Abstract
Tongluojiunao (TLJN) is an herbal medicine consisting of two main components, geniposide and ginsenoside Rg1. TLJN has been shown to protect primary cultured hippocampal neurons. However, its mechanism of action remains unclear. In the present study, primary cultured hippocampal neurons treated with Aβ1–42 (10 µmol/L) significantly increased the release of lactate dehydrogenase, which was markedly reduced by TLJN (2 µL/mL), specifically by the component geniposide (26 µmol/L), but not ginsenoside Rg1 (2.5 µmol/L). The estrogen receptor inhibitor, ICI182780 (1 µmol/L), did not block TLJN- or geniposide-mediated decrease of lactate dehydrogenase under Aβ1–42-exposed conditions. However, the phosphatidyl inositol 3-kinase or mitogen-activated protein kinase pathway inhibitor, LY294002 (50 µmol/L) or U0126 (10 µmol/L), respectively blocked the decrease of lactate dehydrogenase mediated by TLJN or geniposide. Therefore, these results suggest that the non-classical estrogen pathway (i.e., phosphatidyl inositol 3-kinase or mitogen-activated protein kinase) is involved in the neuroprotective effect of TLJN, specifically its component, geniposide, against Aβ1–42-mediated cell death in primary cultured hippocampal neurons.
Keywords: nerve regeneration, neurodegeneration, Alzheimer's disease, cell culture, hippocampus, neurons, Aβ1–42, estrogen signaling pathway, phosphatidyl inositol 3-kinase pathway, mitogen-activated protein kinase pathway, Tongluojiunao injection, geniposide, ginsenoside Rg1, NSFC grant, neural regeneration
Introduction
Alzheimer's disense is a common neurodegenerative disease in the aged population (Maurer et al., 1997; Dong and Chai, 2013). Amyloid-β (Aβ) plaques and neurofibrillary tangles are the main pathological hallmarks of Alzheimer's disense, as well as the loss of neurons and synapses (Bard et al., 2000; Hardy and Selkoe, 2002; Taylor et al., 2002; Ross and Poirier, 2004). Women have a higher risk than men for developing Alzheimer's disense, mainly due to the abrupt decline in estrogen levels during menopause (Winkler and Fox, 2013). Studies have indicated that estrogen is one of the most important signals for maintaining neuronal function (Burek et al., 1995; Morale et al., 2006), and is protective against brain injury, neurodegeneration, and cognitive decline (Dubal et al., 2001). Because of its anti-Aβ action, estrogen was used as a potential drug to treat Alzheimer's disense. (Liang et al., 2010a). In addition to the important role of estrogen in the regulation of reproduction, the immune system, bone maintenance, and cardiovascular disease, recent reports have shown that estrogen exerts neuroprotective effects. However, large clinical trials in postmenopausal women indicated adverse side-effects of estrogens, such as increased incidence of breast cancer and metrocarcinoma, thereby preventing clinical use of estrogen. Therefore, scientists have now turned to exploit phytoestrogens as a potential safer alternative for age-related cognitive decline (Lephart et al., 2002; Sumien et al., 2013).
Two main estrogen signaling transduction pathways exist, the classical and non-classical signaling pathways (Coleman and Smith, 2001; Hall et al., 2001). The classical signaling pathway is mediated by estrogen receptors, ERα and ERβ (Shughrue et al., 1997; Dubal et al., 2001), which are constitutively expressed in many brain regions, initiating gene transcription after specifically binding to estradiol. The non-classical signaling pathway acts mainly via phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) (Marin et al., 2005; Quesada et al., 2008). Studies of the classical estrogen pathway using the estrogen receptor steroidal inhibitor, ICI182780 (fulvestrant), have shown that this inhibitor prevents the translocation of the estrogen receptor into the nucleus where it is downregulated via homodimerization (Jakacka et al., 2001). This inhibitor also interferes with the binding site of the estrogen receptor in the nucleus and reduces the estrogen receptor and estrogen receptor response element, therefore completely blocking the estrogenic classical pathway (Dauvois et al., 1993; Chanda et al., 2000; Björnström and Sjöberg, 2005). In the non-classical estrogen pathway, estrogen requires the participation of intracellular signaling transduction molecules, mainly the PI3K and MAPK pathways. PI3K is a crucial signaling pathway that can be repressed by the inhibitor, LY294002 (Hui et al., 2005; Fan et al., 2006). MAPK is one of most important pathways in eukaryote signaling transmission and plays key roles in regulating gene expression and the functional activity of the cytoplasm. U0126 is an inhibitor of the MAPK pathway (MacDonald et al., 2001; Martin et al., 2003).
Senile plaques are one of the characteristic hallmarks of Alzheimer's disense and are formed predominantly of Aβ, which is a 42-aminoacid peptide and a potential target in the treatment of this disease (Gilbert, 2013; Honig and Boyd, 2013). Accumulating evidence has suggested that Aβ1–42 is neurotoxic and exhibits apoptotic effects in cultured hippocampal neurons (Loo et al., 1993). Therefore, in the present study, Aβ-mediated injury of primary cultured neurons was used as an in vitro model of Alzheimer's disense. Tongluojiunao (TLJN) injection is a traditional Chinese medicine preparation, clinically efficacious in the treatment of ischemic cerebral stroke and dementia (Hui et al., 2005; Hua et al., 2010). The main ingredients of TLJN injection are geniposide and ginsenoside Rg1 (Hua et al., 2010). Ginsenoside Rg1 is one of main components of TLJN, which has been reported to be neuroprotective and reduce toxicity (Gong and Zhang, 1999; Chen et al., 2003; Leung et al., 2007; Gao et al., 2009). Ginsenoside Rg1 is neuroprotective against glutamate-exposed mesencephalic dopaminergic cells (Radad et al., 2004). Geniposide is another main component of Gardenia jasminoides, which is widely used in Chinese traditional medicine, and has been shown be protective against Aβ-, CoCl2-, and H2O2-mediated neuronal apoptosis (Guo et al., 2009; Liu et al., 2009; Liang et al., 2010b). Geniposide protects PC12 cells from hydrogen peroxide-induced cell death via the activation of glucagon-like peptide 1 receptor (Liu et al., 2009). Furthermore, geniposide regulates insulin secretion by activating the glucagon-like peptide 1 receptor (Radad et al., 2004). TLJN has been reported to reduce brain ischemic damage and increase the expression of brain derived neurotrophic factor (Alesheikh et al., 2011). Moreover, previous reports have shown that TLJN is neuroprotective against Aβ-induced injuries (Hua et al., 2010; Liu et al., 2011; Li et al., 2012).
We have previously reported that TLJN improves cognitive performance, primarily via the up-regulation of insulin-degrading enzyme and neprilysin, which promote the degradation of Aβ and clear amyloid plaque from the brain of Alzheimer's disense rats (Liu et al., 2011). Previous unpublished data have shown that improvement of learning and memory by TLJN in Alzheimer's disense rats is gender dependent, in which male Alzheimer's disense rats perform better than female counterparts after drug treatment.
In the present study, we compared the effects of TLJN injection and estrogen on Aβ-exposed primary cultured hippocampal neurons as Alzheimer's disease-like cell models.
Materials and Methods
Animals
Sprague-Dawley rats (gestational age 16–18 days) were obtained from the Institute of Biophysics, Chinese Academy of Science, China (license No. SYXK (SPF) 2007-14). The animals were raised in a specific pathogen-free facility under a 12-hour light/dark cycle with ad libitum access to food and water. All experimental procedures were performed in accordance with the animal research regulation of the Chinese National Natural Science Foundation and the animal care guidelines of the National Institutes of Health.
Drugs
TLJN injection was provided by the Pharmaceutical Factory of Beijing University of Chinese Medicine (Beijing, China; Chinese SFDA: 2004 L01620). The concentrations of geniposide (4.95 mg/mL) and ginsenoside Rg1 (1.02 mg/mL) in TLJN were determined by high performance liquid chromatography (Hua et al., 2010). Purified geniposide (lot: 110749-200714) and ginsenoside Rg1 (lot: 110703-200726) were purchased from the National Institutes for Food and Drug (Beijing, China).
Primary hippocampal neuronal cultures
Primary hippocampal neurons were prepared from Sprague- Dawley rats at embryonic days 16–18. Pregnant rats were anesthetized with 10% chloral hydrate (400 mg/kg) and disinfected with 75% alcohol, and fetuses were removed. After fetuses were sacrificed with an over-dose of chloral hydrate, the skull was opened, the cerebral cortex removed (by ophthalmological forceps), and the separated to reveal the two hippocampi (Joseph, 2008). The hippocampi were dissected, rinsed in Hank's Balanced Salt solution, and then cut into pieces before tissue digestion with 0.25% trypsin for 15 minutes at 37°C. Digestion was stopped by the isometric trypsin inhibitor. The tissue suspension was gently triturated with a pipette and filtered through a 200 mesh screen. To remove the supernatant, the filtrate was centrifuged at 1,580 × g for 5 minutes at 4°C. Cells were plated at a density of 1 × 106 cells/mL and grown in Neurobasal Medium (Invitrogen, Carlsbad, CA, USA) containing B27supplement (Invitrogen), without fetal bovine serum, and maintained in 5% CO2, 90% humidity at 37°C for 4 days (Wu et al., 2012; Quan et al., 2013).
Treatments
Hippocampal cultures were exposed to 10 µmol/L Aβ1–42 (American Peptide, Sunnyvale, CA, USA) for 72 hours (Kashiwaya et al., 2000). The cell medium was then freshly replaced and cells were incubated with 1 µmol/L ICI182780 (Tocris Bioscience, Ellisville, MO, USA) for 30 minutes, 50 µmol/L LY294002 (Cell Signaling, Boston, MA, USA) for 1 hour, or 10 µmol/L U0126 (Cell Signaling) for 2 hours (Chaulet et al., 2001; Lee et al., 2006; Zhang et al., 2010). The inhibitors were removed by replacing the medium with neurobasal medium (Invitrogen, Carlsbad, CA, USA) containing B27 supplement, without fetal bovine serum, and the cells were then treated with 2 µL/mL TLJN, 26 µmol/L geniposide, 2.5 µmol/L ginsenoside Rg1, or 1 nmol/L estradiol (E2) for 72 hours before analyses (Hua et al., 2010; Sun et al., 2013).
Morphology of hippocampal neurons
The morphology of hippocampal neurons was observed in ten randomly selected visual fields under an inverted microscope (NIKON TE2000-S, Tokyo, Japan) 1, 5, 7 and 9 days after treatment.
Immunofluorescence for neurons
Microtubule-associated protein 2 (MAP-2) was used to identify neurons, and Hoechst 33342 was used to stain the nucleus (Gong et al., 2012). Neurons were cultured for 7 days at a density of 1 × 106 cell/mL on 12-mm cover slips coated with poly-L-lysine. Cells were fixed with cold 4% paraformaldehyde for 15 minutes, followed by 10 minute permeabilization in 0.1% Triton X-100 at room temperature, then rinsed three times for 5 minutes with PBS, and blocked with 0.5% bovine serum albumin solution for 30 minutes. Cells were then incubated with rabbit MAP-2 polyclonal antibody (1:500; Chemicon, Billerica, MA, USA) overnight at 4°C followed by three washes with PBS. Cells were incubated with fluorescein isothiocyanate goat anti-rabbit IgG (1:50; Cwbio, Beijing, China) for 1 hour at room temperature, then washed with PBS and double stained with Hoechst 33342 (10 µg/mL) (Beyotime, Haimen, Jiangsu Province, China) for 5 minutes at room temperature. Staining was observed under a confocal laser scanning microscope (Zeiss, LSM 510, Oberkochen, Baden-Württemberg, Germany). Cell and nuclei counts were performed from ten randomly selected visual fields. The purity of hippocampal neurons was calculated as the number of hippocampal neurons/the number of cell nuclei × 100%.
Cell viability analysis
Cell viability was analyzed at day 11 by measuring the release of lactate dehydrogenase (LDH) (Decker and Lohmann-Matthes, 1988) using the LDH kit (Roche, Basel, Switzerland).
Statistical analysis
All data are expressed as mean ± SD (with each experiment in at least triplicate) and were analyzed either by the one-way analysis of variance or the independent samples t-test using GraphPad Prism software (version 6.00; GraphPad Software Inc., CA, USA). A value of P < 0.05 was considered statistically significant.
Results
Morphological observation and identification of hippocampal neurons
Cells began to adhere 2 hours after seeding and were firmly attached within 6–12 hours. The cells were evenly distributed on the bottom of plate 24 hours later, with one or two neurites found in few cells (Figure 1A). On day 5, many neurites extended from the somas and neuronal length increased (Figure 1B). On day 7, the somas were round or oval and the neurites were interweaved into a network. Haloes of the neurons were evident (Figure 1C). On day 9, the neurons matured and aggregated into clumps (Figure 1D). MAP-2 immunofluorescence staining on day 7 (Figure 1E) confirmed that the culture was predominantly (approximately 90%) neuronal (data not shown).
Figure 1.
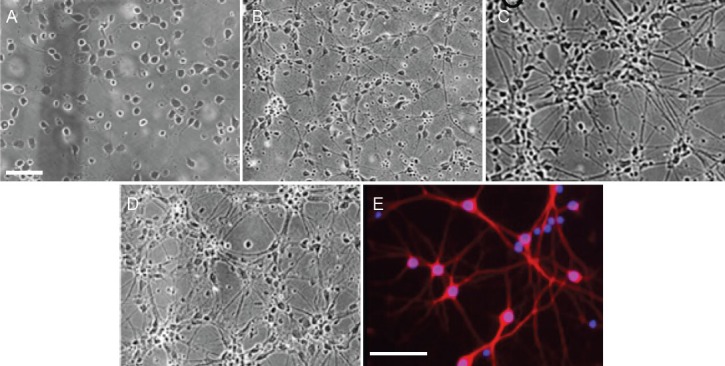
Morphology of hippocampal neurons.
(A–D) Hippocampal neurons were cultured for 1 (A), 5 (B), 7 (C), and 9 days (D). (E) Immunofluorescence for microtubule-associated protein 2 (red) on 7-day-old cultures. Nuclei were stained with Hoechst (blue fluorescence). Scale bars: 100 μm (A–D), 50 μm (E).
TLJN decreased Aβ1–42-induced LDH release independent of the estrogen receptor pathway
Results showed that the release of LDH was significantly (P < 0.01) increased in cultures exposed to Aβ1–42 compared with the control (Figure 2A), indicating that the in vitro Alzheimer's disense. model was reliable. Compared with the Aβ1–42 alone group, these levels were significantly (P < 0.01) reduced in Aβ1–42-exposed cultures with TLJN and E2 (Figure 2A). Furthermore, of the two components of TLJN, geniposide significantly (P < 0.05) reduced LDH in Aβ1–42-exposed cultures (Figure 2B) and ginsenoside Rg1 had no effect (Figure 2C).
Figure 2.
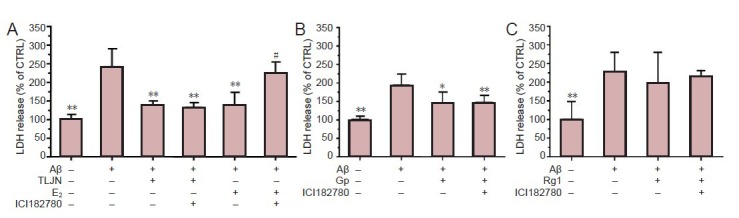
Tongluojiunao (TLJN), including its component, geniposide, reduces amyloid-peptide (1–42) (Aβ1–42)-induced increase of lactate dehydrogenase (LDH) release independent of the classical estrogen pathway.
(A) The classical estrogen pathway inhibitor, ICI182780, does not block (A) TLJN- or (B) geniposide-mediated reduction of Aβ1–42-induced LDH release. (C) Ginsenoside Rg1 does not affect LDH release under Aβ1–42-exposed conditions. Results are presented as a ratio of control cells (CTRL). All data are expressed as mean ± SD (with each experiment in at least triplicate) and were analyzed either by the one-way analysis of variance or the independent samples t-test. *P < 0.05, **P < 0.01, vs. Aβ group; #P < 0.01, vs. Aβ + E2 group. Gp: Geniposide; E2: 17-β estradiol.
Similarly to TLJN, 17-β estradiol markedly (P < 0.01) attenuated the Aβ-induced release of LDH (Figure 2A). Blocking the classical estrogen receptor pathway with ICI182780 significantly (P < 0.01) attenuated the protective effect of 17-β estradiol under Aβ conditions (Figure 2A). However, this effect was significantly (P < 0.01) reversed by the subsequent addition of TLJN (Figure 2A) or geniposide (Figure 2B). These results indicated that the classical estrogen signaling pathway did not participate in TLJN's protective function.
The neuroprotective effect of TLJN was partially attributed to the non-classical estrogen signaling pathway
Because the neuroprotective effect of TLJN was found to be independent of the estrogen receptor classical pathway, we proposed that other signaling cascades could be involved. Therefore, we investigated the effect of the PI3K inhibitor, LY294002. LY294002 plus 17-β estradiol markedly (P < 0.01) attenuated the Aβ1–42-induced release of LDH compared with 17-β estradiol alone under Aβ1–42-exposed conditions (Figure 3A), suggesting that this PI3K inhibitor effectively blocked the neuroprotection mediated through the non-classical estrogen signaling pathway. However, this effect was significantly (P < 0.01) reversed by the subsequent addition of TLJN compared with TLJN alone under Aβ1–42-exposed conditions (Figure 3A), indicating that the PI3K pathway plays a role in the neuroprotective effect of TLJN. However, the protective effect of TLJN protection on cells was not completely blocked by LY294002 because the LDH level was significantly (P < 0.05) lower than that of cells exposed to Aβ1–42 alone (Figure 3A). The effect of TLJN was found to be mainly attributed to geniposide (P < 0.01; Figure 3B). These results suggested that PI3K signaling pathway partially contributed to the neuroprotective effect of TLJN.
Figure 3.
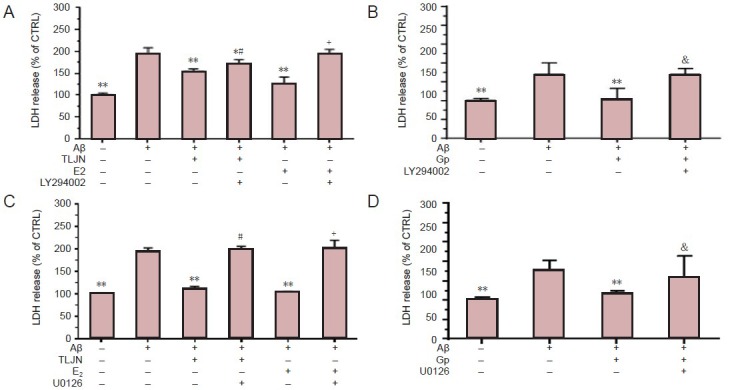
The phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling pathways are partially attributed to the neuroprotective effect of Tongluojiunao (TLJN), including geniposide.
(A) The reduced release of LDH by TLJN is partially blocked by the PI3K inhibitor, LY294002, under Aβ1–42-exposed conditions. (B) The reduced release of LDH by geniposide (Gp) is completely inhibited by LY294002. (C) The neuroprotective effect of TLJN is dependent on the MAPK signal-ing pathway. (D) Gp protects cells via the MAPK estrogen signaling pathway. Results are presented as a ratio of control cells (CTRL). All data are expressed as mean ± SD (with each experiment in at least triplicate) and were analyzed either by the one-way analysis of variance or the indepen-dent samples t-test. *P < 0.05, **P < 0.01, vs. Aβ1–42 group; #P < 0.05, ##P < 0.01, vs. Aβ1–42 + TLJN group; +P < 0.01, vs. Aβ1–42 + E2 group; &P < 0.01, vs. Aβ1–42 + Gp group. E2: 17-β estradiol; U0126: MAPK inhibitor; LDH: lactate dehydrogenase.
Because the non-classical estrogen pathway also includes the MAPK signaling pathway, we next explored if this pathway participated in the protective effect of TLJN under Aβ1–42-exposed conditions using the MAPK inhibitor, U0126 (Duncia et al., 1998). U0126 plus 17-β estradiol significantly (P < 0.01) increased Aβ1–42-induced release of LDH compared with 17-β estradiol alone under Aβ-exposed conditions (Figure 3C), indicating the inhibition of neuroprotection via a non-classical estrogen pathway. LDH release was markedly (P < 0.05) higher with the subsequent addition of TLJN compared with TLJN alone under Aβ1–42-exposed conditions (Figure 3C), indicating that the MAPK pathway participated in the neuroprotective effect of TLJN. Moreover, the protective effect of TLJN was almost completely blocked by U0126 because LDH release was much higher (close to that of Aβ1–42 alone) than that of TLJN treatment under Aβ1–42-exposed conditions. The subsequent addition of geniposide markedly (P < 0.01) attenuated the neuroprotective effect of geniposide (Figure 3D). These findings demonstrated that the MAPK signaling pathway was required for the neuroprotective effect of TLJN.
Discussion
Previous reports have shown that ginsenoside Rg1 alone is neuroprotective (Gong and Zhang, 1999; Chen et al., 2003; Leung et al., 2007; Gao et al., 2009). However, in our study, ginsenoside Rg1 was not protective against the neuronal cells in our model. This discrepancy may be due to its regulated effects in a dose-dependent manner, as well as the differences between primary cultured rat hippocampal neurons and PC12 cells (Chen et al., 2003). Reports have illustrated that the neuroprotective effect of Rg1 through the estrogen classical pathway occurs at a concentration as low as 3 × 10−7µmol/L (Chen et al., 2003). Furthermore, Rg1 has also been shown to be neuroprotective between 5 µmol/L and 20 µmol/L (Chen et al., 2003). However in this study, ginsenoside Rg1 was used at a lower concentration (2.5 µmol/L) than the other studies. The protective effect of ginsenoside Rg1 has been shown to be mediated by the upregulated and downregulated activity of nuclear factor-kappa B in neurons and astrocytes, respectively (Quan et al., 2013), suggesting that ginsenoside Rg1 decreases Aβ1–42 levels by upregulating the expression of peroxisome proliferator-activated receptor γ and insulin-degrading enzyme. Wu et al. (2012) have also demonstrated that ginsenoside Rg1 protects Aβ25–35-induced injury of primary cultures of rat cortical neurons through the mitochondrial-mediated antiapoptotic pathway. These findings suggest that ginsenoside Rg1 induces a neuroprotective effect through a pathway different from that of estrogen. Future studies should aim to determine a concentration-response effect of ginsenoside Rg1 for its neuroprotective action.
In the present study, TLJN, geniposide, and 17-β estradiol exerted different effects when pre-treated with the different kinds of inhibitors. The integrity of cell membranes was preserved and therefore LDH release was decreased in the treatment of ICI182780 plus 17-β estradiol. However, the other two inhibitors had no effect. These findings indicate that 17-β estradiol exerts its effects predominantly through the classical pathway. Interestingly however, the neuroprotective effect of TLJN and geniposide was inhibited by LY294002 and U0126, and not ICI182780. LY294002 only partially blocked the neuroprotective effect of TLJN/geniposide and U0126 completely reversed this response. These results indicate that the neuroprotective effect of TLJN and geniposide against Aβ1–42 occurs through the non-classical (i.e., PI3K and MAPK) signaling pathways instead of the classical (estrogen receptor) signaling pathway.
Although the protective mechanism of TLJN remains unclear, we hypothesized that it exhibits phytoestrogen-like actions and therefore has a neuroprotective effect. Furthermore, elucidating whether the classical or non-classical signaling pathway is involved remains to be determined. The present study explored the effects of TLJN and its components, geniposide and ginsenoside Rg1, on Aβ-mediated neuronal injury and their possible signing pathways as a mechanism of action.
In conclusion, TLJN, and its active ingredient, geniposide, protected hippocampal neurons via a non-classical estrogen signaling pathway, suggesting that geniposide exhibits a phytoestrogen-like function. Therefore, TLJN/geniposide requires further investigation as a possible alternative treatment for neurodegenerative diseases.
Acknowledgments
We would like to thank Dr. Liu KL from Institute of Biophysics, Chinese Academy of Sciences, China for critical review of the manuscript.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by the National Natural Science Foundation of China No. 81072901, the New Teacher Fund for Doctor Station, Ministry of Education, No. 20120013110013, grants from the Nautical Traditional Chinese Medicine Discipline, No. 522/0100604054, and grants from the Nautical Traditional Chinese Medicine Collaborative Innovation Center, No. 522/0100604299.
Peer review: The present study showed that, TLJN injection and its main ingredient, geniposide, exerted similar protective effects to the estrogen against Aβ1–42-induced damage. The underlying mechanism is not mediated by classical estrogen receptor pathway, but by non-classical PI3K and MAPK signaling pathways. Our findings can not only provide important evidence to reveal neuroprotective mechanism of TLJN injection, but also suggests that TLJN is s potential therapy with estrogenic effect.
Copyedited by Mark F, Raye W, Wang RT, Liu Y, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- [1].Alesheikh P, Tang H, Li P, Zhang W, Pan Y, Mashoufi A, Zhao L, Wang R, Di B, Yan Y. The Chinese herbal formula Tongluo Jiunao promotes expression of brain-derived neurotrophic factor/tropomyosin-related kinase B pathways in a rat model of ischemic brain injury. Neural Regen Res. 2011;6:885–891. [Google Scholar]
- [2].Bard F, Cannon C, Barbour R, Burke R, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- [3].Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- [4].Burek M, Nordeen K, Nordeen E. Estrogen promotes neuron addition to an avian song-control nucleus by regulating post-mitotic events. Dev Brain Res. 1995;85:220–224. doi: 10.1016/0165-3806(94)00215-l. [DOI] [PubMed] [Google Scholar]
- [5].Chanda S, Robinette CL, Couse JF, Smart RC. 17β-Estradiol and ICI-182780 regulate the hair follicle cycle in mice through an estrogen receptor-α pathway. Am J Physiol Endocrinol Metab. 2000;278:E202–E210. doi: 10.1152/ajpendo.2000.278.2.E202. [DOI] [PubMed] [Google Scholar]
- [6].Chaulet H, Desgranges C, Renault M, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau A. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- [7].Chen X, Zhu Y, Zhu L, Huang C, Chen Y, Chen L, Fang F, Zhou Y, Zhao C. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- [8].Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–1391. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- [9].Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- [10].Decker T, Lohmann-Matthes M. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- [11].Dong XH, Chai XQ. Alzheimer's disease transgenic animal models:How to get more similar pathological characteristics? Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8075–8082. [Google Scholar]
- [12].Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duncia JV, Santella JB, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- [14].Fan Q, Knight Z, Goldenberg D, Yu W, Mostov K, Stokoe D, Shokat K, Weiss W. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao QG, Chen WF, Xie JX, Wong MS. Ginsenoside Rg1 protects against 6-OHDA-induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-I receptor and estrogen receptor pathways. J Neurochem. 2009;109:1338–1347. doi: 10.1111/j.1471-4159.2009.06051.x. [DOI] [PubMed] [Google Scholar]
- [16].Gilbert BJ. The role of amyloid β in the pathogenesis of Alzheimer's disease. J Clin Pathol. 2013;66:362–366. doi: 10.1136/jclinpath-2013-201515. [DOI] [PubMed] [Google Scholar]
- [17].Gong YS, Zhang JT. Effect of 17-β-Estradiol and Ginsenoside Rg1 on Reactive Microglia Induced by β-Amyloid Peptides. J Asian Nat Prod Res. 1999;1:153–161. doi: 10.1080/10286029908039859. [DOI] [PubMed] [Google Scholar]
- [18].Gong Z, Yang L, Tang H, Pan R, Xie S, Guo L, Wang J, Deng Q, Xiong G, Xing Y. Protective effects of curcumin against human immunodeficiency virus 1 gp120 V3 loop-induced neuronal injury in rats. Reural Regen Res. 2012;7:171–175. doi: 10.3969/j.issn.1673-5374.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo L, Liu J, Xia Z. Geniposide inhibits CoCl2-induced PC12 cells death via the mitochondrial pathway. Chin Med J (Engl) 2009;122:2886–2892. [PubMed] [Google Scholar]
- [20].Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- [21].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [22].Honig LS, Boyd CD. Treatment of Alzheimer's disease: current management and experimental therapeutics. Curr Transl Geriatr Exp Gerontol Rep. 2013;2:174–181. doi: 10.1007/s13670-013-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hua Q, Qing X, Li P, Li W, Hou J, Hu J, Hong Q, Sun P, Zhu X. Brain microvascular endothelial cells mediate neuroprotective effects on ischemia/reperfusion neurons. J Ethnopharmacol. 2010;129:306–313. doi: 10.1016/j.jep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- [24].Hui L, Pei D, Zhang Q, Guan Q, Zhang G. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res. 2005;1052:1–9. doi: 10.1016/j.brainres.2005.05.043. [DOI] [PubMed] [Google Scholar]
- [25].Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- [26].Joseph N. Primary culture of hippocampal neurons from P0 newborn rats. J Vis Exp. 2008 doi: 10.3791/895. pii: 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-β-Hydroxybutyrate Protects Neurons in Models of Alzheimer's and Parkinson's Disease. Proc Natl Acad Sci U S A. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee CM, Fuhrman CB, Planelles V, Peltier MR, Gaffney DK, Soisson AP, Dodson MK, Tolley HD, Green CL, Zempolich KA. Phosphatidylinositol 3-kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin Cancer Res. 2006;12:250–256. doi: 10.1158/1078-0432.CCR-05-1084. [DOI] [PubMed] [Google Scholar]
- [29].Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- [30].Leung K, Yung K, Mak N, Chan Y, Fan T, Wong R. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology. 2007;52:827–835. doi: 10.1016/j.neuropharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [31].Li X, Hou J, Sun P, Li P, He R, Liu Y, Zhao L, Hua Q. Neuroprotective effects of TongLuoJiuNao in neurons exposed to oxygen and glucose deprivation. J Ethnopharmacol. 2012;141:927–933. doi: 10.1016/j.jep.2012.03.042. [DOI] [PubMed] [Google Scholar]
- [32].Liang K, Yang L, Yin C, Xiao Z, Zhang J, Liu Y, Huang J. Estrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin. J Biol Chem. 2010a;285:935–942. doi: 10.1074/jbc.M109.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liang W, Ge S, Yang L, Yang M, Ye Z, Yan M, Du J, Luo Z. Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res. 2010b;1357:19–25. doi: 10.1016/j.brainres.2010.07.091. [DOI] [PubMed] [Google Scholar]
- [34].Liu J, Yin F, Guo L, Deng X, Hu Y. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol Sin. 2009;30:159–165. doi: 10.1038/aps.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Y, Hua Q, Lei H, Li P. Effect of Tong Luo Jiu Nao on Aβ-degrading enzymes in AD rat brains. J Ethnopharmacol. 2011;137:1035–1046. doi: 10.1016/j.jep.2011.07.031. [DOI] [PubMed] [Google Scholar]
- [36].Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, Packer RJ, Cogen P, Stephan DA. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29:143–152. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- [38].Marin R, Guerra B, Alonso R, Ramírez CM, Díaz M. Estrogen Activates Classical and Alternative Mechanisms to Orchestrate Neuroprotection. Curr Neurovasc Res. 2005;2:287–301. doi: 10.2174/156720205774322629. [DOI] [PubMed] [Google Scholar]
- [39].Martin L, Farmer I, Johnston S, Ali S, Marshall C, Dowsett M. Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003;278:30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]
- [40].Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349:1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- [41].Morale MC, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS. Estrogen, neuroinflammation and neuroprotection in Parkinson's disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- [42].Quan Q, Wang J, Li X, Wang Y. Ginsenoside Rg1 Decreases Aβ1–42 Level by Upregulating PPARγ and IDE Expression in the Hippocampus of a Rat Model of Alzheimer's Disease. PLoS One. 2013;8:e59155. doi: 10.1371/journal.pone.0059155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Radad K, Gille G, Moldzio R, Saito H, Rausch W. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- [45].Ross C, Poirier M. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- [46].Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [47].Sumien N, Chaudhari K, Sidhu A, Forster MJ. Does phytoestrogen supplementation affect cognition differentially in males and females? Brain Res. 2013;1514:123–127. doi: 10.1016/j.brainres.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun P, Chen J, Li J, Sun M, Mo W, Liu K, Meng Y, Liu Y, Wang F, He R. The protective effect of geniposide on human neuroblastoma cells in the presence of formaldehyde. BMC Complement Altern Med. 2013;13:152. doi: 10.1186/1472-6882-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- [50].Winkler JM, Fox HS. Transcriptome meta-analysis reveals a central role for sex steroids in the degeneration of hippocampal neurons in alzheimer's disease. BMC Syst Biol. 2013;7:51. doi: 10.1186/1752-0509-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu J, Shen Y, Zhu W, Chen M, Wang Z, Liu Y, Zhu D, Lou Y. Ginsenoside Rg1 antagonizes β-amyloid peptide-induced apoptosis in primarily cultured rat neurons via mitochondrial pathway. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:393–401. [PubMed] [Google Scholar]
- [52].Zhang H, Guo D, Cheng X, Zhao J, Wang C. The action of estrogen receptor in genistein upregulating the expression of endothelial nitric oxide synthase. Zhongguo Dongmai Yinghua Zazhi. 2010;18:792–794. [Google Scholar]


