Abstract
Temporal lobe epilepsy is associated with astrogliosis. Notch1 signaling can induce astrogliosis in glioma. However, it remains unknown whether Notch1 signaling is involved in the pathogenesis of epilepsy. This study investigated the presence of Notch1, hairy and enhancer of split-1, and glial fibrillary acidic protein in the temporal neocortex and hippocampus of lithium-pilocarpine-treated rats. The presence of Notch1 and hairy and enhancer of split-1 was also explored in brain tissues of patients with intractable temporal lobe epilepsy. Quantitative electroencephalogram analysis and behavioral observations were used as auxiliary measures. Results revealed that the presence of Notch1, hairy and enhancer of split-1, and glial fibrillary acidic protein were enhanced in status epilepticus and vehicle-treated spontaneous recurrent seizures rats, but remain unchanged in the following groups: control, absence of either status epilepticus or spontaneous recurrent seizures, and zileuton-treated spontaneous recurrent seizures. Compared with patient control cases, the presences of Notch1 and hairy and enhancer of split-1 were upregulated in the temporal neocortex of patients with intractable temporal lobe epilepsy. Therefore, these results suggest that Notch1 signaling may play an important role in the onset of temporal lobe epilepsy via astrogliosis. Furthermore, zileuton may be a potential therapeutic strategy for temporal lobe epilepsy by blocking Notch1 signaling.
Keywords: nerve regeneration, brain injury, epilepsy, temporal lobe epilepsy, astrogliosis, Notch1, hairy and enhancer of split-1, glial fibrillary acidic protein, LiCl-pilocarpine, zileuton, the Natural Science Foundation of Hubei Province, neural regeneration
Introduction
Temporal lobe epilepsy (a form of focal epilepsy) is one of the most common forms of refractory epilepsy[1]. Approximately 1.2 million people in China with temporal lobe epilepsy have chronic seizures that cannot be controlled by antiepileptic drugs, consequently leading to cognitive deficits or chronic health problems. A considerable population of new-onset patients with epilepsy exhibit seizures originating from the temporal lobe of the brain. The pathogenesis of temporal lobe epilepsy is associated with underlying structural and functional abnormalities, including enhanced adult hippocampal neurogenesis, mossy fiber sprouting, synaptic reorganization, and astrogliosis[2,3,4].
Research evidence indicates that astrogliosis may lead to drug resistance, and is a typical change observed in the hippocampus during epilepsy[5,6,7]. A number of studies have shown that astrocytes are responsive to brain injury. Different types of brain injury lead to the formation of a glial scar, which is the formation of predominately reactive astrocytes and is characterized by the upregulated expression of glial fibrillary acidic protein. This response aims to reduce secondary injury in the surrounding tissue. This protective effect can reduce the efficiency of regeneration and remyelination of renewable axonal fibers, leading to sustained neural injury, which is likely to cause neuronal cell dysfunction at the injured sites[7]. The number of astrocytes in the mammalian brain after brain injury can be controlled by adjusting these injuries in adulthood. De novo regeneration of astrocytes occurs after brain injury to repair functions[8]. Several inflammatory molecules, including tumor necrosis factor-α and interleukins, have been shown to be involved in the development of glial scarring, characterized by astrogliosis[9,10,11].
The Notch signaling pathway regulates cell fate during embryonic development by facilitating short-range signaling between neighboring cells that are in physical contact[12]. Notch signaling coordinates a wide range of fundamental processes and cellular programs, including proliferation, apoptosis, migration, growth, and differentiation in a context-dependent manner[13,14]. The four mammalian Notch receptors are single-pass type-1 trans-membrane proteins that are expressed on the cell surface[15]. The Notch pathway is activated when a signal-sending cell expressing a membrane-bound ligand physically interacts with a signal-receiving cell expressing a Notch receptor[16]. Upon ligand binding, the Notch receptor is cleaved twice, first by an extracellular matrix metalloprotease and then by the trans-membrane protease complex γ-secretase[17], thereby releasing the Notch intracellular domain[18,19]. After dissociating from the cell membrane, the Notch intracellular domain translocates to the nucleus where it interacts with the DNA-binding protein CSL (J kappa-recombination signal-binding protein, suppressor of hairless, lymphocyte activation gene-1) subsequently affecting transcriptional responses. The most prominent targets of the Notch pathway include a set of basic helix-loop-helix factors of the hairy and enhancer-of-split and hairy and enhancer-of-split-related repressor protein transcription factor families[20]. These transcription factors execute Notch signaling functions, including stem cell maintenance, cell fate specification, differentiation, proliferation, and apoptosis[21]. Recent evidence shows that components of Notch signaling are expressed and active in the adult brain[22]. The dysregulation of Notch signaling is associated with diseases with functional mutations in the key components of this pathway, such as Allagile[23], cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy[24] and Hajdu-Cheney syndromes[25]. Moreover, abnormal levels of Notch are found in Down's syndrome[26] and Alzheimer's disease[27] patients. Each of these diseases has a distinct pathogenesis, thus emphasizing the need to understand the functions of Notch in the adult brain. Tanigaki et al.[28] have shown that in isolated neural stem cells from the adult hippocampus, the ectopic expression of Notch signaling intracellular domains triggered the formation of a higher number of astrocytes and fewer numbers of cells expressing neuronal or oligodendrocyte markers. Recent in vitro evidence indicates that the preferential formation of astrocytes occurs after Notch is activated[29]. The Notch pathway is often regarded as a developmental pathway. However, components of Notch signaling are expressed and active in the adult brain. Furthermore, Notch1 signaling plays a more important role than other Notch families in the adult brain[19]. Therefore, our previous studies focused on Notch1 signaling[25]. Zileuton may modulate the expression levels of gamma-secretase to regulate the activity of Notch1 signaling[19].
Glioma is characterized by astrocytic proliferation, and involves the over-activation of the Notch1 signaling pathway[30,31,32]. Astroglial proliferation is found in epilepsy[33]. However, it remains unknown whether Notch1 signaling is also involved in the pathogenesis of epilepsy. Considering the role of Notch1 signaling in the adult brain and glioma, we sought to determine if Notch1 signaling also participates in the plastic changes that occur during the pathogenesis of temporal lobe epilepsy. Therefore, in the present study, we investigated a possible function of Notch1 signaling in temporal lobe epilepsy by examining the expression of Notch1, hairy and enhancer of split-1, and glial fibrillary acidic protein in epileptic animal models, and Notch1 and hairy and enhancer of split-1 in brain tissue of patients with this condition.
Results
Quantitative analysis of experimental animals
A total of 130 rats were used in this study (however, four rats were excluded because their body weight did not meet the requirements). Rats (n = 8) administered with saline served as the control group and the remaining (n = 118) were used to establish epilepsy models. Rats of this group were treated with pilocarpine (intraperitoneal injection), and 74 successfully developed status epilepticus, of which 16 eventually died. 43 rats did not develop status epilepticus and were used as the status epilepticus-free group. Eight rats from the remaining 58 were acute status epilepticus rats and were thus chosen as the status epilepticus group. The remaining 50 status epilepticus rats went into an incubation period. However, seven died. After 33 ± 6.5 days[34], 19 status epilepticus rats that did not have spontaneous recurrent seizures served as the spontaneous recurrent seizures-free group. The 24 remaining rats were exposed to the spontaneous recurrent seizures phase and behavioral observations by video surveillance showed spontaneous recurrent seizures in this group. 21 rats from this group exhibited frequent seizures and more spike-wave discharges (as observed by the electroencephalogram), thereby serving as the intervention group. 12 of these rats were exposed to 200 mg/kg zileuton (zileuton group) and the nine remaining rats were treated with. Three rats died spontaneously in the zileuton and spontaneous recurrent seizures group. All rats were monitored twice by electroencephalogram for 1–4 hours. These rats were also kept under video observation. Eight rats from each group were included in the final statistics. The remaining rats were euthanized.
Spike-wave discharges increased in status epilepticus rats and spontaneous recurrent seizures rats
Quantitative electroencephalogram analysis displayed electroencephalogram waveforms recorded from the temporal lobes. In the control group, α waves (8–10 Hz, 20–120 µV) were followed by low amplitude β waves and a single δ wave. A total of 24 rats experienced spontaneous recurrent seizures. Among the spontaneous recurrent seizures, 21 rats with frequent seizures had more spike-wave discharges. The electroencephalogram showed that the maximal frequency of spike-wave discharges was 28 Hz and the maximal amplitude was 200 µV. Certain spike-wave discharges occurred individually and particular spikes occurred with a short string in status epilepticus-free rats, status epilepticus rats, spontaneous recurrent seizures-free rats, and zileuton-treated rats (Figure 1). However, saline-treated spontaneous recurrent seizures rats did not exhibit a short string (Figure 1).
Figure 1.
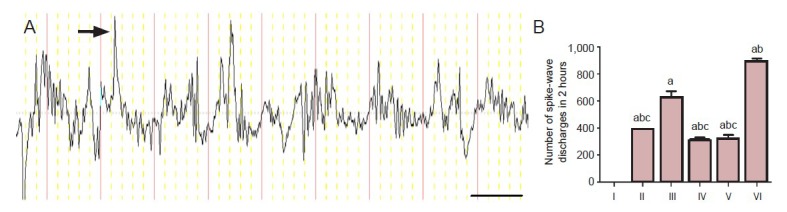
A typical electroencephalogram (EEG) recording and quantification of spike-wave discharges.
(A) A typical EEG (T3) recording from the left temporal cortex of a pilocarpine-induced epileptic rat. The arrow indicates spike-wave discharg-es. Bar: 10 minutes. (B) The number of spike-wave discharges. Values are presented as mean ± SD. One-way analysis of variance was conducted to compare differences among the different groups. aP < 0.05, vs. control group; bP < 0.05, vs. status epilepticus group; cP < 0.05, vs. spontaneous recurrent seizures group. I: Control group; II: status epilepticus-free group; III: status epilepticus group; IV: spontaneous recurrent seizures-free group; V: zileuton group; VI: spontaneous recurrent seizures group.
Behavioral changes in the rats with temporal lobe epilepsy
After pilocarpine treatment, rats experienced several behavioral events, such as oral and masticatory movements, hypokinesia, head nodding, and wet-dog shakes. 74 (65%) rats were eventually affected by status epilepticus. 16 rats died after the onset of status epilepticus. Behavioral observations via video surveillance revealed that 24 (56%) rats that were selected for the chronic phase suffered from spontaneous recurrent seizures. Rats that exhibited spontaneous recurrent seizures were given either zileuton or saline for 1 month. A mild reduction in the frequency of spontaneous recurrent seizures was found in the zileuton-treated group. The frequency of spontaneous recurrent seizures was mildly increased in vehicle-treated. Behavioral observations were subjective, thus statistical analysis was required.
Temporal lobe epilepsy increased the presence of Notch1 receptor, hairy and enhancer of split-1, and glial fibrillary acidic protein
Immunostaining revealed that Notch1 receptor and hairy and enhancer of split-1 were predominantly located in the cortex, hippocampus (mainly in CA3), thalamus, and amygdala of the rats from the control group, status epilepticus-free group, status epilepticus group, spontaneous recurrent seizures-free group, zileuton-treated spontaneous recurrent seizures group, and the vehicle spontaneous recurrent seizures group. Glial fibrillary acidic protein immunostaining was found in the hippocampus from all six groups. Interestingly, the intensity of Notch1 receptor, hairy and enhancer of split-1 and glial fibrillary acidic protein was significantly (P < 0.05) higher in the status epilepticus rats and vehicle spontaneous recurrent seizures rats compared with the other four groups (Figure 2). The presence of Notch1 receptor, hairy and enhancer of split-1, and glial fibrillary acidic protein was significantly P < 0.05) higher in vehicle spontaneous recurrent seizures rats compared with status epilepticus rats (Figure 2). However, no significant differences were found between the vehicle group and the control rats, status epilepticus-free rats, spontaneous recurrent seizures-free rats, and zileuton-treated spontaneous recurrent seizures rats (Figure 2).
Figure 2.
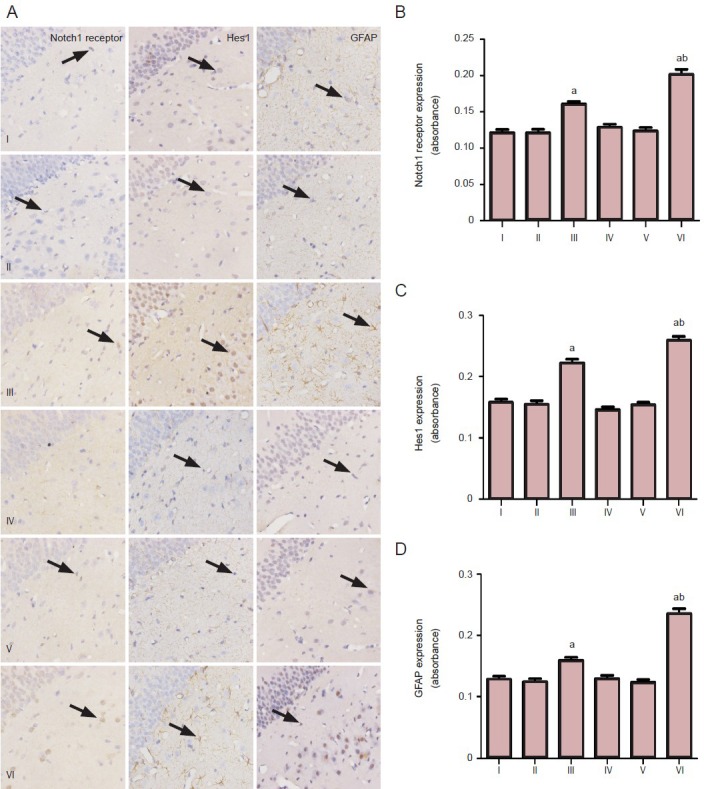
Increased presence of Notch1 receptor, hairy and enhancer of split-1 (Hes1), and glial fibrillary acidic protein (GFAP) in the hippocampal CA3 region of temporal lobe epilepsy rats.
(A) Immunohistochemical staining for Notch1 receptor, Hes1, and GFAP in the hippocampal CA3 region of the temporal lobe epilepsy rats (× 400). Notch1 receptor, Hes1, and GFAP-positive cells are shown by arrows. (B) The intensity of staining of these proteins in the hippocampal CA3 region of temporal lobe epilepsy rats. Values are presented as mean ± SD. One-way analysis of variance was conducted to compare differences among the different groups. aP < 0.05, vs. control group, status epilepticus-free group, spontaneous recurrent seizures-free group and zileuton group; bP < 0.05, vs. status epilepticus group. I: Control group; II: status epilepticus-free group; III: status epilepticus group; IV: spontaneous recur-rent seizures-free group; V: zileuton group; VI: spontaneous recurrent seizures group.
Quantitative analysis and basic data of experimental subjects
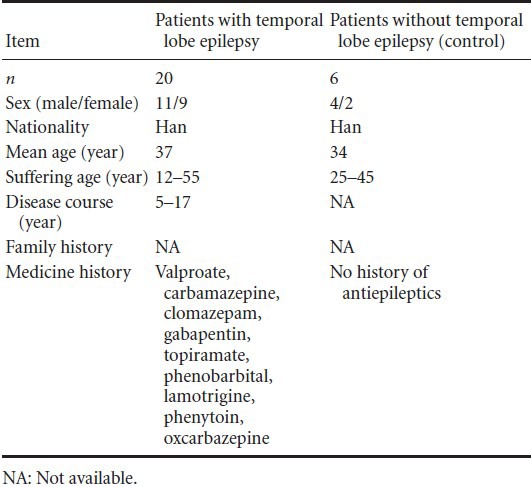
All patients were included in the final analysis. Clinical data of patients in the two groups are listed in Table 1.
Table 1.
Basic data of participants
Prominent presence of Notch1 receptor and hairy and enhancer of split-1 protein in the medial temporal lobe of temporal lobe epilepsy patients
Notch1 receptor and hairy and enhancer of split-1-positive cells were observed in the medial temporal lobe of epileptic and normal slices (Figure 3). Presence of Notch1 receptor and hairy and enhancer of split-1 was significantly (P < 0.05) higher in the medial temporal lobe of epileptic patients compared with control cases.
Figure 3.
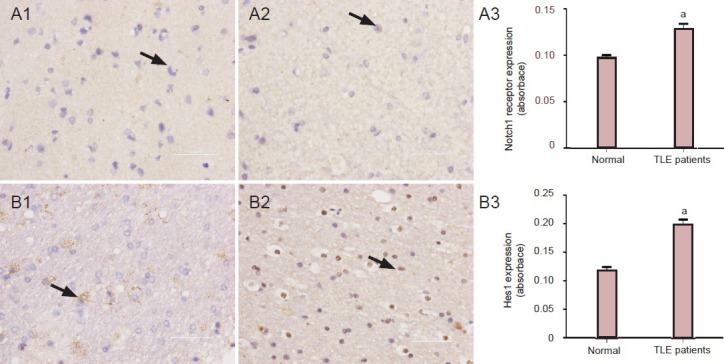
Increased presence of Notch1 receptor and Hes1 in the temporal lobe of temporal lobe epilepsy (TLE) patients.
Immunohistochemistry for Notch1 receptor and Hes1 in the temporal neocortex of a patient without epilepsy (A1, B1) and a patient with intrac-table epilepsy (A2, B2). Notch1 receptor and Hes1 immunoreactive cells are indicated by arrows. Notch1 receptor- (A3) and Hes1 (B3)-immu-noreactivites cells in normal brain tissue and the brain of an intractable epilepsy patient. Values are presented as mean ± SD. Unpaired two-tailed Student's t-test was conducted to compare differences among the different groups. aP < 0.05, vs. control group.
Discussion
Epilepsy is characterized by recurrent spontaneous seizures. Clinically, temporal lobe epilepsy is the most common type of refractory epilepsy and the most common condition in the initial injury response (e.g., status epilepticus)[35]. Among the different types of initial precipitating injuries, intraperitoneal injection of pilocarpine is feasible, simple, and widely-used[36,37]. This model resembles the changes seen in human temporal lobe epilepsy, such as spontaneous recurrent seizures, hippocampal cell loss, and astrogliosis[38]. In addition, the pilocarpine model is established by a simple operation with no need for high-end technology and equipment. Moreover, unlike the stimulation model, this model does not induce as much pain in rats and is thus more accommodating with ethical requirements. In pilocarpine-inducing epileptic rats, the relative regularity of chronic spontaneous seizures can be observed during their lifetime[39]. In view of the above advantages, we used the pilocarpine model to study the pathogenesis of temporal lobe epilepsy.
Astrogliosis is a significant feature of temporal lobe epilepsy[40]. Rapid astrocytic activation occurs in the hippocampus of temporal lobe epilepsy models[41,42]. Such changes cause neuronal damage and are related to abnormal neurotransmission[43]. Recently, an increasing number of studies has demonstrated the role of glial cells in the pathogenesis of epilepsy, including their effects on synaptic transmission[44,45]. Reactive astrogliosis is characterized by molecular, cellular, morphological, and functional changes classified as: (1) changes in gene expression, (2) cell hypertrophy with changes in function, and (3) secondary proliferation of astrocytes and other types of cells, organizational restructuring, and collagen deposition[46]. Changes in astrocytic transporters and receptors in hippocampal sclerosis of temporal lobe epilepsy[7] lead to functional changes of water channels and ion channels in reactive astrocytes, resulting in a deteriorated environment at the onset and development of epilepsy. With the changes in both glutamate transporters and receptors, and other neurotransmitters of glial cells, astrocytic proliferation has been hypothesized to play an important role in hyperexcitability and spontaneous seizures[47].
Receptors of the Notch family are highly conserved trans-membrane receptors that influence the proliferation and apoptosis of diverse types of cells in a variety of organisms[48]. Activation of Notch signaling requires the binding of Jagged and Delta like ligands followed by the release of the Notch intracellular domain by gamma-secretase and its translocation to the nucleus[49]. Notch intracellular domain interacts with CSL transcription factors (CBF1/RBP-Jk, Su(H), Lag-1) and converts them from repressors to activators, promoting the transcription of downstream genes involved in various differentiation programs. In previous studies, astrogliosis has been observed in epilepsy. Interestingly, glioma and epilepsy share a feature of astrogliosis. Some reports have shown that Notch1 signaling may induce astrogliosis. Notch1 signaling plays a more important role than other Notch families in the adult brain[6,19,50]. Therefore, Notch1 was chosen for investigation in the present study.
The 5-lipoxygenase inhibitor, zileuton, can modulate expression levels of gamma-secretase. A previous study reported that zileuton has no regulatory effects on Notch signaling in Alzheimer's disease[19] due to an intact blood brain barrier. The blood brain barrier plays an important role in the homeostasis of the central nervous system, and its breakdown is known to be associated with epileptic seizures[51,52,53,54]. The administration, dosage, and intervention times of zileuton in the present study differed from those of previous studies. Zileuton may play a role in the regulation of the Notch signaling.
In previous studies, the status epilepticus-free group and spontaneous recurrent seizures-free group have often been overlooked. Experimental data from these groups may be helpful in the study of epilepsy. The status epilepticus-free group may help to identify the causal relationship between the research indicators and epilepsy. Spontaneous recurrent seizure-free rats share similar characteristics with patients who have seizures only once. Therefore, these two groups were used in the present study to describe the possible role of Notch1 signaling in the pathogenesis of epilepsy.
More research is required to identify whether zileuton affects Notch signaling, and how this signaling mediates astrogliosis. Moreover, further studies are needed to investigate the effects of modulating the activity Notch signaling activity in temporal lobe epilepsy.
In summary, increased presence of Notch was observed in intractable temporal lobe epilepsy patients and experimental animals. Therefore, Notch signaling may play a role in the pathogenesis of epilepsy in temporal lobe epilepsy and its suppression may provide a new treatment to promote remission in epilepsy.
Materials, Subjects and Methods
Design
A randomized controlled animal study and clinical pathological study.
Time and setting
This study was performed in the Neurological Laboratories of Tongji Medical College, Huazhong University of Science and Technology, China from March 2011 to April 2012.
Materials and Subjects
Animals
A total of 130 clean male Sprague-Dawley rats weighing 200–230 g were obtained from Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology, China. Animals were housed between 24 and 25°C at 50–60% humidity in a 12-hour light/dark cycle, and allowed free access to food and water. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals by the Ministry of Science and Technology of China[55].
Subjects
A total of 26 temporal lobe epilepsy patients from Wuhan Union Hospital in China were enrolled in this experiment. Twenty of the 26 patients (11 males and 9 females; 12–55 years old) with intractable temporal lobe epilepsy had been treated with antiepileptic drugs, including valproate, carbamazepine, clomazepam, gabapentin, topiramate, phenobarbital, lamotrigine, phenytoin, or oxcarbazepine. Pre-surgical assessment included a detailed history and a series of neurological examinations, such as neuropsychological test, ictal electroencephalogram studies, computed tomography scans, and magnetic resonance imaging. Before resection, all patients were subjected to intraoperative electrocorticography. An effective treatment option for intractable epilepsy patients was to remove the epileptogenic zone located in the anterior temporal neocortex. The electrodes for the intraoperative electroencephalogram were placed on the remaining edge of the tissue to ensure that the lesion had been completely removed after its resection. Six head trauma patients (4 males and 2 females; 25–45 years old) were used for comparative purposes in the electrocorticography because their temporal neocortexes were histologically normal. All of these patients had no history of epilepsy or exposure to antiepileptic drugs[56]. All of these patients or their immediate relatives signed written consent forms for the use of brain tissue.
Drug
(±)-N-hydroxy-N-(1-benzo[b]thien-2-ylethyl) urea (Zileuton) (Approval CAS No. 111406-87-2) was purchased from Wuhan GPC-China Chemistry Co., Ltd., Wuhan, Hubei Province, China.
Methods
Establishment of the temporal lobe epilepsy animal model
Rats were treated with lithium chloride (Sigma-Aldrich, Santa Clara, CA, USA; 127 mg/kg) intraperitoneally (i.p.). After 18 hours, animals were injected with pilocarpine (15 mg/kg, i.p.) (Sigma-Aldrich) diluted in physiological saline. Atropine sulfate (1 mg/kg, i.p) (Harvest, Shanghai, China) was injected approximately 30 minutes before pilocarpine administration. Seizure severity was graded according to Racine's standard five-stage scale: Stage 1, mouth and face movements; stage 2, head nods; stage 3, clonus of the unilateral forelimb; stage 4, clonus and rears of the bilateral forelimb; stage 5, falling episodes after a stage 4 seizure[57]. Pilocarpine was administered every 30 minutes if seizure attacks or seizure activities were above stage 4 of the Racine's scale. Rats graded at stage 4 or stage 5 were used in the present study. The maximum dose for pilocarpine was 60 mg/kg. Status epilepticus lasting for 1 hour was stopped by diazepam (10 mg/kg, i.p.) (Jinyao, Tianjin, China)[58,59,60,61]. Rats with status epilepticus who entered the latency period at 24 hours after an acute attack were selected and reared in single cages.
Drug administration
The final concentration of zileuton in the drinking water was 10 mg/mL. Rats in the zileuton group were given zileuton (200 mg/kg, oral gavage, once a day for one month) when they experienced regular spontaneous recurrent seizures.
Electroencephalogram recordings
Cortical electroencephalogram has been more frequently used in the study of animal models of epilepsy in the past than at present[62,63]. Cortical electroencephalogram has many advantages over than scalp electroencephalogram. This technology provides more precise positioning information of the abnormal discharge position and can increase the probability of capturing slight abnormal discharges. However, cortical electroencephalogram is more complicated to operate and can lead to greater suffering of the experimental animal, increasing the risk of death[64]. Interestingly, waveforms recorded by scalp electroencephalogram are similar to those of cortical electroencephalogram. Some studies have also shown that chloral hydrate has little impact on the electroencephalogram waveform at a sedative dose[65,66]. Therefore, we selected scalp electroencephalogram over cortical electroencephalogram in the present study. Rats were anesthetized with chloral hydrate (3 mL/kg, i.p.) to record the electroencephalogram. After rats were fixed, two small silver needles, which served as the electrodes, were inserted underneath the scalp on both sides of the temporal region (0.65 cm in front of the connection of the external ear gate and 0.4 cm beside the center line). The reference electrode was inserted underneath the scalp of the frontal pole mid-point (1.2 cm in front of the external ear gate). Electroencephalogram signals that were filtered below 0.53 Hz and above 30 Hz were subjected to an analog-to-digital conversion by a dynamic electro-encephalograph (Symtop Instrument Co., Ltd., Beijing, China). All rats underwent electroencephalogram monitoring (once a week for 2–4 hours) to record electroencephalogram patterns. The numbers of spike-wave discharges were summarized when rats entered the status epilepticus and the spontaneous recurrent seizures period. Some rats with spontaneous recurrent seizures were given zileuton or vehicle for one month. For the spontaneous recurrent seizure rats not treated with the test drug, electroencephalogram was recorded separately.
Behavioral observation
Fifteen days after the onset of status epilepticus, video surveillance was used to monitor the occurrences of spontaneous recurrent seizures throughout the day. Behavioral parameters, such as latency period (the period between the acute seizure and spontaneous recurrent seizures), seizure frequency, duration of the chronic phase, and mortality during all periods were also recorded.
Tissue processing
After rats were anesthetized (10% chloral hydrate; 0.3 mL/100 g, i.p.), the abdominal cavity was opened and animals were rapidly perfused (via the left ventricle) with saline until the perfusate turned clear. The perfusate was then switched to 4% paraformaldehyde in PBS. Brains were sliced into sections (4 µm) and paraffin-embedded. Human brain tissue samples were taken from the medial temporal lobe. These samples were immediately fixed with 4% paraformaldehyde in PBS followed by processes previously described[60]. All samples from patients were obtained within 30 minutes to 3 hours.
Immunohistochemical staining
Slices were dewaxed at 65°C for 2 hours and rinsed (3 × 5 minutes) in PBS and then incubated with 0.3% hydrogen peroxide at room temperature to block endogenous peroxidase activity. Slices were then incubated with bovine serum albumin at room temperature for 20 minutes. followed by rabbit anti-Notch1 polyclonal antibody (1:100; Cell Signaling, Boston, MA, USA), rabbit anti-rat/human hairy and enhancer of split-1 monoclonal antibody (1:100; Epitomics, Burlingame, CA, USA) or rabbit anti-glial fibrillary acidic protein polyclonal antibody (1:100; ProteinTech Group Inc., Chicago, IL, USA), diluted in PBS (0.01 mol/L, pH 7.4). Sections were stored overnight inside a box at 4°C, and then incubated for 2 hours at 37°C and rinsed in PBS (3 × 5 minutes). The slices were subsequently incubated with goat anti-rabbit IgG (1:50; KPL, Washington, DC, USA) for 30 minutes at 37°C, rinsed in PBS (3 × 5 minutes) then exposed to horseradish peroxidase at 37°C for 30 minutes and rinsed (3 × 5 minutes) in PBS. Sections were stained with 3,3′-diaminobenzidine at room temperature in the dark for 10 minutes. The reaction was terminated with distilled water and the slices were stained with hematoxylin. Sections were then dehydrated in graded ethanol. The same steps were carried out in the negative control group, but the primary antibody was replaced with PBS. Slices were photographed (NIKON ECLIPSE TI-SR, Tokyo, Japan) at the same magnification (× 400) and light intensity. After adjustment of the gray scale, the mean absorbance value of each slice was measured by Image-Pro plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). The presence of positive particles was measured in terms of absorbance values.
Statistical analysis
All values were presented as mean ± SD and were analyzed by the one-way analysis of variance. The unpaired two-tailed student t-test was employed to compare the presence levels of Notch1 receptor and hairy and enhancer of split-1 with patients with or without intractable epilepsy. Statistical analysis was carried out using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
Acknowledgments
We are very grateful to Jiang XB (Department of Cerebral Surgery) and Zheng XB (electroencephalogram Laboratory) of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology in China.
Footnotes
Conflicts of interest: None declared.
Funding: This study was funded by the Natural Science Foundation of Hubei Province in China, No. 02.02.040458.
Copyedited by Farso M, Chen QX, Chen ZY, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16(2):165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- [2].Coiret G, Ster J, Grewe B, et al. Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS One. 2012;7(5):e37320. doi: 10.1371/journal.pone.0037320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li LM, Cendes F, Andermann F, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122(Pt 5):799–805. doi: 10.1093/brain/122.5.799. [DOI] [PubMed] [Google Scholar]
- [4].Hammen T, Hildebrandt M, Stadlbauer A, et al. Non-invasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed. 2008;21(6):545–552. doi: 10.1002/nbm.1222. [DOI] [PubMed] [Google Scholar]
- [5].Spencer SS. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1(6):375–382. doi: 10.1016/s1474-4422(02)00163-1. [DOI] [PubMed] [Google Scholar]
- [6].Fedele DE, Gouder N, Güttinger M, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128(Pt 10):2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- [7].Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- [8].Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994;53(3):213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- [9].Muir EM, Adcock KH, Morgenstern DA, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100(1-2):103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- [10].Yi H, Cho HJ, Cho SM, et al. Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol. 2012;41(1):310–316. doi: 10.3892/ijo.2012.1447. [DOI] [PubMed] [Google Scholar]
- [11].Sandvig A, Berry M, Barrett LB, et al. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46(3):225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- [12].Artavanis-Tsakonas S, Muskavitch MA. Notch: the past, the present, and the future. Curr Top Dev Biol. 2010;92:1–29. doi: 10.1016/S0070-2153(10)92001-2. [DOI] [PubMed] [Google Scholar]
- [13].Sethi N, Kang Y. Notch signaling: mediator and therapeutic target of bone metastasis. Bonekey Rep. 2012;1:3. doi: 10.1038/bonekey.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69(5):840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- [15].Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011;43(11):1550–1562. doi: 10.1016/j.biocel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gray GE, Mann RS, Mitsiadis E, et al. Human ligands of the Notch receptor. Am J Pathol. 1999;154(3):785–794. doi: 10.1016/S0002-9440(10)65325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saxena MT, Schroeter EH, Mumm JS, et al. Murine notch homologs (N1-4) undergo presenilin-dependent proteolysis. J Biol Chem. 2001;276(43):40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- [18].Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- [19].Mumm JS, Schroeter EH, Saxena MT, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5(2):197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- [20].Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194(3):237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- [21].Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer. 2011;105(12):1805–1810. doi: 10.1038/bjc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ables JL, Breunig JJ, Eisch AJ, et al. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12(5):269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fabris L, Cadamuro M, Guido M, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171(2):641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Spinner NB. CADASIL: Notch signaling defect or protein accumulation problem? J Clin Invest. 2000;105(5):561–562. doi: 10.1172/JCI9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simpson MA, Irving MD, Asilmaz E, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43(4):303–305. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- [26].Fischer DF, van Dijk R, Sluijs JA, et al. Activation of the Notch pathway in Down syndrome: cross-talk of Notch and APP. FASEB J. 2005;19(11):1451–1458. doi: 10.1096/fj.04-3395.com. [DOI] [PubMed] [Google Scholar]
- [27].Anderton BH, Dayanandan R, Killick R, et al. Does dysregulation of the Notch and wingless/Wnt pathways underlie the pathogenesis of Alzheimer's disease? Mol Med Today. 2000;6(2):54–59. doi: 10.1016/s1357-4310(99)01640-8. [DOI] [PubMed] [Google Scholar]
- [28].Tanigaki K, Nogaki F, Takahashi J, et al. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29(1):45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- [29].Givogri MI, de Planell M, Galbiati F, et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28(1-2):81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- [30].Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65(6):2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- [31].Kanamori M, Kawaguchi T, Nigro JM, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- [32].Zhang X, Chen T, Zhang J, et al. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation? Cancer Sci. 2012;103(2):181–190. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- [33].Niquet J, Jorquera I, Ben-Ari Y, et al. Proliferative astrocytes may express fibronectin-like protein in the hippocampus of epileptic rats. Neurosci Lett. 1994;180(1):13–16. doi: 10.1016/0304-3940(94)90902-4. [DOI] [PubMed] [Google Scholar]
- [34].Yang Z, Liu X, Yin Y, et al. Involvement of 5-HT7 receptors in the pathogenesis of temporal lobe epilepsy. Eur J Pharmacol. 2012;685(1-3):52–58. doi: 10.1016/j.ejphar.2012.04.011. [DOI] [PubMed] [Google Scholar]
- [35].Lewis DV. Losing neurons: selective vulnerability and mesial temporal sclerosis. Epilepsia. 2005;46(Suppl 7):39–44. doi: 10.1111/j.1528-1167.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- [36].Curia G, Longo D, Biagini G, et al. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172(2):143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Glien M, Brandt C, Potschka H, et al. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43(4):350–357. doi: 10.1046/j.1528-1157.2002.18101.x. [DOI] [PubMed] [Google Scholar]
- [38].Mathern GW, Pretorius JK, Babb TL. Quantified patterns of mossy fiber sprouting and neuron densities in hippocampal and lesional seizures. J Neurosurg. 1995;82(2):211–219. doi: 10.3171/jns.1995.82.2.0211. [DOI] [PubMed] [Google Scholar]
- [39].Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26(1):115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- [40].Hudson LP, Munoz DG, Miller L, et al. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33(6):622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- [41].Hüttmann K, Sadgrove M, Wallraff A, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18(10):2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- [42].Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- [43].Kang TC, Kim DS, Kwak SE, et al. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia. 2006;54(4):258–271. doi: 10.1002/glia.20380. [DOI] [PubMed] [Google Scholar]
- [44].Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- [45].Tian GF, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11(9):973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Binder DK, Steinhäuser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54(5):358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- [48].Xue SL, Wang XS, Li DC, et al. Notch signal pathway effect on receptor protein regulation by transforming growth factor beta in primary human keratinocytes. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(2):296–300. [Google Scholar]
- [49].Herreman A, Van Gassen G, Bentahir M, et al. gamma-Secretase activity requires the presenilin-dependent trafficking of nicastrin through the Golgi apparatus but not its complex glycosylation. J Cell Sci. 2003;116(Pt 6):1127–1136. doi: 10.1242/jcs.00292. [DOI] [PubMed] [Google Scholar]
- [50].Malhotra SK, Luong LT, Bhatnagar R, et al. Up-regulation of reactive astrogliosis in the rat glioma 9L cell line by combined mechanical and chemical injuries. Cytobios. 1997;89(357):115–134. [PubMed] [Google Scholar]
- [51].Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85(2-3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Vliet EA, da Costa Araújo S, Redeker S, et al. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(Pt 2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- [53].Nitsch C, Klatzo I. Regional patterns of blood-brain barrier breakdown during epileptiform seizures induced by various convulsive agents. J Neurol Sci. 1983;59(3):305–322. doi: 10.1016/0022-510x(83)90016-3. [DOI] [PubMed] [Google Scholar]
- [54].Oztas B, Kaya M. The effect of acute hypertension on blood-brain barrier permeability to albumin during experimentally induced epileptic seizures. Pharmacol Res. 1991;23(1):41–46. doi: 10.1016/s1043-6618(05)80104-5. [DOI] [PubMed] [Google Scholar]
- [55].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [56].Fang M, Lu Y, Chen GJ, et al. Increased expression of sonic hedgehog in temporal lobe epileptic foci in humans and experimental rats. Neuroscience. 2011;182:62–70. doi: 10.1016/j.neuroscience.2011.02.060. [DOI] [PubMed] [Google Scholar]
- [57].Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32(3):295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- [58].Carter DS, Harrison AJ, Falenski KW, et al. Long-term decrease in calbindin-D28K expression in the hippocampus of epileptic rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2008;79(2-3):213–223. doi: 10.1016/j.eplepsyres.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Erakovic V, Zupan G, Varljen J, et al. Lithium plus pilocarpine induced status epilepticus--biochemical changes. Neurosci Res. 2000;36(2):157–166. doi: 10.1016/s0168-0102(99)00120-0. [DOI] [PubMed] [Google Scholar]
- [60].Leroy C, Roch C, Koning E, et al. In the lithium-pilocarpine model of epilepsy, brain lesions are not linked to changes in blood-brain barrier permeability: an autoradiographic study in adult and developing rats. Exp Neurol. 2003;182(2):361–372. doi: 10.1016/s0014-4886(03)00122-5. [DOI] [PubMed] [Google Scholar]
- [61].Rigoulot MA, Koning E, Ferrandon A, et al. Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther. 2004;308(2):787–795. doi: 10.1124/jpet.103.057091. [DOI] [PubMed] [Google Scholar]
- [62].Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33(6):635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- [63].Raol YS, Budreck EC, Brooks-Kayal AR. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53(4):503–511. doi: 10.1002/ana.10490. [DOI] [PubMed] [Google Scholar]
- [64].MacDougall KW, Burneo JG, McLachlan RS, et al. Outcome of epilepsy surgery in patients investigated with subdural electrodes. Epilepsy Res. 2009;85(2-3):235–242. doi: 10.1016/j.eplepsyres.2009.03.014. [DOI] [PubMed] [Google Scholar]
- [65].Olson DM, Sheehan MG, Thompson W, et al. Sedation of children for electroencephalograms. Pediatrics. 2001;108(1):163–165. doi: 10.1542/peds.108.1.163. [DOI] [PubMed] [Google Scholar]
- [66].Thoresen M, Henriksen O, Wannag E, et al. Does a sedative dose of chloral hydrate modify the EEG of children with epilepsy? Electroencephalogr Clin Neurophysiol. 1997;102(2):152–157. doi: 10.1016/s0921-884x(96)96509-1. [DOI] [PubMed] [Google Scholar]


