Abstract
The study is designed to find out the biochemical basis of antidiabetic property of Symplocos cochinchinensis (SC), the main ingredient of ‘Nisakathakadi’ an Ayurvedic decoction for diabetes. Since diabetes is a multifactorial disease, ethanolic extract of the bark (SCE) and its fractions (hexane, dichloromethane, ethyl acetate and 90% ethanol) were evaluated by in vitro methods against multiple targets relevant to diabetes such as the alpha glucosidase inhibition, glucose uptake, adipogenic potential, oxidative stress, pancreatic beta cell proliferation, inhibition of protein glycation, protein tyrosine phosphatase-1B (PTP-1B) and dipeptidyl peptidase-IV (DPP-IV). Among the extracts, SCE exhibited comparatively better activity like alpha glucosidase inhibition (IC50 value-82.07±2.10 µg/mL), insulin dependent glucose uptake (3 fold increase) in L6 myotubes, pancreatic beta cell regeneration in RIN-m5F (3.5 fold increase) and reduced triglyceride accumulation (22% decrease) in 3T3L1 cells, protection from hyperglycemia induced generation of reactive oxygen species in HepG2 cells (59.57% decrease) with moderate antiglycation and PTP-1B inhibition. Chemical characterization by HPLC revealed the superiority of SCE over other extracts due to presence and quantity of bioactives (beta-sitosterol, phloretin 2′glucoside, oleanolic acid) in addition to minerals like magnesium, calcium, potassium, sodium, zinc and manganese. So SCE has been subjected to oral sucrose tolerance test to evaluate its antihyperglycemic property in mild diabetic and diabetic animal models. SCE showed significant antihyperglycemic activity in in vivo diabetic models. We conclude that SC mediates the antidiabetic activity mainly via alpha glucosidase inhibition, improved insulin sensitivity, with moderate antiglycation and antioxidant activity.
Introduction
Diabetes mellitus is a global health threat associated with increased morbidity, mortality and poor quality of life which is characterized by chronic hyperglycemia [1]. Hyperglycemia leads to vascular complications via glucose toxicity and oxidative stress [2] and its proper control is an important therapeutic strategy to prevent diabetic complications [3]. Major determinants of postprandial hyperglycemic variations include gut digestion and absorption rate, available insulin response and tissue insulin sensitivity [4]. A medication that can address these abnormalities along with oxidative stress may be quite beneficial to diabetes. Current therapies include insulin and various oral agents such as sulfonylureas, biguanides, alpha-glucosidase inhibitors and gliptins, which are used as monotherapy or in combination to achieve better glycemic regulation [3]. These medications have some undesirable effects [5] and managing diabetes without side effects is still being a challenge. Hence the search for more effective and safer therapeutic agents of natural origin has been found to be valuable.
Traditional medicines are frequently used in urban settings as an alternative in daily healthcare and it recommends complex herbal mixtures and multi-compound extracts [6]. Synergistic properties of herbal medicines due to the presence of variety of components within a single herbal extract are beneficial to multifactorial diseases like diabetes [7]. Herbal medicines have played an important role in treating diabetes in various parts of the world for centuries. Ayurveda, a system of traditional medicine native to Indian subcontinent always plays major role in primary health care of both rural and urban populations of India [8]. Symplocos cochinchinensis (Lour.) S. Moore. (SC) from the family Symplocaceae, is a medicinal plant with anti-inflammatory, antitumor, antimicrobial and antidiabetic properties [9], [10]. The bark of SC is one of the key ingredients of Nisakathakadi Kashayam (decoction); a very effective Ayurvedic preparation for diabetes mentioned in the ancient script ‘Sahasrayogam’ [11]. For wider acceptability of the health benefits of SC, a detailed scientific investigation on its mode of action on various biochemical targets relevant to diabetes is mandatory. But any thorough study illustrating the mechanism of action of SC or its biochemical targets relevant to diabetes is not available in literature. Here, attempts are made to see the main bioactives responsible for its antidiabetic property and to elucidate the mode of action of SC using selected biochemical targets relevant to diabetes.
Materials and Methods
Chemicals and Reagents
Streptozotocin (≥98%), 2,2 diphenyl-1-1-picryl hydrazyl (DPPH), 4-nitro phenyl alpha-D- glucopyranoside, yeast alpha-glucosidase, acarbose, gallic acid, tannic acid, quercetin, trolox, diprotin A, suramin, beta-sitosterol, phloretin 2′glucoside, oleanolic acid, rosiglitazone, metformin, cytochalasin B, 2-deoxyglucose, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, insulin, dimethyl sulphoxide (DMSO) and all other chemicals and biochemicals unless otherwise noted were from Sigma (St. Louis, MO, USA). 2-deoxy-d-[3H]-glucose (2-DG) was from GE Healthcare, UK. All the positive controls used were of HPLC grade.
Ethics statement
No specific permission was required for the collection of the plant material. This plant is plenty available in this specific area (Palode, Thiruvanathapuram) and there is no restriction for the collection of the plant. It is not an endangered or protected species. The location is not privately-owned or protected in any way. According to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) formed by the Government of India in 1964, proper sanction had been obtained for animal experiments from CSIR-CDRI institutional animal ethics committee (Ethics Committee Approval Reference No. IAEC/2008/63/Renewal 04 dated 16.05.2012). Approval was obtained specifically for the animal experiments of this study from CSIR-CDRI institutional animal ethics committee. Animals were sacrificed by cervical dislocation under light ether anaesthesia as per ethics committee guidelines.
Plant material
The bark of SC was collected from Palode, Thiruvananthapuram (8°29′N, 76°59′E) during July 2011 and authenticated by Dr. Biju Haridas, Taxonomist, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Thiruvananthapuram, Kerala. A voucher specimen (No. 66498) was stored at the herbarium of JNTBGRI. 2 kg dry powder was extracted by maceration at 35–37°C; five times for 18 to 20 hrs with 70% ethanol [12]. Then it was filtered under vacuum and dried using rotary evaporator (Heidolph, Schwabach, Germany) at 35–40°C. This Symplocos cochinchinensis hydroethanol extract was designated as SCE. SCE was fractionated using 4 different solvents based on polarity; n-hexane (SCH), dichloromethane (SCD), ethyl acetate (SCEC) & 90% ethyl alcohol (SCEL). The SCE and its fractions were stored at 4°C, protected from light and humidity.
HPLC analysis
The HPLC analysis was carried out as described previously with slight modifications [13] on LC-20AD HPLC system (Shimadzu, Tokyo, Japan) equipped with the PDA detector, SPD-M20A and LC solutions software. The chromatographic separations were performed using Phenomenex Luna C-18 Column (150 mm×4.6 mm I. D, 5 µm), with a flow rate of 0.5 mL/min and a sample injection volume of 20 µL. The mobile phase used was acetonitrile (A) and water (B) with an isocratic elution ratio of 85∶15 (A∶B (v/v)) in 20 min. The sample was monitored with UV detection at 210 nm at 40°C.
Atomic Absorption Spectrophotometer (AAS) analysis
SCE (25 mg/mL) was digested in dilute HCl (7∶3). The concentration of minerals was quantified (mg/g of sample) by atomic absorption spectrophotometer (Perkin Elmer Inc. USA).
Quantification of Total Phenolic Content (TPC), Total Tannin Content (TTC) and Total Flavonoid Content (TFC)
TPC was determined as described previously [14], and were expressed as milligram gallic acid equivalents per gram of extract (mg GAE/g). Tannin estimation was done by the indirect method [15]. TTC was expressed as milligram tannic acid equivalents per gram of extract (mg TAE/g). TFC estimation was done as described previously [16] and expressed as milligram quercetin equivalents per gram of extract (mg QE/g).
In vitro alpha glucosidase (AG), dipeptidyl peptidase-IV (DPP-IV) & protein tyrosine phosphatase-1B (PTP-1B) inhibition assay
Yeast and rat intestinal AG (EC 3.2.1.20) inhibitory property of the extracts were determined as described previously [17] using acarbose as standard. All the extracts were checked for DPP-IV (EC 3.4.14.5) inhibition using the kit from Cayman chemicals (Ann Arbor, MI, USA). Diprotin A was used as the standard. PTP-1B (EC 3.3.3.48) inhibitory property of extracts was evaluated using the kit from Calbiochem (Darmstadt, Germany). Percentage inhibition values were plotted against the corresponding concentrations of the sample to obtain IC50 value.
Determination of antioxidant potential and metal chelation activity
The antioxidant activity of extracts was assessed by DPPH method [18] with gallic acid as standard. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity was determined using assay kit (Zen-Bio Inc., NC, USA) and trolox was the standard. The hydroxyl radical scavenging activity was measured by the deoxyribose method [19] with catechin as standard. The chelation of ferrous ions by the extracts was estimated using ferrozine method [20] and EDTA was used as the standard. IC50 values were calculated and compared with the respective standards.
Determination of antiglycation activity
Advanced glycation end products (AGEs) derived from bovine serum albumin (BSA) were quantified using the previous method [21]. BSA in the presence of ribose in phosphate buffered saline was served as control. AGE fluorescence (λex370 nm; λem 440 nm) was measured in terms of relative fluorescence unit (RFU) after 24 h and 7 days of incubation. Investigations after 24 h and 7 days incubation are designated as day1 and day7 experiments respectively for future references. The data was compared with the reference compound quercetin (100 µM). AGEs formed were also processed for complexity analysis to check whether test material has capacity to block the formation of glycated products [21] using scanning electron microscope (SEM; Carl Zeiss, Munich, Germany).
Cell culture
HepG2 and L6 cell lines were obtained from National Centre for Cell Science, Pune, India. The HepG2 cells were maintained in low glucose (5.5 mM) DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution (10,000 U/mL penicillin G, 10 mg/mL streptomycin, 25 µg/mL amphotericin B), with 5% CO2 at 37°C. L6 skeletal muscle cells were maintained in alpha-MEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution at 5% CO2 at 37°C. Differentiation was induced by switching confluent cells to medium supplemented with 2% FBS. Experiments were performed in differentiated myotubes. RIN-m5F cells (ATCC, USA) were cultured in RPMI-1640 supplemented with 10% FBS and 1% antibiotic/antimycotic solution at 5% CO2 at 37°C. MIN-6 cells (ATCC, USA) were maintained in DMEM supplemented with 10% FBS, 1% antibiotic/antimycotic solution, 100 µg/mL L-glutamine, 10 µL/L beta - mercaptoethanol at 5% CO2 at 37°C. 3T3-L1 murine preadipocytes (ATCC, USA) were cultured in DMEM supplemented with 10% FBS and antibiotics. Differentiation was induced by switching to DMEM with 500 µM 3-isobutyl-1-methylxanthine (IBMX), 10 µM dexamethasone and 500 nM insulin (MDI) for 48 h. Differentiation was then maintained in DMEM containing 10% FBS and 500 nM insulin for 8 days.
Determination of cell viability
The extracts were dissolved in DMSO for application to cell cultures and final concentration of DMSO was fixed at 0.1% for all cell based assays. The cytotoxicity was checked by MTT assay kit (Cayman chemicals, Ann Arbor, MI, USA). HepG2, RIN-m5F, MIN-6, L6 and 3T3-L1 cells were seeded at a density of 4×104 cells/well in 24 well plate and incubated for 24 h. Cells were treated with various concentrations of extract and incubated for 24 h. Then, cell viability in HepG2, L6 and 3T3-L1 or proliferation in RIN-m5F and MIN-6 was evaluated.
Hyperglycemia induced oxidative stress
The cells were maintained in low glucose medium (5.5 mM) for the initial 24 h, then switched over to high glucose (25 mM) medium with or without the extracts or quercetin (positive control) to check whether test material prevent the generation of oxidative stress. The intracellular reactive oxygen species (ROS) production was monitored with the fluorescent probe CM-H2DCFDA [22].
Glucose uptake
The 2-deoxy glucose uptake in L6 myotubes was performed as described previously [23]. Glucose uptake measured in triplicate and normalized to total protein, was expressed as fold induction with respect to unstimulated cells. Rosiglitazone and metformin were the standards.
Adipocyte differentiation
The adipogenic potential of all the extracts (30 µg/mL) was assessed in 3T3-L1 preadipocyte over untreated cells by quantifying the accumulation of triglycerides using oil red O staining on day 8 [24], [25]. Rosiglitazone was used as standard. The cell lysate from all experimental groups was prepared according to the previous method [21] and assayed for GPDH (EC 1.1.1.8) activity using a Takara GPDH Assay Kit (Takara Bio Inc, Otsu, Japan). The membrane fraction for DGAT1 assay was collected as described previously [26]. DGAT1 activity was measured using the kit from MyBioSourse.com (San Diego, CA, USA). Total cellular TG was extracted as reported previously [27]. TG content was assayed using a TG assay kit (Cayman Chemicals). The protein content was measured and normalized for GPDH, DGAT 1 and TG assays using a bicinchoninic acid kit (Pierce, Rockford, IL USA). The adiponectin level in the residual media was measured using adiponectin assay kit (Cayman Chemicals).
Animals
Male albino rats of Sprague Dawley (SD) strain (7–8 weeks old, 160±20 g), bred at animal facility of CSIR-CDRI, Lucknow were selected for this study. Rats were housed in polypropylene cages (5 rats per cage) under an ambient temperature of 23±2°C; 50–60% relative humidity; light 300 lux at floor level with regular 12 h light/dark cycle. Animals were maintained on a standard pellet diet and water ad libitum.
Oral Sucrose Tolerance Test (OSTT) in normal rats
For this normal SD rats were fasted for 16 h. Animals showing fasting blood glucose level (BGL) between 70 to 90 mg/dL were divided into 6 groups containing 6 animals each. Animals of experimental groups were orally administered SCE (100, 250 and 500 mg/kg body weight (bw)), metformin (100 mg/kg bw) or acarbose (50 mg/kg bw) dissolved in 1.0% gum acacia. The dose of SCE was selected on the basis of dosage of ‘Nisakathakadi Kashayam’ for human use. 10–15 mL of this preparation containing approximately 3.5 g of SC bark including other 7 herbs in equal amount, thrice in a day is generally prescribed for patients [11]. Ethanol extract has been used for in vivo study due to the yield of more bioactive molecules and less toxicity of the solvent [28]. Since its selective nature, 70% ethanol is the most suitable solvent for in vivo pharmacological evaluation compared to other solvents; it will dissolve only the required bioactive constituents with minimum amount of the inert materials [28]. Animals of control group were given an equal volume of 1.0% gum acacia. Rats were loaded with sucrose (10 g/kg bw) orally 30 min after administration of test sample or vehicle. BGL was estimated at 30, 60, 90 and 120 min post administration. Food but not water was withheld during the course of experimentation [29].
OSTT in sucrose loaded mild diabetic rat model (SLM)
Animals were made diabetic by injecting streptozotocin (60 mg/kg in 100 mM citrate buffer-pH 4.5) intraperitoneally after overnight fasting. Animals showing fasting BGL<200 mg/dL after 72 h were selected, termed as mild diabetic [30] and divided into 4 groups of 6 animals each. Animals of experimental group were administered SCE (500 mg/kg bw), metformin (100 mg/kg bw) or acarbose (50 mg/kg bw). Mild diabetic control group were given an equal amount of 1.0% gum acacia. A sucrose load (10 g/kg) was given to each animal orally 30 min after test sample or vehicle. BGL was determined at 30, 60, 90 and 120 min post-administration of sucrose [29].
OSTT in sucrose-challenged streptozotocin-diabetic rat model (STZ-S)
Like SLM, rats were made diabetic. Animals of BGL>350 mg/dL after 72 h were selected, termed as diabetic [30], and divided into 4 groups of 6 animals each. Experimental groups were administered SCE, metformin or acarbose like SLM. Diabetic control group received equal amount of 1.0% gum acacia. Rats were loaded with sucrose (3 g/kg bw) orally 30 min after test sample or vehicle. BGL was checked at 30, 60, 90, 120, 180, 240, 300 and 1440 min (24 h), respectively [29]. Acarbose has been selected as one of the positive control as it is the alpha-glucosidase inhibitor which can improve long term glycemic control in patients with diabetes [31]. Metformin is the widely used antidiabetic to treat the cardinal symptoms of diabetes like polyphagia, polydipsia, polyuria and insulin resistance due to its pleiotropic effect via various targets and it shows wide tolerance and less toxicity compared to other antidiabetics [32]. Due to the wider acceptability of metformin as an antidiabetic drug, we used it as a positive control.
Statistical analysis
Quantitative glucose tolerance of each group was calculated by the area under the curve (AUC) method using GraphPad Prism software version 3 (GraphPad Software Inc., La Jolla, CA, USA). All other results were analyzed using a statistical program SPSS/PC+, version 11.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SD, from 3 independent experiments with triplicates. P≤0.05 was considered to be significant.
Results
Phytochemical characterization
HPLC analysis confirmed the presence of beta-sitosterol (111.62±4.12 mg/g), phloretin 2′glucoside (98.32±4.87 mg/g) and oleanolic acid (63.89±3.03 mg/g) in Symplocos cochinchinensis ethanolic extract (SCE); phloretin 2′glucoside (508.46±11.63 mg/g) and oleanolic acid (39.09±1.73 mg/g) in ethyl acetate fraction of SCE (SCEC); beta-sitosterol (145.56±4.63 mg/g) in hexane fraction of SCE (SCH); beta-sitosterol (152.29±6.31 mg/g) and phloretin 2′glucoside (188.97±6.41 mg/g) in dichloromethane fraction of SCE (SCD); phloretin 2′glucoside (273.65±7.63 mg g−1) in ethyl acetate fraction of SCE (SCEL) (Fig. S1 and S2 in Supporting Information S1) [33]. Analysis of minerals by AAS for micro-nutrients revealed presence of various minerals like zinc (0.014±0.0005 mg/g) manganese (0.096±0.0041 mg/g), iron (0.147±0.005 mg/g), sodium (1.387±0.062 mg/g), potassium (2.496±0.11 mg/g), magnesium (4.368±0.203 mg/g) and calcium (46.799±2.15 mg/g). The dry yield, TPC, TTC and TFC of the extracts were shown in Table 1. Since SCE exhibited comparatively better activity with respect to various in vitro targets and its high content of bioactives, SCE was taken forward for in vivo study. Moreover, in Indian traditional system of medicine (Ayurveda) most of the decoctions are hydroalcohol based (eg. Arishta and Kashaya).
Table 1. Dry yield, Total Phenolic Content (TPC), Total tannin Content (TTC) and Total Flavonoid Content (TFC) of test materials.
| Sl no. | Sample | Dry yield as % weight of dry plant material | Total phenolic content (TPC) in mg GAE/g | Total flavonoid content (TFC) in mg QE/g | Total tannin content (TTC) in in mg TAE/g |
| 1 | SCE | 12.35 | 53.72 | 19.35 | 10.47 |
| 2 | SCH | 0.50 | 13.40 | 8.56 | - |
| 3 | SCD | 0.32 | 36.27 | 19.85 | - |
| 4 | SCEC | 0.55 | 57.28 | 26.35 | 17.54 |
| 5 | SCEL | 2.91 | 54.68 | 22.85 | 13.26 |
In vitro AG, DPP-IV and PTP1-B inhibitory property
The extracts were evaluated for AG inhibition utilizing rat intestinal and yeast enzymes. SCEC, SCD and SCE showed significant yeast AG inhibition with IC50 values of 62.30±1.53, 71.26±1.94 and 82.07±2.10 µg/mL respectively (Fig. 1A). Rat intestinal AG inhibition (IC50) of the extracts was found to be 194.93±2.67 (SCEC), 143.02±2.91 (SCD) and 232.05± 3.34 µg/mL (SCE) (Fig. 1B). Acarbose showed an IC50 of 45±1.12 for yeast and 49.78±1.45 µg/mL for rat AG enzymes. SCEC fraction showed DPP-IV inhibition with an IC50 of 87.63±1.88 µg/mL while IC50 of SCE was 269.98±2.95 µg/mL (Fig. 2A). Standard compound diprotin A showed an IC50 of 1540±11.2 µg/mL. PTP-1B inhibition was noticed in SCEC fraction with an IC50 of 55.83 µg/mL and SCE exhibited an IC50 of 159.10 µg/mL (Fig. 2B). Standard was suramin (IC50 14.01 µg/mL (10.8 µM)).
Figure 1. SCE SCD and SCEC exhibited alpha-glucosidase inhibitory property.
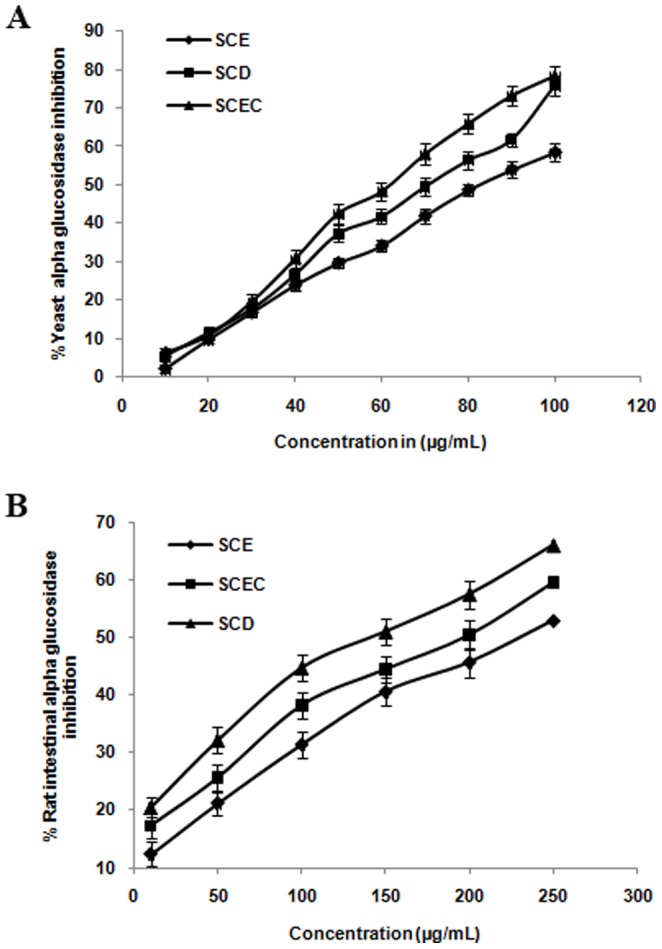
(A) Yeast alpha glucosidase inhibition. (B) Rat intestinal alpha glucosidase inhibition. Values are means ± SD; n = 3. SCE, S. cochinchinensis (SC) ethanol extract; SCD, SC dichloromethane fraction and SCEC, SC ethyl acetate fraction.
Figure 2. DPP-IV and PTP-1B inhibitory property of SCE and SCEC.
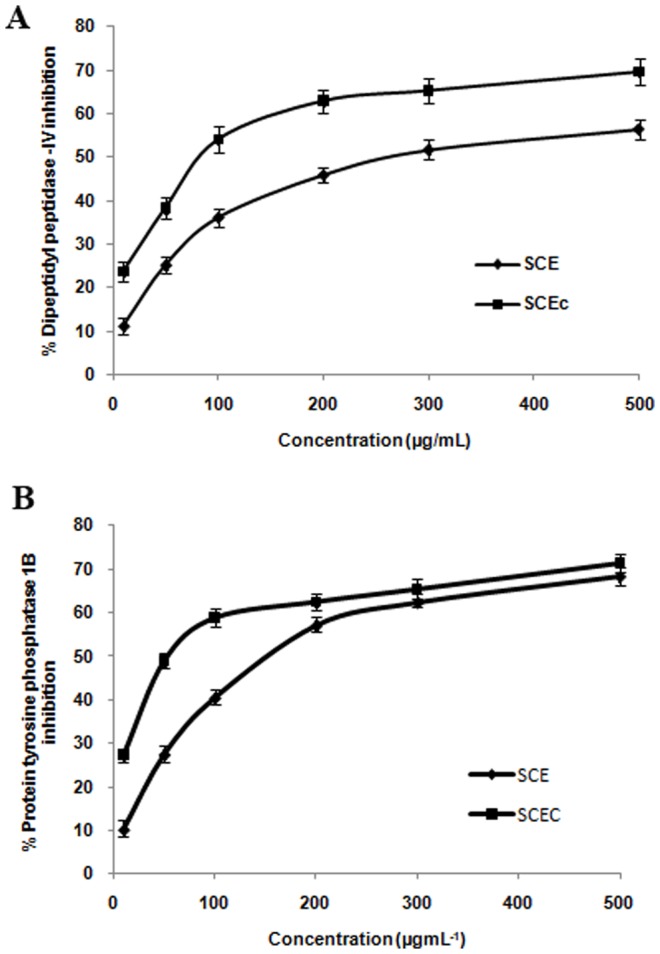
(A) DPP-IV inhibition by SCE & SCEC. SCEC IC50- 87.63±1.88 µg/mL and SCE IC50- 269.98±2.95 µg/mL. Values are means ± SD; n = 3. (B) PTP-1B inhibitory property of SCE & SCEC. SCEC IC50- 55.83±1.24 µg/mL and SCE IC50- 159.10±1.91 µg/mL. Values are means ± SD; n = 3. SCE, S. cochinchinensis (SC) ethanol extract and SCEC, SC ethyl acetate fraction.
SC fractions exhibited antioxidant and metal chelation potential
SCEC, SCEL and SCE showed better DPPH radical scavenging property compared to SCH and SCD (Table 2). IC50 of gallic acid was 6.5±0.73 µg/mL. Similarly SCEC, SCEL and SCE exhibited promising ABTS cation decolorization potential compared to SCH and SCD (Table 2). IC50 of the standard trolox was 5±0.51 µg/mL. SCEC, SCEL and SCE showed potent hydroxyl radical scavenging and metal chelation activity compared to SCH and SCD (Table 2). IC50 of catechin was 9±0.86 µg/mL and that of EDTA was 4.67±0.36 µg/mL.
Table 2. IC50 values of antioxidant (DPPH, ABTS and hydroxyl radical scavenging) and metal chelation assays.
| Sl no. | Samples | IC50 values of DPPH radical scavenging assay in µg/mL | IC50 values of ABTS radical scavenging assay in µg/mL | IC50 values of hydroxyl radical scavenging assay in µg/mL | IC50 values of metal chelation activity in µg/mL |
| 1 | SCE | 133.20±2.45 | 54.95±1.12 | 34.74±1.06 | 89.31±1.82 |
| 2 | SCH | 402.62±3.41 | 321.12±2.94 | 364.23±3.17 | 295.21±4.67 |
| 3 | SCD | 541.65±3.61 | 96.29±1.90 | 164.37±2.56 | 211.38±3.61 |
| 4 | SCEC | 129.43±1.84 | 35.72±1.02 | 31.64±0.98 | 86.49±1.76 |
| 5 | SCEL | 130.04±1.92 | 36.47±1.21 | 42.81±1.52 | 94.38±2.04 |
Antiglycation property was observed in SCE, SCEC and SCEL
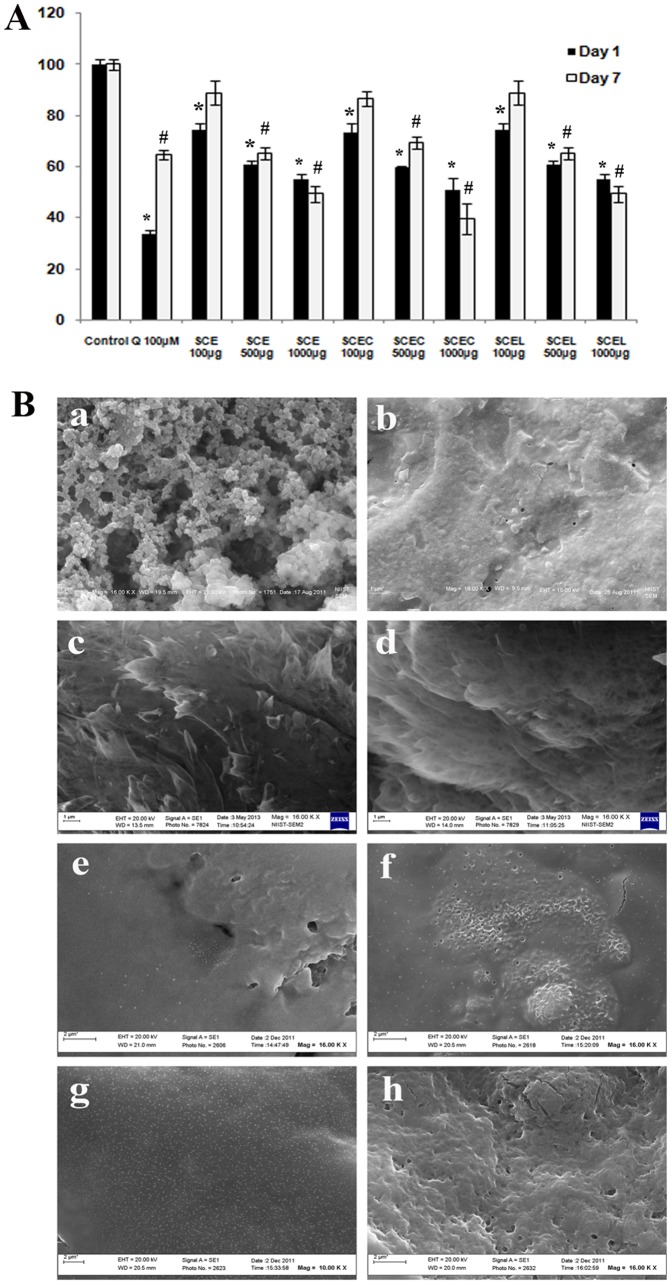
AGEs derived from BSA was analysed using 2 methods; by RFU measurements and SEM analysis. There were 22 groups under day1 and day7 experiments. In detail, 2 untreated control groups (one each with day1 and day7 experiments), 2 quercetin treated groups (day1 and day7 experiments) and 18 extract treated groups (3 doses- 100, 500 & 1000 µg/mL of SCE, SCEC and SCEL under day1 and day7). Quercetin (100 µM) showed significant (P≤0.05) antiglycation property in RFU measurement and also in SEM analysis (Fig. 3B b). Significant decrease (P≤0.05) in fluorescence in dose dependent manner was observed in day1 and day7 experiments at 500 and 1000 µg/mL doses of three extracts, indicative of antiglycation property (Fig. 3A). SEM analysis of the microstructure of control group of day1 showed highly granular agglomeration with uneven pores and highly complex cross linking (Fig. 3B a). 500 and 1000 µg/mL doses of SCE, SCEC and SCEL reduced highly complex microstructure to simple membranous structure without any cross linking in day1 and day7 experiments (Fig. 3B c–h; day7 SEM data not shown). The SEM results were analyzed based on the previous report [21].
Figure 3. Fluorescence quantification and SEM microstructure analysis of advanced glycation end products revealed the antiglycation property of SCE SCEC and SCEL.
(A) Quantification of fluorescence intensity of glycated products in presence of various concentrations of SCE, SCEC and SCEL (100, 500, 1000 µg/mL) under 2 different time intervals (day1 & day7) in terms of relative fluorescence units (RFU). Quercetin (100 µM) was used as reference compound. RFU are normalized to 100. Values are means ± SD; n = 3. *represents groups differ significantly from day 1 control group (P≤0.05) and ≠represents groups differ significantly from day 7 control group (P≤0.05). (B) Representative SEM microstructures of glycated products formed under various groups of day1 experiments (a–h), (a, control; b, quercetin 100 µM, c & d, SCE 100 µg/mL and SCE 1000 µg/mL; e & f, SCEC 100 µg/mL and SCEC 1000 µg/mL and g & h, SCEL 100 µg/mL and SCEL 1000 µg/mL. All samples were visualized at 16000× magnification.
Protection from hyperglycemia induced oxidative stress
High glucose treatment induced the generation of significant amount of ROS in HepG2 cells (64.23%; Fig. 4A and B, b), but co-treatment with SCE or SCEC significantly attenuated ROS in a dose dependent manner (P≤0.05). SCE and SCEC were selected on the basis of their potent in vitro antioxidant property. Results showed that 41.94, 51.28 and 59.57% decrease of ROS level with 10, 50 and 100 µg/mL SCE respectively (Fig. 4B, d–f) compared to high glucose control group. Similarly SCEC caused 34.92, 45.79 and 56.72% decrease of ROS level with 10 and 50 and 100 µg/mL dose respectively (Fig. 4B, g–i). Quercetin (25 µM) showed significant (P≤0.05) decrease (60.04%) of ROS (Fig. 4B, c). All extracts were found to be absolutely safe up to 100 µg/mL in all 5 cell lines; HepG2, L6, 3T3L1, RIN-m2F and MIN-6 (data not shown).
Figure 4. SCE and SCEC fractions protected HepG2 cells against ROS generation during hyperglycemia.
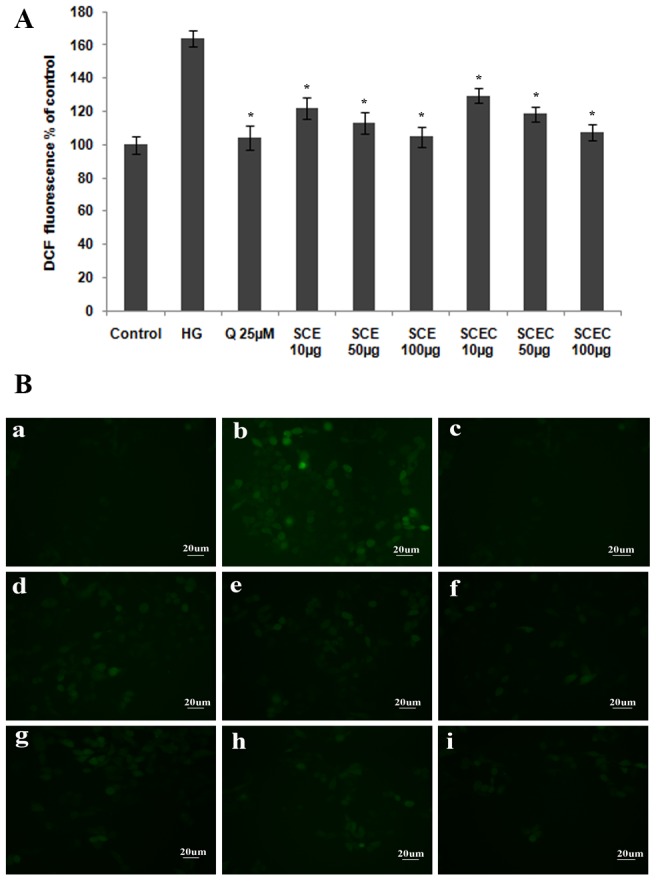
Analysis of high glucose induced intracellular ROS levels in HepG2 cells by DCFDA method. Cultured HepG2 cells were treated with SCE or SCEC in the presence of high glucose (HG; 25 mM) for 24 h and then incubated with H2DCFDA. The results are shown as (A) the quantitative analysis of fluorescence from three independent experiments. Values are means ± SD; n = 3. *represents groups differ significantly from HG group (P≤0.05). (B) Representative microscopic scans a–i (a, vehicle control; b, high glucose (HG); c, HG+Quercetin; d–f, HG+10 µg, 50 µg and 100 µg SCE; g–i, HG+10 µg, 50 µg and 100 µg SCEC). All samples were visualized at 20× magnification.
Proliferation potential of pancreatic beta cells in RIN-m5F and MIN-6 cell lines
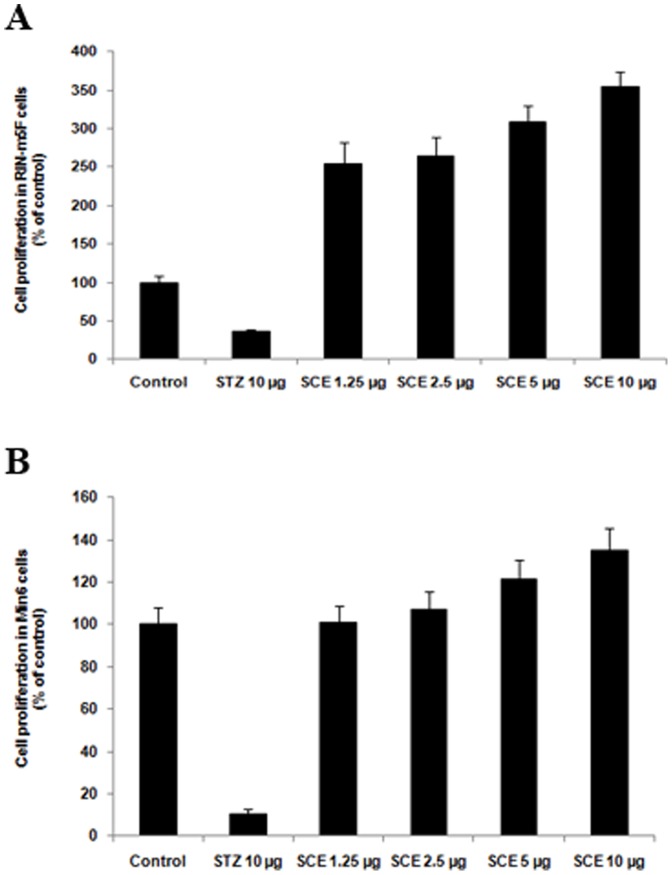
Treatment with SCE (10 µg/mL) exhibited significant cell proliferation rate; 3.5 fold and 0.5 fold respectively compared to control both in RIN-m5F and MIN-6 cells (Fig. 5) and other extracts did not show any positive effect.
Figure 5. Pancreatic beta cell proliferation potential of SCE in RIN-m5F and MIN-6 cells.
(A) Beta cell proliferation potential of SCE in RIN-m5F cells. (B) Beta cell proliferation potential of SCE in MIN-6 cells. Results are normalised to 100 based on control readings. Values are means ± SD; n = 3. *represents groups differ significantly from control group (P≤0.05).
Enhancement of glucose uptake in L6 myotubes
Pre-treatment of myotubes with SCE and its fractions for 16 h with insulin (100 nM) resulted in increase of glucose uptake in an additive manner (Fig. 6A, P≤0.05). Among various fractions studied, both SCE and SCEL exhibited better activity both in the absence and presence of insulin in a dose dependent manner (Fig. S3 in Supporting Information S1; P≤0.05). Insulin alone showed a significant increase in glucose uptake (1.9 fold of basal, P≤0.05) in L6 myotubes. Metformin and rosiglitazone were standards (Fig. 6A).
Figure 6. Glucose uptake and adipocyte differentiation studies in all 5 extracts.
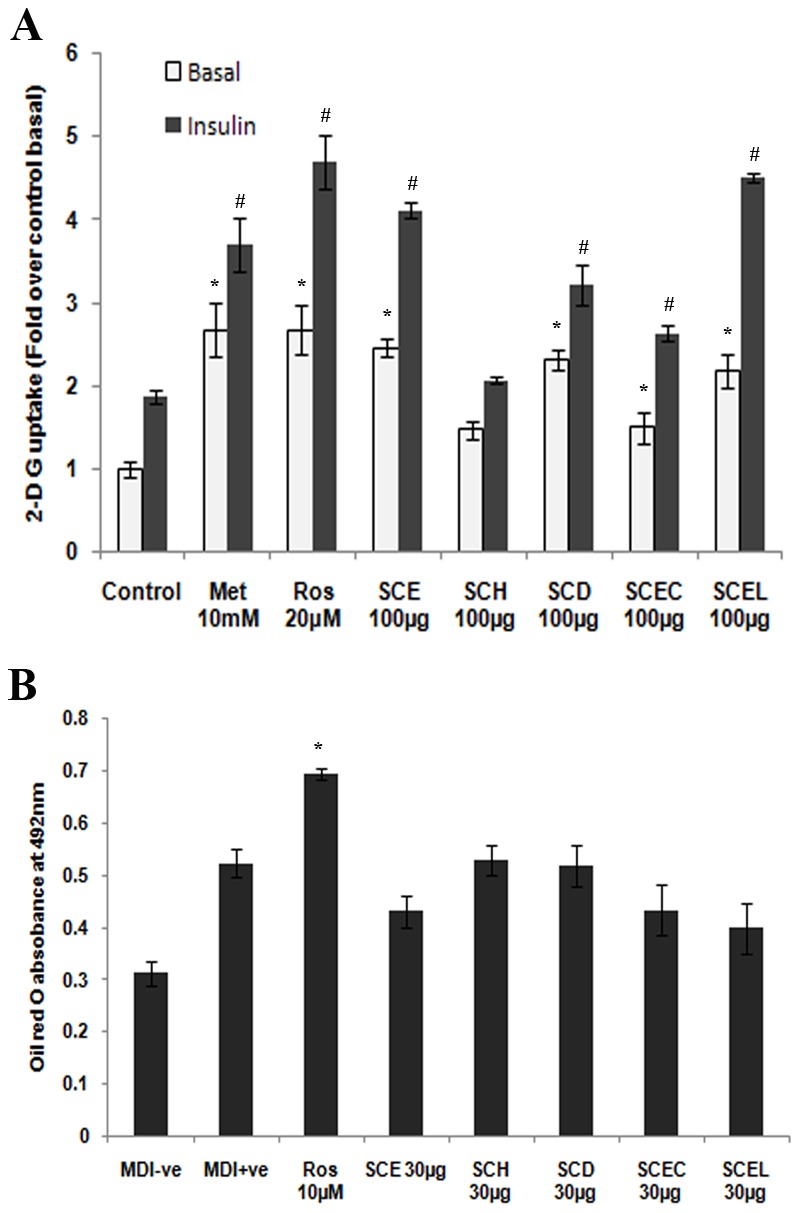
(A) 2-deoxy glucose uptake in L6 myotubes. Cells were incubated for 16 h with different extracts (100 µg/mL) or standards. After incubation myotubes were left untreated (white bars) or stimulated with 100 nM insulin (black bars) for 20 min, followed by the determination of 2-DG uptake. Results are expressed as fold stimulation over control basal. Metformin (10 mM) & rosiglitazone (20 µM) were the standards. Values are means ± SD; n = 3. *represents groups differ significantly from basal control group (P≤0.05). ≠represents groups differ significantly from insulin control group (P≤0.05). (B) Quantification of triglyceride content in differentiating 3T3-L1 adipocytes treated with different extracts (30 µg/mL) or rosiglitazone (10 µM) for 8 days by oil red O staining. Data are expressed as the means ± SD; n = 3; * represents groups differ significantly from MDI positive group (P≤0.05). MDI−ve, media without 3-isobutyl-1-methylxanthine (IBMX), dexamethasone & insulin; MDI+ve, media with IBMX, dexamethasone & insulin and Ros 10 µM, rosiglitazone 10 µM.
Adipogenesis
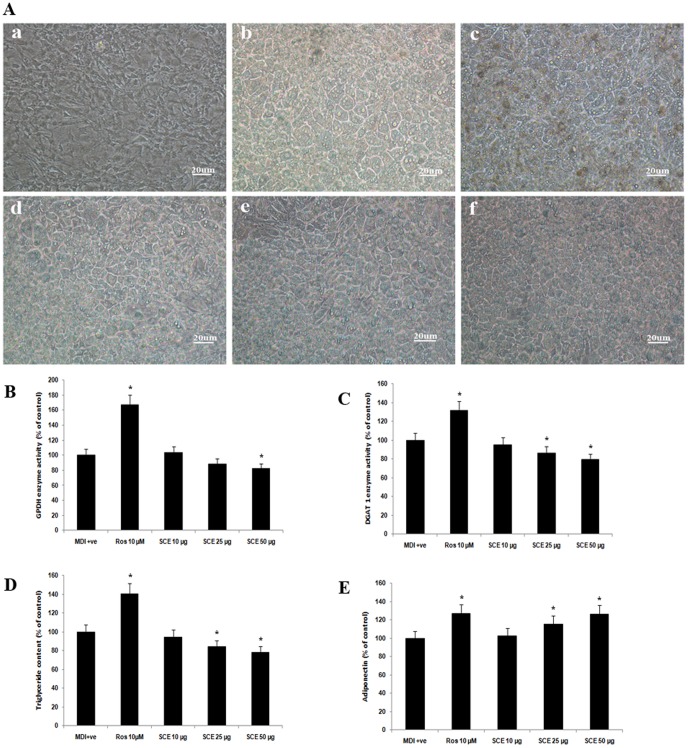
The treatment with SCE and its fractions (30 µg/mL) induced a moderate level of differentiation of 3T3-L1 preadipocytes to adipocytes, but less than rosiglitazone. This was based on the morphological observation and quantification of triglycerides by oil red O staining (Fig. 6B). SCE at 50 µg/mL dose exhibited a significant decrease in GPDH activity compared to MDI positive group (P<0.05, Fig. 7B), at the same time 25 and 50 µg/mL doses of SCE exhibited a significant decrease in DGAT1 activity and TG content compared to MDI positive group (P<0.05, Fig. 7C and D). However, adiponectin level was significantly increased by SCE treatment (25 and 50 µg/mL) compared to MDI positive group (P<0.05, Fig. 7 E). Rosiglitazone was the reference standard.
Figure 7. Estimation of adipogenesis in SCE tretment.
(A) Cellular morphology. (Panels a–f) Micrographs (×10) showing (a) MDI negative, (b) MDI positive (vehicle control) (c) differentiating 3T3-L1 adipocytes treated for 8 days with rosiglitazone (10 µM), and (d–f) various concentrations of SCE (10, 25 and 50 µg/mL, respectively). DMSO (0.1%, vehicle) in differentiation media served as the vehicle control group i.e MDI positive. (B) Glycerol-3-phosphate dehydrogenase activity in various groups (MDI positive, rosiglitazone at 10 µM) and various concentrations of SCE (10, 25 and 50 µg/mL, respectively). (C) Diacyl glycerol -3 phosphate activity in various groups (MDI positive, rosiglitazone at 10 µM) and various concentrations of SCE (10, 25 and 50 µg/mL, respectively). (D) The triglyceride content in various groups (MDI positive, rosiglitazone at 10 µM) and various concentrations of SCE (10, 25 and 50 µg/mL, respectively). (E) Adiponectin level in various groups (MDI positive, rosiglitazone at 10 µM) and various concentrations of SCE (10, 25 and 50 µg/mL, respectively). Results are normalised to 100 based on control readings. Data are expressed as the means ± SD; n = 3. *Represents groups that differ significantly from the MDI positive (vehicle control) group (P≤0.05).
Antihyperglycemic effect of SCE in normal and diabetic in vivo models
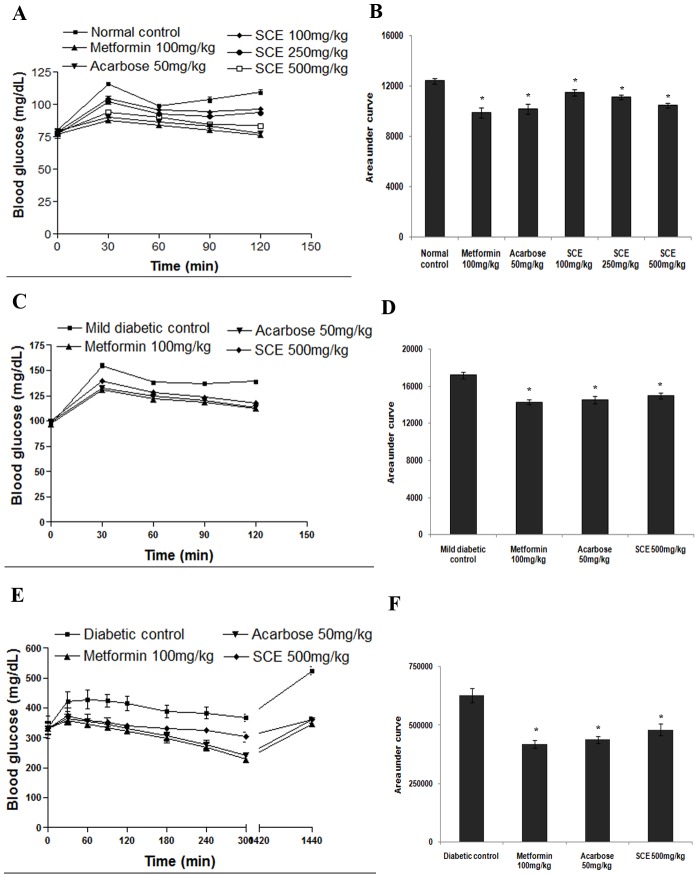
In acute toxicity study, SCE did not show any observable toxic effects in behaviour or physiology of animals up to 2 g/kg bw. In normal and SLM, the rise in BGL at 30 min of oral sucrose load was significantly reduced in SCE treated group compared to control group. SCE treatment at doses of 100, 250 and 500 mg/kg bw exhibited 7.56, 10.23 and 15.53% reduction respectively in plasma glucose in normal sucrose loaded rats and 18.18 and 20.42% by acarbose and metformin treatment (Fig. 8A and B). In SLM, treatment with 500 mg/kg bw of SCE reduced the whole glycemic response by 12.88% while acarbose and metformin caused 15.73 &and 17.12% reduction respectively (Fig. 8C and D). SCE treatment (500 mg/kg bw) in STZ-S caused 23.48% improvement in blood glucose profile after 5 h of treatment and acarbose and metformin showed 30.27 and 33.18% respectively (Fig. 8E and F).
Figure 8. The antihyperglycemic effect of SCE in normal rats, mild diabetic rat model (SLM) and streptozotocin-induced diabetic rat model (STZ-S) after sucrose administration.
(A) The glycemic response curve and (B) incremental AUC0–120 min in normal rats. (C) The glycemic response curve and (D) incremental AUC0–120 min in SLM model. (E) The glycemic response curve and (F) incremental AUC0–1440 min in STZ-S model. Data are expressed as the mean ± SD, n = 6. * represents groups differ significantly from control group (p<0.05). SCE, S. cochinchinensis (SC) ethanol extract.
Discussion
The pathogenesis of diabetes mellitus is complex and involves many mechanisms leading to several complications and demands a multiple therapeutic approach. Nowadays, medicinal plants have re-emerged as an effective source for the treatment of diabetes as it hold diverse group of compounds. Metformin exemplifies an efficacious oral glucose lowering agent derived from the research based on medicinal plants [34]. To date many antidiabetic medicinal plants have been reported although only a small number of these have received scientific evaluation to elucidate their mechanism of action. The World Health Organisation Expert Committee on diabetes has stressed the need of research on traditional medicine for future drugs [35]. In this study, phytochemically characterized SC was subjected to investigation on various biochemical targets relevant to diabetes like AG, glycation, DPP-IV, PTP-1B and hyperglycemia induced oxidative stress along with pancreatic beta cell proliferation, insulin dependent glucose uptake and adipogenesis using in vivo and in vitro models. Cell line based in vitro models are very much important in diabetic research as it is helpful to determine the mechanism of action of a plant extract with traditional use and/or human or in vivo data to support the antidiabetic effect [36]. In addition, the cell line based model allows the use of less amount of test material with reduced variability in results [36].
Oxidative stress due to hyperglycemia and dyslipidemia is one of the physiological parameter evident in diabetes [37]. Depletion of antioxidant level has been demonstrated in diabetic patients and extra administration of antioxidants to compensate the depletion, had helped to prevent diabetes complications [38]. Hyperglycemia induces accelerated hydroxyl radical generation and reactive oxygen species production which could represent the key event in the development of diabetic complications [39], [40]. So we had analysed the antioxidant potential and hydroxyl radical scavenging activity of various extracts and the ability of extracts to prevent ROS generation under hyperglycemia. The results revealed significant antioxidant potential of SCE, SCEC and SCEL in an in vitro cell free system (Table 2) and protected HepG2 cells from hyperglycemia induced oxidative stress by preventing generation of ROS (Fig. 4A & B, P≤0.05). This result is in line with the reported protective effect of SCE on hepatic oxidative stress markers in STZ diabetic animal model [41]. It has been suggested that during hyperglycemic conditions, a non-enzymatic reaction occur between proteins and monosacharides (glycation) leading to the formation of pathologically significant AGEs [42]. Due to far reaching consequences of AGEs in the body, the estimation of glycated haemoglobin (% HbA1c) has been advised by clinicians in addition to glucose in diagnosing metabolic syndrome. Biologically AGEs alter enzyme activity, modify protein and are main culprit in diabetes induced cardiomyopathy, retinopathy and neuropathy. Moreover, AGEs induce oxidative stress and vice versa [42]. So there is a tremendous interest in aniglycation agents for diabetes therapy. But as of today no specific drug is available with antiglycation potential. Our study revealed significant antiglycation activity of SCE, SCEC & SCEL (Fig. 5A and B) which could possibly one prominent mechanism of its known antidiabetic property. The two categories of antiglycation agents (AGE inhibitors and AGE breakers) act primarily as chelators by inhibiting metal-catalyzed oxidation reactions that catalyze AGE formation [43]. From the SEM microstructure analysis of AGEs, it is clear that SCE, SCEC and SCEL exhibited antiglycation via its AGE inhibitor property [44]. The in vitro method had shown potent metal chelation capacity of SC which may be the mechanism behind the better antiglycation potential of this plant and the reported diminished %HbA1c level in the SCE treated STZ diabetic animal model [41].
PTP-1B is an abundant and widely expressed enzyme localized in endoplasmic reticulum. Theoretically, inhibition of action of PTP-1B that terminates insulin signalling would be expected to increase insulin sensitivity [45]. SCEC and SCE showed better PTP -1B inhibitory property (Fig. 2B). DPP-IV is a serine exopeptidase which regulates the half- life of two key glucoregulatory incretin hormones like glucose dependent insulinotropic polypeptide (GIP) and glucagone like peptide-1 (GLP-1) [46]. Inhibition of DPP-IV prolongs and enhances the activity of endogenous GIP and GLP-1, which serve as important prandial stimulators of insulin secretion in response to glucose and it inhibit glucagon secretion and conserve beta cell mass [46]. SCEC & SCE exhibited moderate DPP-IV inhibitory potential (Fig. 2A).
Significantly enhanced pancreatic beta cell proliferation was noticed in RIN-m5F and MIN-6 cells by SCE treatment. This pancreatic beta cell proliferation potential of test material represent an extremely useful criteria to evaluate anti-diabetic activity, which could protect the beta cells from degeneration due to gluco-lipotoxicity during type 2 diabetes mellitus or protect from autoimmune mediated destruction as in the case of type 1 diabetes mellitus [47]. We had seen the protective property of SCE against streptozotocin induced toxicity in pancreas [41]. With this result, we strongly believe that this beneficial property contribute significantly to its antidiabetic efficiency.
Since insulin resistance is a major metabolic abnormality of type 2 diabetes, there has been considerable interest in insulin sensitizing agents to counteract insulin resistance for the treatment of this disease [3]. The result of the present study showed significant insulin dependent and independent glucose uptake proving insulin sensitizing property of SCE (Fig. 6A, P≤0.05). Further studies are required to find out the mechanism behind this effect. The peroxisome proliferator activated receptor (PPAR) gamma, the master regulator of adipogenesis is abundantly present in adipocytes which can maintain whole body insulin sensitivity and thiazolidinedione group of drugs (rosiglitazone and pioglitazone) act as PPAR modulators [48], [49]. Analysis of the effect of SCE treatment on various markers of adipogenesis such as diminished activity of GPDH and reduced TG content compared to rosiglitazone, the full PPAR gamma agonist allude partial PPAR gamma agonist property of SCE (Fig. 6B and D). Adiponecin, solely secreted from adipocytes acts as a hormone with anti-inflammatory and insulin sensitizing properties [50]. There are reports to suggest the risk of T2DM appeared to decrease monotonically with increasing adiponectin level by several mechanisms [51]. So the potential of SCE to increase adiponectin level in 3T3-L1, suggest a role in its antidiabetic property and this is the first report in this regard (Fig. 6E). But detailed study on transactivation is required to confirm this [52]. Rosiglitazone is effective insulin sensitizer [48], act through its PPAR agonism. It enhances glucose uptake and adipocyte differentiation in a variety of insulin-resistant states [53]. So rosiglitazone has been taken as positive control for both glucose uptake and adipocyte differentiation studies.
Obesity is characterized by the accumulation of triacylglycerol in adipocytes and is an important risk factor for diabetes. Diacylglycerol acyltransferase (DGAT) catalyzes the final reaction of triacylgycerol synthesis and has two isoforms DGAT1 and DGAT2. DGAT1 plays a role in VLDL synthesis; increased plasma VLDL concentrations may promote obesity and thus DGAT1 is considered a potential therapeutic target of obesity and associated complications [54]. Here, a decrease in the DGAT1 activity by the treatment of SCE was observed in the study may attribute to its potential to reduce development of obesity as well hyperglycemia induced dis/hyperlipidemia (Fig. 6C).
The postprandial hyperglycemia (PPH) became a relevant target clinically and scientifically due to the importance in cardiovascular diseases and other complications [55]. The enzyme AG, present in the intestinal brush border cells hydrolyses complex carbohydrates to simple sugars. Inhibition of AG modulate carbohydrate digestion rate and prolong overall carbohydrate digestion time, causing a reduction in the rate of glucose absorption and consequently blunting PPH and insulin levels [56]. Additional therapeutic properties of AG inhibitors include protection against pancreatic beta cell apoptosis, inhibition of attachment of macrophage to vascular endothelium and amelioration of development of atherosclerosis [57]. Initial in vitro screening using yeast AG is required to see whether the study material has some alpha glucosidase inhibitory property [58]. Our in vitro studies showed promising AG inhibitory activity against both yeast derived and rat intestinal enzymes by SCE and its fractions SCD and SCEC (Fig. 1A and B). In sucrose loaded normal and SLM models, SCE prevented acute PPH effectively compared to normal control and mild diabetic control (Fig. 8A–D, P≤0.05). This reveals the efficacy of SCE to control sucrose induced PPH significantly. This antihyperglycemic activity of SCE at 500 mg/kg bw was comparable with the existing drugs like acarbose and metformin. So we selected only 500 mg/kg dose for SLM and STZ-S studies. In streptozotocin models of diabetes, due to the destruction of pancreatic beta-cells, insulin secretion has been impaired and cause blood glucose elevation [55]. SCE treatment in STZ model resulted in attenuation of PPH, whereas diabetic control animals showed elevated blood glucose even after 5 h of sucrose load (Fig. 8E and F, P≤0.05). From this it is clear that SCE negate PPH by inhibiting AG that modify sucrose breakdown rate in small intestine in normal and diabetic rats.
Deficiency of specific vitamins and minerals play important roles in glucose metabolism and insulin signalling contribute to the development of diabetes [59]. In the present investigation, SCE was found to have high amount of calcium, moderate amount of sodium, potassium and magnesium and traces of manganese and zinc. There are also reports to link the role of these minerals in ameliorating complications arising from diabetes [59]. In addition, our TPC and TFC measurement showed the presence of high content of phenolics and flavanoids (Table 1). Accordingly, HPLC analysis revealed the presence of beta-sitosterol, phloretin 2′glucoside and oleanolic acid. All these compounds are reported to have beneficial role in diabetes as well as to attenuate diabetes induced complications via different ways: beta-sitosterol improves glucose uptake and lipid metabolism [60], [61] and alpha glucosidase inhibition [62]; phloretin enhances glucose uptake [63], [64] and oleanolic acid improves insulin response [65], [66] and possesses alpha glucosidase inhibitory property [67]. The results exhibited by SC in the present study may be due to the synergistic action of these three compounds in addition to other polyphenolic components.
Conclusion
Overall results reveal potent antihyperglycemic activity via inhibition of alpha glucosidase and enhanced insulin sensitivity with moderate antiglycation and antioxidant potential of SC which contribute significantly to its antidiabetic property. The presence of known insulin sensitizers and AG inhibitors like phloretin-2 glucoside, oleanolic acid and beta-sitosterol in SC play an important role in these multifaceted activities of SC with respect to diabetes.
Supporting Information
Figures S1–S3, Antidiabetic property of Symplocos cochinchinensis is mediated by inhibition of alpha glucosidase and enhanced insulin sensitivity.
(DOCX)
Acknowledgments
We thank Director, CSIR-NIIST, Thiruvananthapuram & Director, CSIR-CDRI, Lucknow for providing necessary laboratory facilities via networking research programme of NaPAHA CSC 0130 of CSIR 12th FYP.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funder for this work was the Indian Council of Medical Research, Govt. of India, as Research Fellowship (3/1/3/JRF-2009/MPD-34 (21404)) of Antu KA. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 2. Fowler MJ (2008) Microvascular and macrovascular complications of diabetes. Clin Diabetes 26: 77–82. [Google Scholar]
- 3. Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414: 821–827. [DOI] [PubMed] [Google Scholar]
- 4.Hanfeld M (2008) Alpha glucosidase inhibitors. In: Goldstein BJ, Dirk MW, editors. Type 2 diabetes Principles and Practice. New York: Informa Healthcare. 121.
- 5. Cheng AY, Fantus IG (2005) Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ 172: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leonti M, Casu L (2013) Traditional medicines and globalization: current and future perspectives in ethnopharmacology. Front Pharmacol 4: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graziose R, Lila MA, Raskin I (2010) Merging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foods. Curr Drug Discov Technol 7: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meena AK, Bansal P, Kumar S (2009) Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J Tradit Med 4: 152–170. [Google Scholar]
- 9. Sunil C, Ignacimuthu S, Agastian P (2011) Antidiabetic effect of Symplocos cochinchinensis (Lour.) S. Moore. in type 2 diabetic rats. J Ethnopharmacol 134: 298–304. [DOI] [PubMed] [Google Scholar]
- 10. Sunil C, Agastian P, Kumarappan C, Ignacimuthu S (2012) In vitro antioxidant, antidiabetic and antilipidemic activities of Symplocos cochinchinensis (Lour.) S. Moore bark. Food Chem Toxicol 50: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 11.Krishnanvaidyan KV, Pillai SG (Eds.) (2000) Sahasrayogam-Sujanapriya Commentary. Alappuzha: Vidyarambham Publishers. 93p.
- 12. Jones WP, Kinghorn AD (2012) Extraction of plant secondary metabolites. Methods Mol Biol 864: 341–366. [DOI] [PubMed] [Google Scholar]
- 13. Pellati F, Orlandini G, Benvenuti S (2012) Simultaneous metabolite fingerprinting of hydrophilic and lipophilic compounds in Echinacea pallida by high performance liquid chromatography with diode array and electron spray ionization mass spectrometry detection. J Chromatogr A 1242: 43–58. [DOI] [PubMed] [Google Scholar]
- 14. Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144–158. [Google Scholar]
- 15. Makkar HPS, Bluemmel M, Borowy NK, Becker K (1993) Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric 61: 161–165. [Google Scholar]
- 16. Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10: 178–182. [Google Scholar]
- 17. Apostolidis E, Kwon YII, Shetty K (2007) Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol 8: 46–54. [Google Scholar]
- 18. Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of Xantan on the autooxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40: 945–948. [Google Scholar]
- 19. Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: A simple test tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165: 215–219. [DOI] [PubMed] [Google Scholar]
- 20. Stookey LL (1970) Ferrozine - A new spectrophotometric reagent for iron. Anal Chem 42: 779–781. [Google Scholar]
- 21. Riya MP, Antu KA, Vinu T, Chandrakanth KC, Anilkumar KS, et al. (2014) An in vitro study reveals nutraceutical properties of Ananas comosus (L.) Merr. var. Mauritius residue beneficial to diabetes. J Sci Food Agric 94: 943–950. [DOI] [PubMed] [Google Scholar]
- 22. Sankar V, Pangayarselvi B, Prathapan A, Raghu KG (2013) Desmodium gangeticum (Linn.) DC exhibits antihypertrophic effect in isoproterenol-induced cardiomyoblasts via amelioration of oxidative stress and mitochondrial alterations. J Cardiovasc Pharmacol 61: 23–34. [DOI] [PubMed] [Google Scholar]
- 23. Tamrakar AK, Schertzer JD, Chiu TT, Foley KP, Bilan PJ, et al. (2010) NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology 151: 5624–5637. [DOI] [PubMed] [Google Scholar]
- 24. Shi C, Wang X, Wu S, Zhu Y, Chung LW, et al. (2008) HRMAS 1H-NMR measured changes of the metabolite profile as mesenchymal stem cells differentiate to targeted fat cells in vitro: implications for non-invasive monitoring of stem cell differentiation in vivo . J Tissue Eng Regen Med 2: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nerurkar PV, Lee YK, Nerurkar VR (2010) Momordica charantia (bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes. BMC Complement Altern Med 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu YH, Zhang Y, Oelkers P, Sturley SL, Rader DJ, et al. (2002) Post transcriptional control of the expression and function of diacyl glycerol acyl transferase-1 in mouse adipocytes. J Biol Chem 277: 50876–50884. [DOI] [PubMed] [Google Scholar]
- 27. Zou C, Shen Z (2007) One-step intracellular triglycerides extraction and quantitative measurement in vitro . J Pharmacol Toxicol Methods 56: 63–66. [DOI] [PubMed] [Google Scholar]
- 28.Trease GE, Evans WC (1989) Pharmacognosy I, 12th ed, London: WB Saunders Co. Ltd.
- 29. Singh AB, Yadav DK, Maurya R, Srivastava AK (2009) Antihyperglycaemic activity of alpha-amyrin acetate in rats and db/db mice. Nat Prod Res 23: 876–882. [DOI] [PubMed] [Google Scholar]
- 30. Thomson M, Al-Amin ZM, Al-Qattan KK, Ali M (2007) Hypoglycemic effects of ginger in mildly and severely diabetic rats. FASEB J 21: 103.4. [Google Scholar]
- 31. Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, et al. (1994) The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med 121: 928–935. [DOI] [PubMed] [Google Scholar]
- 32. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, et al. (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122: 253–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbasi MA (2004) Bioactive chemical constituents of Symplocos recemosa and Comiphora mukul [Dissertation]. Pakistan: University of Karachi.
- 34. Bailey CJ, Day C (2004) Metformin: its botanical background. Pract Diab Int 21: 115–117. [Google Scholar]
- 35. Bailey CJ, Day C (1989) Traditional plant medicines as treatments for diabetes. Diabetes Care 12: 553–564. [DOI] [PubMed] [Google Scholar]
- 36.Soumyanath A (2006) Traditional medicines for modern times - Antidiabetic plants. Boca Raton, Florida: CRC press, Taylor & Francis Group.
- 37. Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622. [DOI] [PubMed] [Google Scholar]
- 38. Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, et al. (2012) Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: a randomized double-blinded placebo-controlled study. Asia Pac J Clin Nutr 21: 12–21. [PubMed] [Google Scholar]
- 39. Winiarska K, Drozak J, Wegrzynowicz M, Fraczyk T, Bryla J (2004) Diabetes-induced changes in glucose synthesis, intracellular glutathione status and hydroxyl free radical generation in rabbit kidney-cortex tubules. Mol Cell Biochem 261: 91–98. [DOI] [PubMed] [Google Scholar]
- 40. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, et al. (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790. [DOI] [PubMed] [Google Scholar]
- 41. Antu KA, Riya MP, Mishra A, Sharma S, Srivastava AK, et al. (2014) Symplocos cochinchinensis attenuates streptozotocin-diabetes induced pathophysiological alterations of liver, kidney, pancreas and eye lens in rats. Exp Toxicol Pathol 66: 281–291. [DOI] [PubMed] [Google Scholar]
- 42. Ahmed N (2005) Advanced glycation end products: role in pathology of diabetic complications. Diabetes Res Clin Pract 67: 3–21. [DOI] [PubMed] [Google Scholar]
- 43. Nagai R, Murray DB, Metz TO, Baynes JW (2012) Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 61: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vasan S, Foiles PG, Founds HW (2001) Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin Investig Drugs 10: 1977–1987. [DOI] [PubMed] [Google Scholar]
- 45. Shilpa K, Sangeetha KN, Muthusamy VS, Sujatha S, Lakshmi BS (2009) Probing key targets in insulin signaling and adipogenesis using a methanolic extract of Costus pictus and its bioactive molecule, methyl tetracosanoate. Biotechnol Lett 31: 1837–1841. [DOI] [PubMed] [Google Scholar]
- 46. Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 47. Vetere A, Choudhary A, Burns SM, Wagner BK (2014) Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov 13: 278–289. [DOI] [PubMed] [Google Scholar]
- 48. Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed M (2001) Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 86: 280–288. [DOI] [PubMed] [Google Scholar]
- 49. Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPAR gamma. Annu Rev Biochem 77: 289–312. [DOI] [PubMed] [Google Scholar]
- 50. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li S, Shin HJ, Ding EL, van Dam RM (2009) Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302: 179–188. [DOI] [PubMed] [Google Scholar]
- 52. Atanasov AG, Blunder M, Fakhrudin N, Liu X, Noha SM, et al. (2013) Polyacetylenes from Notopterygium incisum - new selective partial agonists of peroxisome proliferator activated receptor-gamma. PLoS One 8: e61755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nugent C, Prins JB, Whitehead JP, Savage D, Wentworth JM, et al. (2001) Potentiation of glucose uptake in 3T3-L1 adipocytes by PPAR gamma agonists is maintained in cells expressing a PPAR gamma dominant-negative mutant: evidence for selectivity in the downstream responses to PPAR gamma activation. Mol Endocrinol 15: 1729–1738. [DOI] [PubMed] [Google Scholar]
- 54. Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, et al. (2005) Increased very low density lipoprotein secretion and gonadal fat mass in mice over expressing liver DGAT 1. J Biol Chem 280: 21506–21514. [DOI] [PubMed] [Google Scholar]
- 55. Chiasson JL, Rabasa-Lhoret R (2004) Prevention of type 2 diabetes: insulin resistance and beta-cell function. Diabetes 53: S34–S38. [DOI] [PubMed] [Google Scholar]
- 56. Ross SA, Gulve EA, Wang M (2004) Chemistry and biochemistry of type 2 diabetes. Chem Rev 104: 1255–1282. [DOI] [PubMed] [Google Scholar]
- 57. Osonoi T, Saito M, Mochizuki K, Fukaya N, Muramatsu T, et al. (2010) The alpha-glucosidase inhibitor miglitol decreases glucose fluctuations and inflammatory cytokine gene expression in peripheral leukocytes of Japanese patients with type 2 diabetes mellitus. Metabolism 59: 1816–1822. [DOI] [PubMed] [Google Scholar]
- 58. Brindis F, González-Trujano ME, González-Andrade M, Aguirre-Hernández E, Villalobos-Molina R (2013) Aqueous extract of Annona macroprophyllata: a potential α-glucosidase inhibitor. Biomed Res Int 2013: 591313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martini LA, Catania AS, Ferreira SR (2010) Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev 68: 341–354. [DOI] [PubMed] [Google Scholar]
- 60. Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS (2011) Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes 3: 29–37. [DOI] [PubMed] [Google Scholar]
- 61. Chai JW, Lim SL, Kanthimathi MS, Kuppusamy UR (2011) Gene regulation in β-sitosterol-mediated stimulation of adipogenesis, glucose uptake, and lipid mobilization in rat primary adipocytes. Genes Nutr 6: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tiabou Tchinda A, Nahar Khan S, Fuendjiep V, Ngandeu F, Ngono Ngane A, et al. (2007) Alpha-glucosidase inhibitors from Millettia conraui . Chem Pharm Bull (Tokyo) 55: 1402–1403. [DOI] [PubMed] [Google Scholar]
- 63. Masumoto S, Akimoto Y, Oike H, Kobori M (2009) Dietary phloridzin reduces blood glucose levels and reverses SGLT1 expression in the small intestine in streptozotocin - induced diabetic mice. J Agric Food Chem 57: 4651–4656. [DOI] [PubMed] [Google Scholar]
- 64. Najafian M, Jahromi MZ, Nowroznejhad MJ, Khajeaian P, Kargar MM, et al. (2012) Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol Biol Rep 39: 5299–5306. [DOI] [PubMed] [Google Scholar]
- 65. Wang X, Liu R, Zhang W, Zhang X, Liao N, et al. (2013) Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol 376: 70–80. [DOI] [PubMed] [Google Scholar]
- 66. Castellano JM, Guinda A, Delgado T, Rada M, Cayuela JA (2013) Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 62: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ali MS, Jahangir M, Hussan SS, Choudhary MI (2002) Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 60: 295–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3, Antidiabetic property of Symplocos cochinchinensis is mediated by inhibition of alpha glucosidase and enhanced insulin sensitivity.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.