Abstract
Shuganjieyu capsule has been approved for clinical treatment by the State Food and Drug Ad-ministration of China since 2008. In the clinic, Shuganjieyu capsule is often used to treat mild to moderate depression. In the rat model of depression established in this study, Shuganjieyu capsule was administered intragastrically daily before stress. Behavioral results confirmed that depressive symptoms lessened after treatment with high-dose (150 mg/kg) Shuganjieyu capsule. Immunohistochemistry results showed that high-dose Shuganjieyu capsule significantly increased phosphorylation levels of phosphorylation cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor expression in the medial prefrontal cortex and hippocampal CA3 area. Overall, our results suggest that in rats, Shuganjieyu capsule effec-tively reverses depressive-like behaviors by increasing expression levels of neurotrophic factors in the brain.
Keywords: nerve regeneration, Shuganjieyu capsule, depression, neurotrophic factor, brain-derived neurotrophic factor, phosphorylation cyclic adenosine monophosphate response element binding protein, chronic unpredictable mild stress, NSFC grant, neural regeneration
Introduction
Depression is a common mental disorder afflicting 20% of the population, and characterized by a number of signs and symptoms including depressed mood, anhedonia, insomnia, anorexia, concentration difficulties and suicidal thoughts (Hamet and Tremblay, 2005). The majority of depressed patients require long-term treatment. However, because of the involvement of multiple pathogenic factors, many anti-depressant drugs have low response rates and some cause adverse side effects such as nausea, headache, anger, weight loss, insomnia, yawning, diarrhea, weakness, fatigue, and dyspepsia (Park et al., 2007; Guadarrama-Cruz et al., 2008; Kiss, 2008). Therefore, it is necessary to develop safer, better tolerated, and more effective antidepressant drugs.
Neurotrophins are capable of augmenting neurogenesis in the adult brain (Pencea et al., 2001). Neurotrophic defects are thought to be one of the pathogenic mechanisms of depression. There is evidence that shortage of neurotrophic factors is the main cause of pathological change in the brain of patients with depression (Castrén and Rantamäki, 2010). Brain-derived neurotrophic factor is the most widely expressed member of the nerve growth factor family of neurotrophins. Brain-derived neurotrophic factor is a downstream target gene of cyclic adenosine monophosphate response element binding protein, and a candidate molecule for the structural changes associated with depression (Koponen et al., 2005). Cyclic adenosine monophosphate response element binding protein is a nuclear protein regulated by diverse signaling pathways, controlling integration of numerous external stimuli, including antidepressants. Chronic antidepressant administration not only affects cyclic adenosine monophosphate response element binding protein expression, but also activates cyclic adenosine monophosphate response element binding protein and mediates cyclic adenosine monophosphate response element binding protein transcription (Gur et al., 2007). Cyclic adenosine monophosphate response element binding protein expression and phosphorylation levels are closely associated with pathological and pharmacological mechanisms of depression.
Many traditional Chinese medicines induce anti-depressive effects with fewer side effects, and are a good choice for maintenance treatment of depression. Shuganjieyu capsule is a traditional Chinese medicine compound preparation made from St. John's wort and Siberian ginseng extracts. Shuganjieyu capsule has been approved for clinical application by the State Food and Drug Administration of China since 2008. In the clinic, Shuganjieyu capsule is often used to treat mild to moderate depression in China. Clinical studies have found that Shuganjieyu capsule has definite curative efficacy for mild to moderate depressive symptoms with very few adverse effects (Sun et al., 2009; Qiu et al., 2011). Moreover, St. John's wort extract is used worldwide to treat mild and moderate depression (Rychlik et al., 2001). Hyperforin, a phloroglucinol derivative of St. John's wort extract, is the main anti-depressant constituent. Hyperforin may exert anti-depressive effects through multiple pathways, with one of the recognized antidepressant mechanisms being inhibition of synaptosomal reuptake of serotonin, norepinephrine, and dopamine, thereby increasing synaptic concentrations of these neurotransmitters (Muller et al., 1997). Recent studies suggest that the anti-depressant effects of St. John's wort extract may be related to brain-derived neurotrophic factor levels and phosphorylated cyclic adenosine monophosphate response element binding protein (Gibon et al., 2013; Heiser et al., 2013; Leuner et al., 2013). Siberian ginseng extract, prepared from the root or stem bark of Acanthopanax senticosus, has anti-fatigue, anti-stress, immunoenhancing and anti-depressive effects (Deyama et al., 2001). Studies have shown that Siberian ginseng extract increases levels of acetylcholine, norepinephrine, and serotonin, and brain-derived neurotrophic factor expression in the hippocampus of rat models of depression (Huang and Liu, 2008; Li et al., 2012; Zhu et al., 2012). However, the anti-depressant mechanism of Shuganjieyu capsule remains poorly understood. It has been suggested that the anti-depressant mechanism of Shuganjieyu capsule is related to increased levels of monoaminergic neurotransmitters including noradrenaline, serotonin, and dopamine (Jiang et al., 2006; Chen et al., 2013). Although it is not known if the effect of Shuganjieyu capsule as an antidepressant traditional Chinese medicine is associated with increased brain-derived neurotrophic factor and p-CERB expression levels.
Therefore, we examined the effect of Shuganjieyu capsule on behavioral changes in a rat model of depression. In addition, we investigated the underlying mechanisms by examining brain-derived neurotrophic factor and phosphorylated cyclic adenosine monophosphate response element binding protein expression levels in the medial prefrontal cortex and hippocampal CA3 area.
Materials and Methods
Animals
A total of 74 pathogen-free male Sprague-Dawley rats aged 6–8 weeks and weighing 180–200 g, were provided by Shilaikejingda Co., Ltd., Shanghai, China (license No. HNASLKJ20120005). Animals were maintained on a 12-hour light/dark cycle (lights on at 8:00 a.m. and off at 8:00 p.m.) under controlled temperature conditions (22 ± 1°C), and allowed free access to standard food and water. They were acclimatized for 3 days before use. Simultaneously, they were trained to drink 1% sucrose, and sucrose consumption levels and open-field baselines measured. All tests were performed during the hours of 9:00–17:00. All experimental procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85–23, revised 1996), and approved by the Animal Experimentation Ethics Committee of the Central South University in China.
Drugs
Shuganjieyu capsule used in the present study was generously provided by Chengdu Kanghong Pharmaceutical Group Co., Ltd., Chengdu, Sichuan Province, China. Shuganjieyu capsule is composed of St. John's wort and Siberian ginseng extracts, and has been approved by the State Food and Drug Administration of China for mild to moderate depression, and proven potent in clinical practice since 2008 (drug batch No. Z20080580).
Animal groups for experiments
Of the 74 rats, 50 rats were randomly selected, and equally and randomly divided into five groups: control, model, low-dose Shuganjieyu capsule, high-dose Shuganjieyu capsule, and fluoxetine. They were subjected to open-field, sucrose consumption, and forced swimming tests. In order to exclude the effects of behavioral testing on brain-derived neurotrophic factor and phosphorylated cyclic adenosine monophosphate response element binding protein expression in the brain, the remaining 24 rats were used for a mechanistic study. They were equally and randomly divided into control, model, high-dose Shuganjieyu capsule, and fluoxetine groups, and then immunohistochemical analysis of brain-derived neurotrophic factor and phosphorylated cyclic adenosine monophosphate response element binding protein expression performed. As low-dose Shuganjieyu capsule showed little effect on depressive-like behavior in rats, the low-dose Shuganjieyu capsule group was not included in the mechanistic study.
Chronic unpredictable mild stress procedure
The chronic unpredictable mild stress procedure was performed as described by Willner (1997). Briefly, chronic unpredictable mild stress consisting of 10 types of unpredictable stressors, including 24-hour food deprivation, 24-hour water deprivation, 5-minute cold water swim (at 4°C), 5-minute hot condition (at 45°C), 1-minute tail pinch (1 cm from the end of the tail), foot shock (voltage 35 V, 10-second duration, average 1 shock/50 s) for 30 times, vibration (160 T/min for 5 minutes), bind (2 hours), day and night inversion (24 hours), and overnight illumination. One of these stressors (in random order) was given every day between 9:00 a.m. and 12:00 a.m. for 21 days. All stresses were applied unexpectedly to prevent adaptive responses. Control (unstressed) rats were undisturbed except for necessary procedures such as routine cage cleaning. The control group was raised together in a cage, while the other groups were raised separately in cages.
Drug treatment
Experimental doses of Shuganjieyu capsule were 50 or 150 mg/kg per day, equivalent to approximately 2- or 6-fold, respectively, the clinical dose (24 mg/kg per day, according to Shuganjieyu capsule instructions). Shuganjieyu capsule was dissolved in 0.5% carboxymethyl cellulose suspension, at concentrations of 5 or 15 mg/mL. The groups received appropriate drug doses, specifically, low-dose Shuganjieyu capsule (50 mg/kg per day), high-dose Shuganjieyu capsule (150 mg/kg per day), and fluoxetine (1.54 mg/kg per day, 20 mg/tablet dissolved in carboxymethyl cellulose at a concentration of 0.154 mg/mL; Lilly Company, Indianapolis, IN, USA). Control and model groups were administered 0.5% carboxymethyl cellulose suspension (10 mL/kg per day) intragastrically, 30 minutes before each stressor once a day for 21 days.
Open-field test
Open-field tests were performed at the beginning and end of the 21-day modeling period. Open-field tests were used to assess the rats’ spontaneous activity and exploration (or seeking) abilities (Thiel et al., 1999). Each animal was placed at the center of a dimly illuminated square area (44 cm × 44 cm) with walls 47 cm in height. The ENV-520 video tracking analysis system (Med Associates Inc., St. Albans, VT, USA) was used to observe spontaneous activity. Data were continuously recorded for 3 minutes, and cumulative locomotion paths analyzed. The cage was thoroughly cleaned before the next test.
Sucrose consumption test
Sucrose preference tests were performed at the beginning and end of the 21-day modeling period. The test was performed as described previously (Luo et al., 2008), with minor modifications. Briefly, before the test, rats were trained to adapt to sucrose solution (1%, w/v). Two bottles of sucrose solution were placed in each cage for 24 hours, and then one bottle replaced with water for 24 hours. After the adaptation procedure, rats were deprived of water and food for 24 hours. Next, rats were housed in individual cages and given free access to two bottles containing 100 mL of sucrose solution (1%, w/v) and 100 mL of water. After 60 minutes, volumes of both consumed sucrose solution and water were recorded, and sucrose preference calculated using the following formula: sucrose preference = sucrose consumption/(water consumption + sucrose consumption) × 100%.
Forced swimming test
Rats were individually forced to swim twice at 24-hour intervals in a cylinder (60 cm high, 23 cm diameter, self-manufactured) filled with water (25 ± 1°C) up to 45 cm. Before the test, rats were subjected to 12-hour fasting without water deprivation. The previous day, rats were forced to swim for 15 minutes, taken back to their cages, before another forced swim for 5 minutes within 24 hours. We recorded the time taken for 5 minutes immobility to appear. Immobility time is noted by floating of the rat in the water, without struggle or only slight body movement (enough to keep the head above water). In the forced swimming test, the immobility status was referred to as a depression symptom in rat models.
Tissue preparation
After model establishment, rats were perfused with 0.9% 200 mL PBS using a catheter inserted into the carotid artery, followed immediately by perfusion with 4% paraformaldehyde for fixing the brain. Next, brains were separated from the skull and placed into 4% paraformaldehyde for 4 hours, dehydrated in 30% sucrose, 4% paraformaldehyde and embedded in paraffin. Fixed brains were cut into 30 µm thick sections. Using a rat atlas of anatomy (George and Charles, 2005), we selected brain slices of the medial prefrontal cortex and hippocampal CA3 area for immunohistochemistry.
Immunohistochemistry for phosphorylated cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor expression in the rat medial prefrontal cortex and hippocampal CA3 area
Immunohistochemistry was performed according to the avidin-biotin peroxidase complex procedure. Brain slices were rinsed in 0.01 mol/L PBS and inactivated with peroxidase, followed by antigen retrieval and 5% bovine serum albumin sealing. Sections were incubated with rabbit anti-rat phosphorylated cyclic adenosine monophosphate response element binding protein monoclonal antibody (1:400; Abcam, Cambridge, MA, USA) and rabbit anti-rat brain-derived neurotrophic factor monoclonal antibody (1:200; Abcam) at 4°C overnight. Next, sections were treated with biotinylated goat anti-rabbit IgG (1:200; Vector Company, Burlingame, CA, USA) and incubated in avidin-biotin peroxidase complex liquid, followed by 3,3′-diaminobenzidine coloration (Vector Company) and H2O2 staining. Sections were covered, dehydrated, permeabilized, and mounted. Positive comparison film in the kit was used as a positive control, and an alternative primary antibody (0.01 mol/L PBS) as the negative control. Four brain slices from the medial prefrontal cortex or hippocampal CA3 area of each rat were randomly selected. Average grayscale values were measured using the Motic pathology analysis system (Motic China group Co., Ltd., Xiamen, Fujian Province, China).
Statistical analysis
Data were expressed as mean ± SEM, and analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Multiple group comparisons were performed using one-way analysis of variance followed by post hoc least significant difference tests to detect intergroup differences. P values < 0.05 were considered statistically significant.
Results
High-dose Shuganjieyu capsule improved spontaneous activity in rats with depressive-like behaviors
The open-field test showed that before chronic unpredictable mild stress, there was no significant difference in cumulative locomotion path between groups (P > 0.05). However, it was significantly decreased in rats with depressive-like behaviors compared with the control group (P < 0.01). Low-dose Shuganjieyu capsule (50 mg/kg) induced a minimal response, and there was no significant difference compared with the model group (P > 0.05). Similar to fluoxetine, high-dose Shuganjieyu capsule (150 mg/kg) produced a significant increase in cumulative locomotion path compared with the model group (P < 0.05; Figure 1A).
Figure 1.

Effect of Shuganjieyu capsule (SGJYC) on behavioral changes in depressive-like rats.
(A) Effect of SGJYC on cumulative locomotion path in the open-field test. Longer cumulative locomotion paths show greater spontaneous activity and exploration (or seeking) abilities in rats. (B) Effect of SGJYC on total liquid and sucrose consumption. (C) Effect of SGJYC on sucrose prefer-ence percentage. Sucrose preference = sucrose consumption/(water consumption + sucrose consumption) × 100%. (D) Effect of SGJYC on immo-bility time in the forced swimming test. Longer immobility times indicate more severe depressive-like behavior in rats. Values are expressed as mean ± SEM (10 rats per group). Multiple group comparisons were performed using one-way analysis of variance. Intergroup differences were compared using post hoc least significant difference tests. *P < 0.05, **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. model group.
High-dose Shuganjieyu capsule increased sucrose consumption in rats with depressive-like behaviors
Before chronic unpredictable mild stress treatment, there was no significant difference in total liquid consumption, sucrose consumption, and sucrose preference in the sucrose consumption test, between the groups (P > 0.05). After 21 days of chronic unpredictable mild stress, there was no significant difference in total liquid consumption (P > 0.05), although compared with the control group, significant decreases in sucrose consumption and preference were detected in the model group (P < 0.05 and P < 0.01, respectively). There was little change in sucrose consumption and preference in the low-dose Shuganjieyu capsule group compared with the model group (P > 0.05). However, similar to fluoxetine, high-dose Shuganjieyu capsule caused significant increases in sucrose consumption and preference (P < 0.05 and 0.01; Figure 1B, C).
High-dose Shuganjieyu capsule shortened immobility time in rats with depressive-like behaviors
The forced swimming test showed that 21-day chronic unpredictable mild stress significantly increased immobility time (P < 0.05) compared with control rats. Compared to the model group, high-dose capsule and fluoxetine both produced similar shortened immobility times (P < 0.01), but not low-dose Shuganjieyu capsule (P > 0.05; Figure 1D).
High-dose Shuganjieyu capsule increased phosphorylated cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor levels in the medial prefrontal cortex and hippocampal CA3 area
Immunohistochemical staining of phosphorylated cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor expression in the medial prefrontal cortex and hippocampal CA3 area was significantly decreased in rats with depressive-like behaviors compared with control rats (P < 0.05). However, high-dose Shuganjieyu capsule and fluoxetine significantly increased phosphorylated cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor expression in the medial prefrontal cortex and hippocampal CA3 area of rats with depressive-like behaviors (P < 0.05), nearly restoring them to normal levels (P > 0.05; Figures 2, 3).
Figure 2.
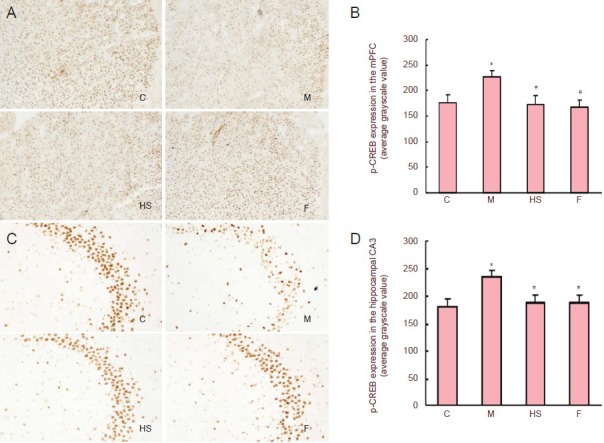
Effect of Shuganjieyu capsule treatment on p-CREB expression in mPFC and hippocampal CA3 area of rats with depressive-like behaviors.
(A, C) Expression of p-CREB in mPFC and hippocampal CA3 area (magnification: mPFC, × 40; hippocampal CA3 area, × 100). p-CREB-positive cells are brown. (B, D) Semi-quantitative analysis of p-CREB expression in mPFC and hippocampal CA3 area. Values are presented as mean ± SEM (six rats per group). Multiple group comparisons were performed using one-way analysis of variance. Intergroup differences were compared using post hoc least significant difference tests. *P < 0.05, vs. control group; #P < 0.05, vs. model group. C: Control group; M: model group; HS: high-dose Shuganjieyu capsule group; F: fluoxetine group; p-CREB: phosphorylation cyclic adenosine monophosphate response element binding protein; mPFC: medial prefrontal cortex.
Figure 3.
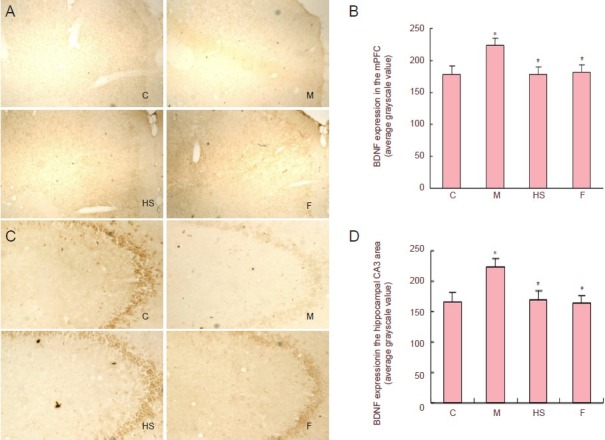
Effect of Shuganjieyu capsule on BDNF expression in mPFC and hippocampal CA3 area of rats with depressive-like behaviors.
(A, C) BDNF expression in rat mPFC and hippocampal CA3 area (magnification: mPFC, × 40; hippocampal CA3 area, × 100). BDNF-positive cells are brown. (B, D) Semi-quantitative analysis of BDNF expression in rat mPFC and hippocampal CA3 area. Values are presented as mean ± SEM (six rats per group). Multiple group comparisons were performed using one-way analysis of variance. Intergroup differences were compared using post hoc least significant difference tests. *P < 0.05, vs. control group; #P < 0.05, vs. model group. C: Control group; M: model group; HS: high-dose Shuganjieyu capsule group; F: fluoxetine group; BDNF: brain-derived neurotrophic factor; mPFC: medial prefrontal cortex.
Discussion
It is well-known that long-term chronic stress is a pathogenic mechanism in depression. In this study, we used chronic unpredictable mild stress to produce a rat model of depressive-like behaviors. This model has been widely used for studying the pathological and pharmacological mechanisms of depression (Vry and Schreiber, 1997; Mao et al., 2010). Moreover, optimization of the chronic unpredictable mild stress method was performed using isolated-living conditions, housing the rats separately, which is closer to a depressive-like model (Willner, 1984; Fenli et al., 2013; Xia et al., 2013). We found that cumulative locomotion path, sucrose consumption and percentage are significantly reduced by 76.1%, 38.3% and 27.4%, respectively, and immobility time extended by 53% in model rats compared with controls, suggesting successful establishment of the model.
According to recent evidence, in depression there is dysfunction of specific neuroanatomical foci, notably the hippocampus and the prefrontal cortex (Sheline et al., 2003; Warner-Schmidt and Duman, 2006). The hippocampus and frontal cortex are affected structurally and functionally by stress responses, and critically involved in regulation of mood and learning/memory function (Luo et al., 2008; Qi et al., 2008). Neuronal atrophy/destruction in the hippocampus and frontal cortex correlates with the incidence of major depressive disorders (Manji and Duman, 2001; Fuchs et al., 2004).
In this study, high-dose (150 mg/kg) Shuganjieyu capsule significantly increased cumulative locomotion path by 2.4 fold, and sucrose consumption and percentage by 49.3% and 24%, respectively, and decreased immobility time by 43%, compared with the model group. However, there was minimal change at low Shuganjieyu capsule doses. These results provide evidence that Shuganjieyu capsule improves depressive-like behaviors in rats.
Shuganjieyu capsule is extensively employed to treat mild and moderate depression in China. A previous study found that compared with sertraline, Shuganjieyu capsule had similar anti-depressive actions and less side effects in depressed patients (Qiu et al., 2011). A randomized, double-blind, placebo controlled trial also suggested Shuganjieyu capsule was effective and safe in treating patients with mild or moderate depression (Sun et al., 2009). These reports are consistent with the results of our study.
Cyclic adenosine monophosphate response element binding protein is an important nuclear transcription factor, widely expressed in neurons. Cyclic adenosine monophosphate response element binding protein expression and phosphorylation levels are associated with depression. In depressed patients and animal models of depression, phosphorylated cyclic adenosine monophosphate response element binding protein expression is significantly decreased in the prefrontal cortex and hippocampus (Bilang-Bleuel et al., 2002; Wang et al., 2007; Yuan et al., 2010). Many antidepressants have effects on cyclic adenosine monophosphate response element binding protein expression and phosphorylation (Nibuya et al., 1996; Manji and Duman, 2001; Laifenfeld et al., 2005; Nair and Vaidya, 2006; Li et al., 2009). Gibon et al. (2013) found hyperforin, the active antidepressant component of St. John's wort, increased cyclic adenosine monophosphate response element binding protein, phosphorylated cyclic adenosine monophosphate response element binding protein, and TrkB expression in the cortex but not the hippocampus. Moreover, hippocampal neurogenesis remained unchanged in cultured cortical neurons and brains of adult mice. Hyperforin acts on the cortical brain-derived neurotrophic factor/TrkB pathway, leaving adult hippocampal neurogenesis unaffected. A recent study found that hyperforin activated different pathways, including Ras/MEK/ERK, PI3K/Akt and CAMKIV, causing cyclic adenosine monophosphate response element binding protein phosphorylation in PC12 cells and primary hippocampal neurons (Heiser et al., 2013). We found that chronic unpredictable mild stress decreased phosphorylated cyclic adenosine monophosphate response element binding protein expression in the hippocampal CA3 area and medial prefrontal cortex of rats. These effects were significantly reversed by Shuganjieyu capsule, suggesting that the antidepressant actions of Shuganjieyu capsule in the medial prefrontal cortex and hippocampal CA3 region are related to phosphorylated cyclic adenosine monophosphate response element binding protein expression. Our results are consistent with previous reports that antidepressants increase cyclic adenosine monophosphate response element binding protein expression and phosphorylation (Nibuya et al., 1996; Laifenfeld et al., 2005; Li et al., 2009; Heiser et al., 2013). However, another study found hyperforin increased cyclic adenosine monophosphate response element binding protein and phosphorylated cyclic adenosine monophosphate response element binding protein expression in the cortex, but not the hippocampus (Gibon et al., 2013). Result inconsistencies between the studies may be attributed to different methods for measuring expression, animal species, and medicines used.
Brain-derived neurotrophic factor is an important neurotrophin, and thought to be one of the pathogenic factors of depression. Brain-derived neurotrophic factor is responsible for proliferation and maintenance of central nervous system neurons by modulating neuronal plasticity, inhibiting cell death cascades, and increasing cell survival (Aydemir et al., 2006; Yulug et al., 2009). Physical stress may reduce hippocampal brain-derived neurotrophic factor expression in adult rats. For example, binding stress may decrease brain-derived neurotrophic factor expression levels in the rat dentate gyrus, indicating that stress-related depression is closely related to hippocampal brain-derived neurotrophic factor expression (Ishida et al., 2011). Brain-derived neurotrophic factor shortage is the main agent of pathological change in depressed patients (Castrén and Rantamäki, 2010). A recent study reported that region-specific brain-derived neurotrophic factor knock-down in the dentate gyrus induces depressive-like behavior (Taliaz et al., 2010). Infusing brain-derived neurotrophic factor into the hippocampal dentate gyrus or midbrain produces obvious anti-depressant effects (Pae et al., 2008). Chronic antidepressant treatment may reduce or reverse hippocampal neuronal atrophy and damage by increasing brain-derived neurotrophic factor expression, increasing hippocampal neuronal generation, and promoting nerve fiber germination (Tardito et al., 2006; Cattaneo et al., 2010). In addition, it has been suggested that the antidepressant actions of St. John's wort are mediated via a similar mechanism to brain-derived neurotrophic factor (Leuner et al., 2013). Siberian ginseng extract increases brain-derived neurotrophic factor expression in the hippocampus of depressed rats (Li et al., 2012). Consistent with these reports, our results show that chronic unpredictable mild stress decreases brain-derived neurotrophic factor expression in the medial prefrontal cortex and hippocampal CA3 area of rats, which is attenuated by treatment with Shuganjieyu capsule.
The cyclic adenosine monophosphate response element binding protein signal pathway is involved in brain-derived neurotrophic factor activation (Fukuchi et al., 2005). Enhancing the cyclic adenosine monophosphate response element binding protein pathway up-regulates brain-derived neurotrophic factor expression. Chen and Russo-Neustadt (2009) found that cyclic adenosine monophosphate response element binding protein overexpression in mice leads to transcriptional activation of brain-derived neurotrophic factor, and consequent promotion of hippocampal neuronal generation, survival, maturity, and differentiation. Cyclic adenosine monophosphate response element binding protein is also activated by Ca2+-dependent and microtubule related kinases, through which cyclic adenosine monophosphate response element binding protein regulates brain-derived neurotrophic factor expression (Zhao et al., 2005). Anti-depressants do not increase brain-derived neurotrophic factor expression in the brain of cyclic adenosine monophosphate response element binding protein knockout mice (Conti et al., 2002). In addition, there are reported interactions between cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor. Cyclic adenosine monophosphate response element binding protein promotes brain-derived neurotrophic factor generation, while brain-derived neurotrophic factor induces cyclic adenosine monophosphate response element binding protein phosphorylation (Vinet et al., 2004). A previous study found that brain-derived neurotrophic factor induced cyclic adenosine monophosphate response element binding protein protein phosphorylation in cultured neurons from rat visual cortex (Tommaso et al., 2000). Our study indicates that in chronic unpredictable mild stress-exposed rats, Shuganjieyu capsule effectively reverses depressive-like behaviors and increases both brain-derived neurotrophic factor and phosphorylated cyclic adenosine monophosphate response element binding protein expression levels in the medial prefrontal cortex and hippocampal CA3 area. This anti-depressant mechanism may be related to increased neuronal brain-derived neurotrophic factor expression via phosphorylated cyclic adenosine monophosphate response element binding protein activation, and subsequent activation of the cyclic adenosine monophosphate response element binding protein-brain-derived neurotrophic factor signaling pathway. It is interesting to note the crosslink between cyclic adenosine monophosphate response element binding protein and brain-derived neurotrophic factor following antidepressant therapy (Sanjukta, 2012). How Shuganjieyu capsule affects the cyclic adenosine monophosphate response element binding protein-brain-derived neurotrophic factor cascade in depression requires further study.
In summary, the results presented in this study demonstrate for the first time that Shuganjieyu capsule effectively reverses depressive-like behaviors by increasing brain- derived neurotrophic factor and phosphorylated cyclic adenosine monophosphate response element binding protein expression levels in the medial prefrontal cortex and hippocampal CA3 area of depressive-like rats. The anti-depressant mechanism of Shuganjieyu capsule may be associated with up-regulation of brain-derived neurotrophic factor expression through increased phosphorylated cyclic adenosine monophosphate response element binding protein phosphorylation.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81071093, 81171268.
Copyedited by James R, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- [2].Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul J. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- [3].Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- [4].Cattaneo A, Bocchio-Chiavetto L, Zanardini R, Milanesi E, Placentino A, Gennarelli M. Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol. 2010;13:103–108. doi: 10.1017/S1461145709990812. [DOI] [PubMed] [Google Scholar]
- [5].Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen Z, Ye Q, Tan K. A comparison study on suganjieyu capsule and denixit in treatment of depressive symptoms of CHF patients. Zhongguo Jiankang Xinli Xue Zazhi. 2013;21:27–29. [Google Scholar]
- [7].Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deyama T, Nishibe S, Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin. 2001;22:1057–1070. [PubMed] [Google Scholar]
- [9].Fenli S, Feng W, Ronghua Z, Huande L. Biochemical mechanism studies of venlafaxine by metabonomic method in rat model of depression. Eur Rev Med Pharmacol Sci. 2013;17:41–48. [PubMed] [Google Scholar]
- [10].Fuchs E, Czeh B, Kole MHP, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14:S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [11].Fukuchi M, Tabuchi A, Tsuda M. Transcriptional regulation of neuronal genes and its effect on neural functions: Cumulative mRNA expression of PACAP and BDNF genes controlled by calcium and cAMP signals in neurons. J Pharmacol Sci. 2005;98:212–218. doi: 10.1254/jphs.fmj05001x4. [DOI] [PubMed] [Google Scholar]
- [12].George P, CHarles W. Amsterdam: Elsevier Academic Press; 2005. The rat brain in stereotaxic coordinates. [Google Scholar]
- [13].Gibon J, Deloulme JC, Chevallier T, Ladevèze E, Abrous DN, Bouron A. The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int J Neuropsychopharmacol. 2013;16:189–198. doi: 10.1017/S146114571100188X. [DOI] [PubMed] [Google Scholar]
- [14].Guadarrama-Cruz G, Alarcon-Aguilar FJ, Lezama-Velasco R, Vazquez- Palacios G, Bonilla-Jaime H. Antidepressant-like effects of Tagetes lucida Cav. in the forced swimming test. J Ethnopharmacol. 2008;120:277–281. doi: 10.1016/j.jep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [15].Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27:7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamet P, Tremblay J. Genetics and genomics of depression. Metabolism. 2005;54:10–15. doi: 10.1016/j.metabol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [17].Heiser JH, Schuwald AM, Sillani G, Ye L, Müller WE, Leuner K. TRPC6 ch annel-mediated neurite outgrowth in PC12 cells and hippocampal neurons involves activation of RAS/MEK/ERK, PI3K, and CAMKIV signaling. J Neurochem. 2013;127:303–313. doi: 10.1111/jnc.12376. [DOI] [PubMed] [Google Scholar]
- [18].Huang D, Liu X. Effects of acanthopanax senticosus injectionon learning and memory impairmentand monoamine neurotransmitter of hippocampus in aging model rat. Hubei Minzu Xueyuan Xuebao: Yixue Ban. 2008;25:1–4. [Google Scholar]
- [19].Ishida A, Ueda Y, Ishida K, Misumi S, Masuda T, Fujita M, Hida H. Minor neuronal damage and recovered cellular proliferation in the hippocampus after continuous unilateral forelimb restraint in normal rats. J Neurosci Res. 2011;89:457–465. doi: 10.1002/jnr.22566. [DOI] [PubMed] [Google Scholar]
- [20].Jiang RH, Du B, Zhang HY, Shu L, Huang SZ, Xu XF, Zhang XB, Zhao JP. Randomized, doubleblind, double-dummy, multicenter, parallel control clinical trial of Kaiyuanshen in the treatment of depressive disorder. Zhongguo Linchuang Yaoli Xue Zazhi. 2006;22:15–17. [Google Scholar]
- [21].Kiss JP. Theory of active antidepressants: a nonsynaptic approach to the treatment of depression. Neurochem Int. 2008;52:34–39. doi: 10.1016/j.neuint.2007.04.006. [DOI] [PubMed] [Google Scholar]
- [22].Koponen E, Rantamaki T, Voikar V, Saarelainen T, MacDonald E, Castren E. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005;25:973–980. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Laifenfeld D, Karry R, Grauer E, Klein E, Ben-Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- [24].Leuner K, Li W, Amaral MD, Rudolph S, Calfa G, Schuwald AM, Harteneck C, Inoue T, Pozzo-Miller L. Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca2+-permeable TRPC6 channels. Hippocampus. 2013;23:40–52. doi: 10.1002/hipo.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li GB, Lei N, Long J, Chen DS, Long J. The effects of acanthopanacis senticosi pill on the expression of TH and TPH in hippocamp of depressed rats. Shengwu Yixue Jinzhan. 2012;12:1078–1080. [Google Scholar]
- [26].Li YC, Wang FM, Pan Y, Qiang LQ, Cheng G, Zhang WY, Kong LD. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:435–449. doi: 10.1016/j.pnpbp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [27].Luo D, An S, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull. 2008;77:8. doi: 10.1016/j.brainresbull.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [28].Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- [29].Mao QQ, Xian YF, Ip SP, Tsai SH, Che CT. Long-term treatment with peony glycosides reverses chronic unpredictable mild stress-induced depressive-like behavior via increasing expression of neurotrophins in rat brain. Behav Brain Res. 2010;210:171–177. doi: 10.1016/j.bbr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- [30].Muller WE, Rolli M, Schafer C, Hafner U. Effects of hypericum extract (LI 160) in biochemical models of antidepressant activity. Pharmacopsychiatry 30 Suppl. 1997;2:102–107. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- [31].Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: Molecules that modulate our mood? J Biosci. 2006;31:423–434. doi: 10.1007/BF02704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pae CU, Marks DM, Han C. Does neurotropin-3 have a therapeutic implication in major depression? Int J Neurosci. 2008;118:1515–1522. doi: 10.1080/00207450802174589. [DOI] [PubMed] [Google Scholar]
- [34].Park SW, Kim YK, Lee JG, Kim SH, Kim JM, Yoon JS, Park YK, Lee YK, Kim YH. Antidepressant-like effects of the traditional Chinese medicine kami-shoyo-san in rats. Psychiatry Clin Neurosci. 2007;61:401–406. doi: 10.1111/j.1440-1819.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- [35].Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [37].Qiu TW, Liu XW, Zhu HX. A comparative study of shuganjieyu capsule and sertraline in treatment of depression. Hunan Zhongyiyao Daxue Xuebao. 2011;31:60–61. [Google Scholar]
- [38].Rychlik R, Siedentop H, von den Driesch V, Kasper S. St. John's wort extract WS 5572 in minor to moderately severe depression. Effectiveness and tolerance of 600 and 1200 mg active ingredient daily. Fortschr Med Orig. 2001;119:119–128. [PubMed] [Google Scholar]
- [39].Sanjukta M. Cyclic AMP response element binding protein (CREB) in depression: A new role of an old molecule. Curr Neurobiol. 2012;3:3–6. [Google Scholar]
- [40].Sheline Y, Gado M, Kraemer H. Unwanted depression and hippocampal volumen loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- [41].Sun XY, Chen AQ, Xue XF. Randomized, double blind, placebo controlled trial of Shuganjieyu capsule in the treatment of mild or moderate depression. Zhongguo Xinyao Zazhi. 2009;18:413–416. [Google Scholar]
- [42].Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- [44].Thiel CM, Muller CP, Huston JP, Schwarting RK. High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience. 1999;93:243–251. doi: 10.1016/s0306-4522(99)00158-x. [DOI] [PubMed] [Google Scholar]
- [45].Tommaso P, Gian MR, Elena P. Brain-derived neurotrophic factor causes cAMP response element-binding protein phoshorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. Neuroscience. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vinet J, Carra S, Blom JMC, Brunello N, Barden N, Tascedda F. Chronic treatment with desipramine and fluoxetine modulate BDNF, CaMKK alpha and CaMKK beta mRNA levels in the hippocampus of transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuropharmacology. 2004;47:1062–1069. doi: 10.1016/j.neuropharm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- [47].Vry JD, Schreiber R. The chronic mild stress depression model: future developments from a drug discover perspective. Psychophanncology. 1997;134:349–350. doi: 10.1007/s002130050464. [DOI] [PubMed] [Google Scholar]
- [48].Wang JL, Liu P, Tu HH, Wang ZX, Chen GY. Effects of kaixinsan on behavior and expression of p-CREB in hippocampus of chronic stress rats. Zhongguo Zhong Yao Za Zhi. 2007;32:1555–1558. [PubMed] [Google Scholar]
- [49].Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- [50].Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- [51].Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- [52].Xia J, Ye H, Zhou YC. Establishment and evaluation of rat model of chronic unpredictable stress depression. J Huazhong Univ Sci Technolog Med Sci. 2013;34:493–495. [Google Scholar]
- [53].Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, Guitart X, Manji HK. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yulug B, Ozan E, Goenuel AS, Kilic E. Brain-derived neurotrophic factor stress and depression: A minireview. Brain Res Bull. 2009;78:267–269. doi: 10.1016/j.brainresbull.2008.12.002. [DOI] [PubMed] [Google Scholar]
- [55].Zhao L, Chen S, Wang JM, Brinton RD. 17 beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic amp response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- [56].Zhu L, Zhang R, Li T. Effects of acanthopanax on learning and memory and monoamine neurotransmitters in hippocampus of sleep deprived rats. Zhongguo Shiyan Fangji Xue Zazhi. 2012;18:219–223. [Google Scholar]


