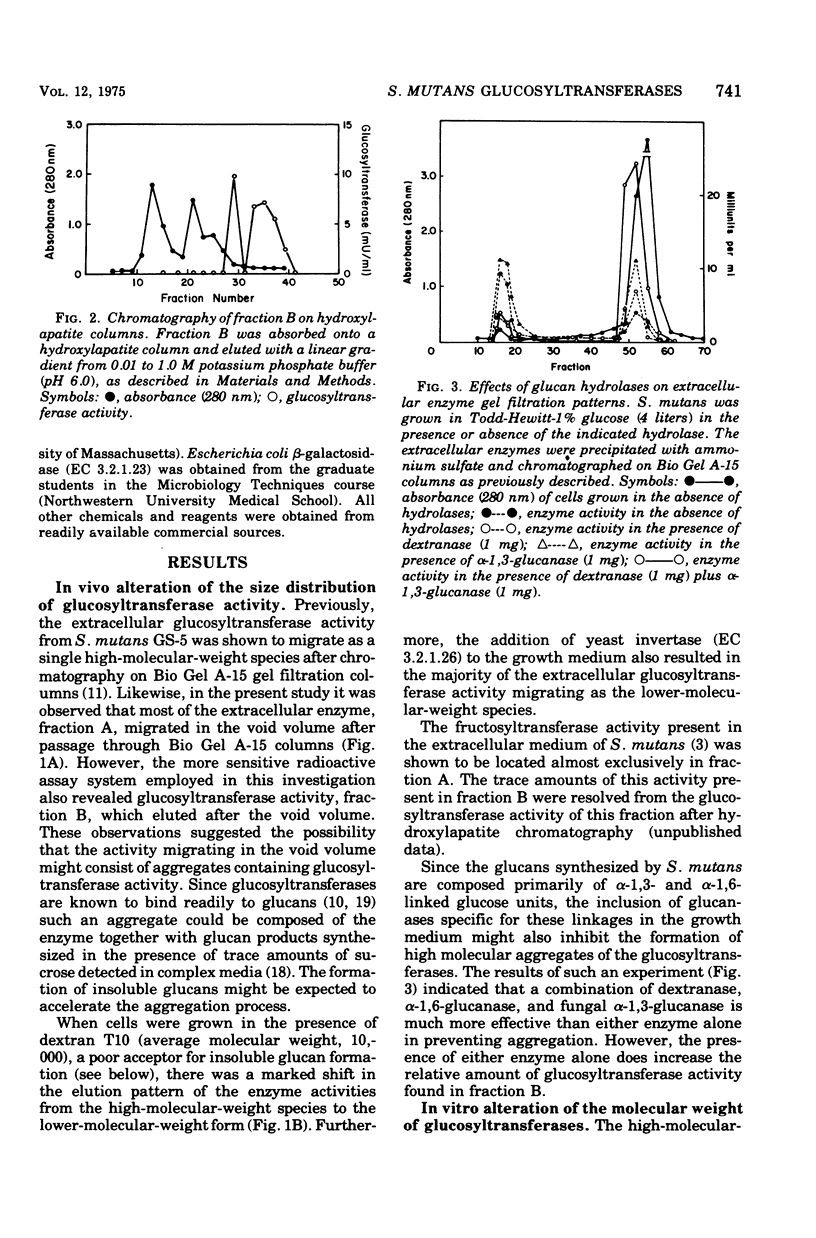
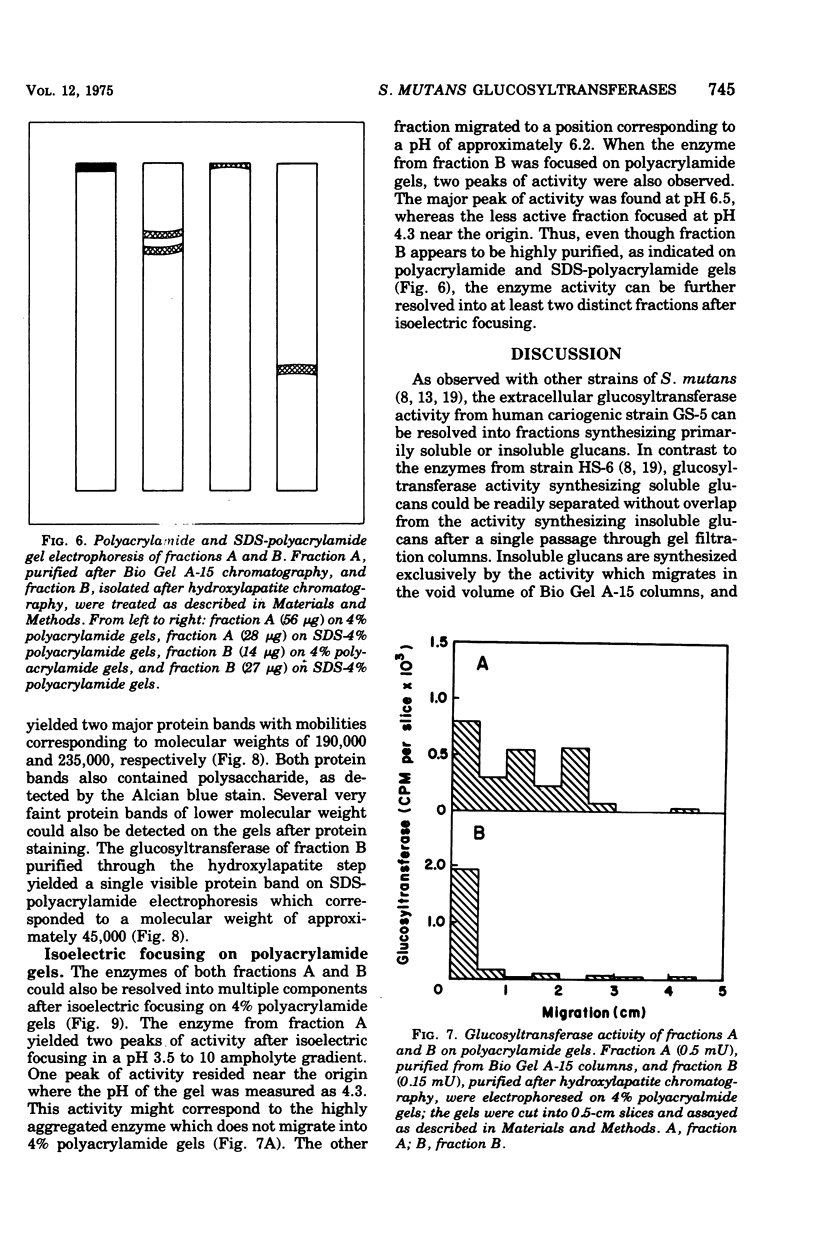
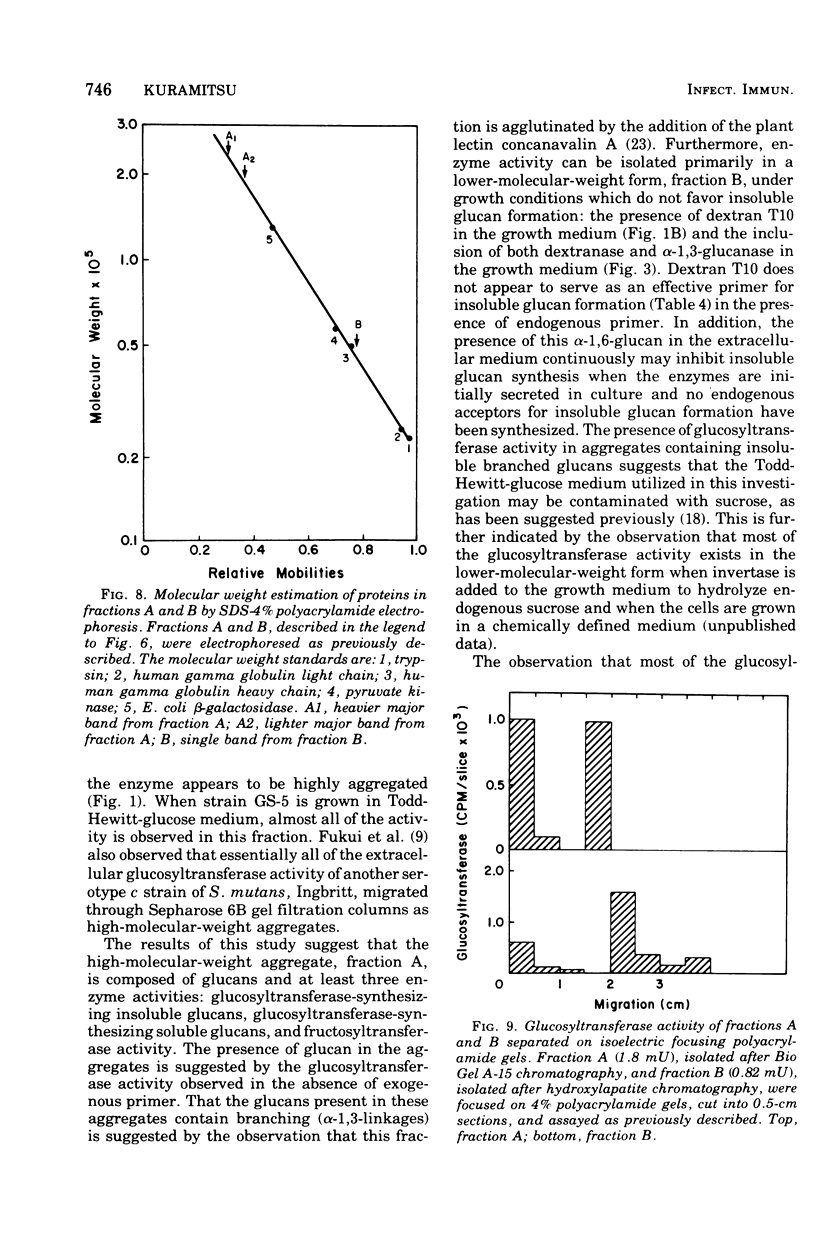
The extracellular glycosyltransferase activity of Sterptococcus mutans GS-5 has been resolved into two non-overlapping fractions after gel filtration chromatography on Bio Gel A-15 columns. The activity eluting in the void volume, fraction A, was highly aggregated and synthesized both soluble and insoluble glucans. The activity retarded by the resin, fraction B, synthesized only soluble glucan. Almost all of the extracellular glucosyltransferase activity was eluted in the void volume when the cells were grown in Todd-Hewitt medium. However, most of the activity migrated as the lower-molecular-weight species when cells were grown under conditions which inhibit insoluble glucan formation. The activities in both fractions had identical temperature and pH optima as well as similar Km values for sucrose. Fraction A synthesized both alpha-1,3- and alpha-1,6- linked glucans, whereas fraction B catalyzed alpha-1,6-glucan formation. Fraction B has been purified to near homogeneity and is also aggregated with a subunit molecular weight of 45,000. The properties of the glucosyltransferases in both fractions are discussed in terms of the role of the enzymes in both soluble and insoluble glucan formation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Carlsson J. A levansucrase from Streptococcus mutans. Caries Res. 1970;4(2):97–113. doi: 10.1159/000259632. [DOI] [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Kobayashi M., Matsuda K. The dextransucrase isoenzymes of Leuconostoc mesenteroides NRRL B-1299. Biochim Biophys Acta. 1974 Dec 29;370(2):441–449. doi: 10.1016/0005-2744(74)90105-3. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. 13. The interaction of concanavalin A with alpha-mannans from a variety of microorganisms. J Biol Chem. 1968 Apr 25;243(8):2003–2007. [PubMed] [Google Scholar]
- Wardi A. H., Michos G. A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal Biochem. 1972 Oct;49(2):607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



