Abstract
Ankylosing spondylitis (AS) is a highly familial rheumatic disorder and is considered as a chronic inflammatory disease. Genetic factors are involved in the pathogenesis of AS. To identify genes which render people susceptible to AS in a Taiwanese population, we selected six single-nucleotide polymorphisms (SNPs) from previous genome-wide association studies (GWASs) which were associated with AS in European descendants and Han Chinese. To assess whether the six SNPs contributed to AS susceptibility and severity in Taiwanese population, 475 AS patients fulfilling the modified New York Criteria and 527 healthy subjects were recruited. We found that rs10865331 was significantly associated with AS susceptibility and with Bath AS Function Index (BASFI). The AA and AG genotypes of rs10865331 were also significantly associated with a higher erythrocyte sedimentation rate. Our findings provided evidence that rs10865331 is associated AS susceptibility and with disease activity (BASFI) in a Taiwanese population.
Background
Ankylosing spondylitis (AS) is a systemic, autoimmune disease that causes inflammation of the area where ligaments and tendons insert into the bone, and includes sacroiliitis, spondylitis, spondylodiscitis, and spondylarthritis. The prevalence of AS in Taiwan is about 0.2%∼0.3% which is similar to those in Europe and USA [1], [2]. The male-to-female ratio is about 3∶1 in AS. Twin and family studies have indicated that genetic factors contribute over 90% to the overall AS susceptibility [3], [4]. Human leucocyte antigen-B 27 (HLA-B27) gene is the best-known genetic susceptibility marker for AS, and frequencies of HLA-B27 are approximately 5.7% and 95% in the general Taiwanese population and Taiwanese AS patients, respectively [5]. Wei et al., showed that HLA-B60 and HLA-B61 are associated with AS in HLA-B27-negative patients in Taiwan [6]. Despite the strong association between HLA and AS, only 1%∼5% of HLA-B27 carriers develop AS [4]. Many non-major histocompatibility complex (non-MHC) regions were found to be significantly associated to AS in genome-wide association studies (GWASs) [7]–[9]. Additionally, genetic polymorphisms of ORAI1 (rs12313273 and rs7135617) and STIM1 (rs3750996) were reported to be associated with the pathogenesis of HLA-B27-positive (HLA-B27(+)) AS patients [10], [11].
Previous GWAS reports in European descent indicated that AS development was strongly associated with the 2p15 (rs10865331), 21q22 (rs2242944), anthrax toxin receptor 2 (ANTXR2) (rs4333130), and interleukin (IL)-23 receptor (IL23R) (rs2310173) loci [7]. John Reveille et al. confirmed the associations of IL23R (rs11209026) and endoplasmic reticulum aminopeptidase 1 (ERAP1) (rs27037 and rs27434) with AS [12]. Other significant loci, including RUNX3 (rs11249215), LTBR-TNFRSF1A (rs11616188), and IL12B (rs6556416), were also found in a combined discovery and replication study of Europeans [8]. Furthermore, Evans et al., showed a new susceptibility loci of PTGER4 (rs10440635), TBKBP1 (10781500), ANTXR2 (rs4389526), and CARD9 (rs10781500) that were also involved in the disease pathogenesis. In addition, the ERAP1 polymorphisms (rs30187) was found to associate with the risk of HLA-B27(+) AS patients [8].
The GWAS conducted in Han Chinese identified new AS susceptibility loci at 6q21 (rs13210693), HAPLN1-EDIL3 (rs4552569), and ANO6 (rs17095830). However, there is no strong evidence to indicate the association between ANTXR2 and IL23R polymorphisms and AS in Han Chinese population [9], [13]. Wang et al. confirmed the findings of previous studies [14]–[16] that ERAP1 is a risk factor for AS susceptibility in a Taiwanese population [17]. However, susceptibility loci between EDIL3, HAPLN1 at 5q14.3 and within ANO6 at 12q12 discovered in a Han Chinese GWAS were negatively associated with Taiwanese population [18], suggesting that genetic factors underlying the susceptibility may differ between these two populations. In this study, we selected SNPs from previous GWAS reports to test whether candidate genetic variations contribute to AS susceptibility and severity in a Taiwanese population.
Methods
Subjects
The design of the work was conformed to the Declaration of Helsinki. The study was approved by the Institute Review Board of Chung Shan Medical University Hospital. Before any data were collected, we received informed consent from all subjects. All subjects provided a written consent form. AS patients who fulfilled the selection criteria were sequentially solicited at Chung Shan Medical University Hospital in Taichung, Taiwan. The criteria included (a) patients being aged 17∼82 years; (b) cognitive performance not being influenced by other diseases such as dementia; and (c) an AS diagnosis having been made by modified New York criteria developed in 1984. AS diagnosed by qualified rheumatologist. Patients with sacroiliitis were confirmed by a qualified radiologist. The detailed clinical history included extra-spinal manifestations, age on initial symptoms, and laboratory parameters of inflammation, i.e., the erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Family history of AS was also recorded. The age of onset of AS symptom was defined as the time when the first symptom (axial symptom, peripheral arthritis, uveitis, or enthesitis) was noted. Peripheral arthritis was defined as the presence of at least one swollen joint. All AS patients in this study had sacroiliitis. These symptoms were recorded in medical record reviews, and were ascertained by a rheumatologist, ophthalmologist, and gastroenterologist.
Potential controls were randomly selected from sequential patients with no significant medical histories or abnormal laboratory results. To exclude controls with potential risks of getting AS, we used the HLA-B (rs13202464) polymorphism as a screening factor. Forty-three control subjects carrying the heterozygous AG genotype were excluded. Meanwhile, our data confirmed that HLA-B was significantly associated with AS in a Taiwanese population (P<0.0001). The Bath AS Disease Activity Index (BASDAI), Bath AS Functional Index (BASFI), and Bath AS Global (BAS-G) were respectively applied to evaluate disease activity, physical function, and global wellbeing. Modified Chinese versions of the BASDAI, BASFI, and BAS-G had good intra-class correlations and Cronbach's alpha values.
Candidate SNPs
We included SNPs which showed a significant association with AS in three previous GWAS studies (with trend P-values of less than or near 10−7) [7]–[9] and excluded those hadpublished on Taiwanese populations. The reason for including ERAP1 (rs27434) is because the other three SNPs on ERAP1 are reported as predisposing factors for AS in Taiwanese [17]; therefore, we test whether SNP, rs27434, has the same effect or not.
DNA extraction
Blood cells were subjected to DNA extraction by first treating them with 0.5% sodium dodecylsulfate lysis buffer and then protease K (1 mg/ml) to digest nuclear proteins for 4 h at 60°C. Total DNA was harvested using a Gentra (Qiagen, Valencia, CA) extraction kit followed by 70% alcohol precipitation.
Genotyping
Genotyping for the seven SNPs was carried out using the TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster City, CA). A polymerase chain reaction (PCR) used a 96-well micro-plate with an ABI9700 Thermal Cycler (Applied Biosystems). After the PCR, StepOne software vers. 2.2.2 (Applied Biosystems) was used to detect and analyze the fluorescence. Possession of the HLA-B27 polymorphism was assessed by flow cytometry as previously described [19].
Statistical analysis
JMP, Version 8.0 SAS Institute Inc., Cary, NC, 1989–2007 was applied for statistical analyses. Hardy-Weinberg equilibrium (HWE) was used to test the SNPs' allelic frequencies by using χ2-test. Statistical differences between the patient and control groups in genotype frequencies were evaluated by χ2-test with one degree of freedom or Fisher's exact test. Kruskal-Wallis test was applied to compare the mean of continuous variables (BASDAI, BASFI, and BAS-G). Differences in the values of the ESR, and CRP among AS patients stratified by different genotypes were computed by Wilcoxon rank sum test. Analysis of covariance (ANCOVA) was used to adjust for age, gender, and disease duration. The correlation coefficient was examined between inflammatory biochemical results (ESR and CRP) and BASDI, BASFI, BAS-G. P-value of <0.05 was considered statistical significant.
Results
Clinical features
Six selected SNPs are shown in Table 1. 475 patients with AS and 527 control subjects were recruited (Table 2). All AS patients were diagnosed according to the modified New York criteria. Their mean age was 39 years; 69.3% were men and 90.7% were HLA-B27 (+). Their mean BASDAI, BASFI and BAS-G scores were 4.3±2.2, 2.1±2.2, and 4.4±2.8, respectively. No significant different distribution of genotypes was found between HapMap CHB population and our control subjects (Table S1 in File S1).
Table 1. Six previously reported single nucleotide polymorphisms associated with ankylosing spondylitis.
| SNP | Population | Chr.a position | Candidate gene | No. Sample (Case/Control) | Trend p value | Ref.b |
| rs27434 | Han Chinese | 5p15 | ERAP1 | 1,837/4,231 | 6.68E-4 | 9 |
| rs3734523 | European | 6p22.2 | MHC | 2,053/5,140 | 1.60E-8 | 7 |
| rs4672495 | European | 2p15 | - | 2,053/5,140 | 3.30E-9 | 7 |
| rs10865331 | Han Chinese | 2p15 | - | 1,837/4,231 | 1.98E-8 | 9 |
| rs11209032 | British, Australian | 1p31 | IL23R | 4,810/13,579 | 2.3E-17 | 8 |
| rs13210693 | Han Chinese | 6q21 | - | 1,837/4,231 | 9.31E-7 | 9 |
. Chr., Chromosome.
. Reference number (Ref.) is the same as that in the text.
Table 2. Characteristics of the Taiwanese population.
| Characteristic | AS patient | Control |
| Number of subjects | 475 | 527 |
| Gender: male No (%) | 323 (68.0) | 365 (69.3) |
| Age: mean ± SD (years) | 39.0±11.3 | 39.0±11.9 |
| BASDAI | 4.3±2.2 | |
| BASFI | 2.1±2.2 | |
| BAS-G | 4.4±2.8 |
rs10865331 is associated with AS susceptibility
All genotype distributions of the polymorphisms fulfilled the criteria of HWE (P>0.05). Table 3 showed a comparison between AS cases and controls for each genotype and individual allele for all six SNPs. The rs10865331 A allele carrier (contained AA and AG genotypes) had 1.65 fold risk compared with GG genotype carrier (OR (95% CI) = 1.65 (1.21–2.23), P-value = 0.001). G allele of rs13210693 at 6q21, had a significant correlation with AS susceptibility (OR (95% CI) = 1.38 (1.96–2.06), P-value = 0.039). Since P-value was considered significant when it was less than 0.0083, after applying Bonferroni correction, only rs10865331 polymorphism showed significant association with AS.
Table 3. Association risk SNPs in previous study and current study.
| European cohort7 | British, Australia cohort8 | Han Chinese cohort9 | Current study | |||||
| SNP | Minor allele | Allele frequencya | Allele frequencya | Allele frequencya | Allele frequencya | OR (95% CI)b | P-valueb | HWEc |
| rs3734523 | A | 0.09/0.12 | 0.02/0.04 | 0.58 (0.33–1.00) | 0.052 | 0.265 | ||
| rs4672495 | G | 0.36/0.32 | 0.17/0.19 | 0.89 (0.67–1.17) | 0.400 | 0.632 | ||
| rs10865331 | A | 0.43/0.36 | 0.45/0.37 | 0.54/0.48 | 0.53/0.47 | 1.65 (1.21–2.23) | 0.001 ** | 0.817 |
| rs11209032 | G | 0.36/0.33 | 0.49/0.49 | 1.04 (0.26–1.96) | 0.794 | 0.073 | ||
| rs27434 | G | 0.26/0.21 | 0.55/0.53 | 0.46/0.50 | 0.86 (0.94–1.96) | 0.347 | 0.336 | |
| rs13210693 | G | 0.47/0.44 | 0.52/0.48 | 1.38 (1.96–2.06) | 0.039 | 0.867 | ||
χ2-test was applied for testing genotype frequencies of SNPs in controls and patients with AS.
**Significant (p<0.0017) value is in bold.
Allele frequency in case/control.
The OR and P-value were showed for dominant model of SNPs.
HWE were performed by chi-square.
The reference numbers are as the same as that in the text.
rs10865331 is associated with AS severity
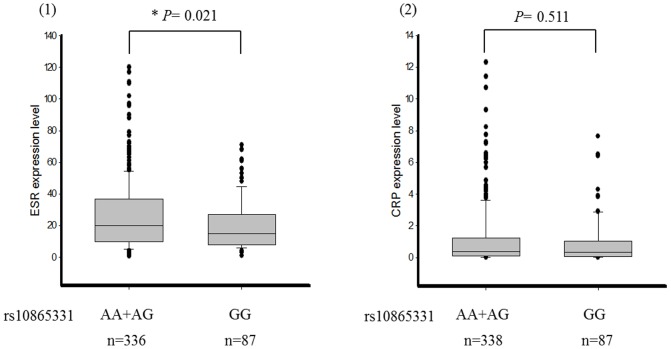
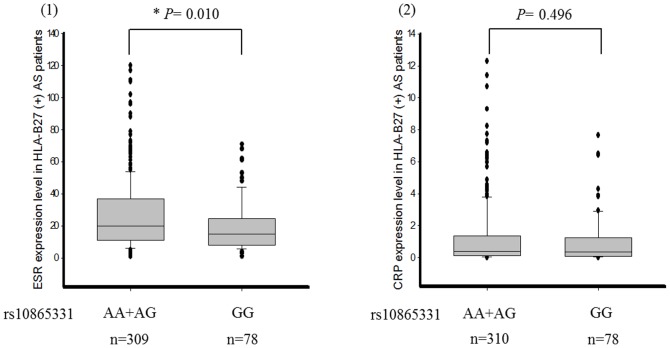
We investigated the relationship between genetic polymorphisms and clinical phenotypes including the BASDAI, BASFI, and BAS-G. We found that rs10865331 was highly associated with the BASFI (P-value = 0.033). However, the results showed no statistical significance after applying the Bonferroni correction and adjusting for gender and disease duration (Table 4). We further analyzed the association between inflammatory biochemical results (ESR and CRP) and the rs10865331 genetic polymorphism. rs10865331 AA and AG genotypes were significantly correlated with an increased ESR compared to the GG genotype in AS patients (P-value = 0.021) (Figure 1). The means±standard deviations of the ESR for the combined AA and AG genotypes and GG genotype were 26.00±21.74 and 20.07±16.26, respectively. However, the risk allele (A allele) of rs10865331 showed no correlation with CRP (P-value = 0.511). CRP values were 1.17±1.89 for the combined AA and AG genotypes and 0.96±1.49 for the GG genotype. In addition, the A allele of rs10865331 in HLA-B27(+) AS patients had a significant association with the ESR (P-value = 0.010) (Figure 2).
Table 4. Difference in disease activity scores in the AS patients stratified by genotypes.
| SNP | Genotype | BASDAI | BASFI | BAS-G |
| rs3734523 | AA | 3.84 | 4.01 | 5.00 |
| AG | 3.61±2.48 | 1.35±2.23 | 3.79±3.07 | |
| GG | 4.32±2.19 | 2.03±2.16 | 4.41 ± 2.73 | |
| Unadjusted p value | 0.289 | 0.170 | 0.538 | |
| Adjusted p value | 0.361† | 0.194§ | 0.573† | |
| rs4672495 | GG | 3.99±2.63 | 0.79±0.82 | 4.13 ± 2.80 |
| GT | 4.22±2.31 | 2.06±2.25 | 4.31 ± 2.76 | |
| TT | 4.32±2.15 | 2.08±2.24 | 4.42 ± 2.76 | |
| Unadjusted p value | 0.847 | 0.210 | 0.862 | |
| Adjusted p value | 0.737† | 0.275§ | 0.888† | |
| rs10865331 | AA | 4.66±2.09 | 2.35±2.34 | 4.66 ± 2.73 |
| AG | 4.16±2.22 | 2.13±2.32 | 4.31 ± 2.75 | |
| GG | 4.22±2.25 | 1.47±1.69 | 4.22 ± 2.68 | |
| Unadjusted p value | 0.132 | 0.033* | 0.391 | |
| Adjusted p value | 0.104† | 0.133§ | 0.500† | |
| rs11209032 | AA | 4.61±2.10 | 2.11±2.36 | 4.56 ± 2.55 |
| AG | 4.09±2.10 | 1.97±2.12 | 4.32 ± 2.77 | |
| GG | 4.34±2.44 | 2.12±2.32 | 4.36 ± 2.90 | |
| Unadjusted p value | 0.096 | 0.941 | 0.604 | |
| Adjusted p value | 0.156† | 0.467§ | 0.737† | |
| rs27434 | GG | 4.56±2.12 | 2.05±2.08 | 4.53 ± 2.50 |
| AG | 4.17±2.28 | 2.03±2.14 | 4.50 ± 2.85 | |
| AA | 4.33±2.11 | 1.61±1.92 | 3.84 ± 2.60 | |
| Unadjusted p value | 0.593 | 0.364 | 0.083 | |
| Adjusted p value | 0.407† | 0.331§ | 0.125† | |
| rs13210693 | AA | 4.37±2.30 | 1.93±2.20 | 4.10 ± 2.79 |
| AG | 4.18±2.20 | 1.95±2.21 | 4.26 ± 2.66 | |
| GG | 4.60±2.07 | 2.28±2.09 | 4.83±2.75 | |
| Unadjusted p value | 0.203 | 0.209 | 0.127 | |
| Adjusted p value | 0.271† | 0.368§ | 0.100† | |
BAS, Bath Ankylosing Spondylitis; DAI, Disease Activity Index; FI, Function Index; G, Global. Data are presented as the mean ± standard deviation. The Kruskal-Wallis test is applied to exam the difference in disease activity scores in the AS patients stratified by genotypes.
Adjusted for the effects of age and gender.
Adjusted for the effects of age, gender, and disease duration. Significant (p<0.05) values are in bold.
Figure 1. Comparison of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels among different genotypes of rs10865331 in ankylosing spondylitis (AS) patients.
Figure 2. Comparison of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels among different genotypes of rs10865331 in ankylosing spondylitis (AS) patients positive for HLA-B27.
ESR and CRP showed positive correlations with the Bath indices
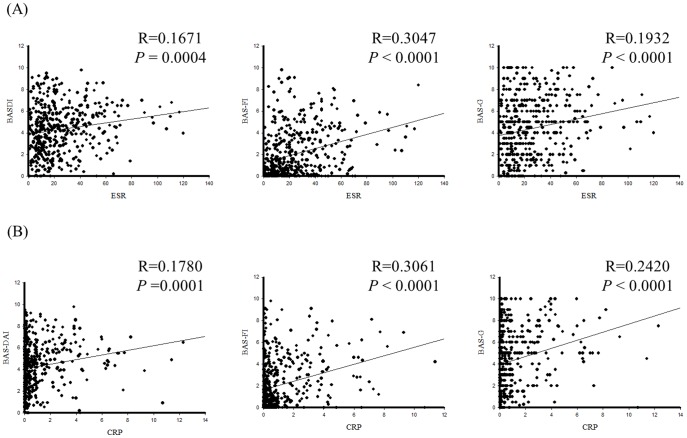
Table 4 indicated the relationship between rs10865331 and BASFI. We, therefore, further tested the correlation between inflammatory biochemical data (ESR and CRP) and disease activity (BASDI, BASFI, BAS-G). As shown in Figure 3A, ESR associated with BASDI (R = 0.1671, P-value = 0.0004), BASFI (R = 0.3047, P-value<0.0001), and BAS-G (R = 0.1932, P-value<0.0001). Regarding to CRP, positive correlations between CRP and BASDI (R = 0.178, P-value<0.0001), BASFI (R = 0.3061, P-value<0.0001), BAS-G (R = 0.2420, P-value<0.0001) were found. (Figure 3B)
Figure 3. Correlation analysis between erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and Bath Indices (BASDI, BASFI and BAS-G).
Discussion
In the case-control study, rs10865331 was significantly associated with a genetic predisposition for AS and also affected patients' daily functional activities represented by BASFI. However, the association between AS and the BASFI became insignificance after adjusting for age, gender, and the duration of the disease, this is possibly caused by the variation of scores among patients. We, therefore, used clinically important measures, including the ESR and CRP, to support the correlation between candidate SNPs and disease severity. We found an association between inflammatory biochemical lab data, i.e., the ESR, and disease severity in AS patients. After excluding HLA-B27(−) AS patients, the same trend was also found. This is the first study to confirm that the intergenic SNP, rs10865331, on chromosome 2p15 is highly associated with AS disease in a Taiwanese population.
The intergenic region, 2p15, of the associated rs10865331 SNP was replicated in several studies of Spanish, Korean and Han Chinese populations [14], [15], [20]. The rs10865331 polymorphism located 99 kb upstream of B3GNT2 and 182 kb downstream of TMEM17. Although the mechanism and functions leading to progression of AS still need to be elucidated, an SNP on B3GNT2 was found to be a susceptibility marker for rheumatoid arthritis in a Japanese GWAS meta-analysis [21]. In addition, a polymorphism (rs6545946) at 2p15 could be a plausible candidate SNP for Crohn's disease, which is a common comorbid condition of AS in Ashkenazi Jewish patients [22]. The evidence implies that intergenic regions of chromosomes 2p15 are somehow associated with certain immune diseases.
ERAP1 involves peptide trimming as presented by HLA class I molecules within the endoplasmic reticulum and only affects AS risk in HLA-B(+) individuals [23]. ERAP1 may play an important role in the shedding of proinflammatory cytokine receptors and downregulation of inflammatory responses [24]. Nonetheless, the association between ERAP1 polymorphisms and AS is divergent in different ethnic groups in genetic studies. In a meta-analysis study, rs27434 showed no significant correlation with AS, which corresponded with our results [16]. Wang et al. identified another ERAP1 SNP, rs27037, which can predict AS susceptibility and syndesmophyte formation [17]. rs27434 is a synonymous SNP, and rs27037 is located in the intron possibly affecting the regulation of MHC I heavy chain homodimers leading to unfolded protein responses [25]. This may explain the different effects of the two SNPs.
In 2013, evidence from high-density genotyping of immune-related loci indicated that major histocompatibility complex (MHC) class I presentation and IL-23 pathway are key elements in the development of ankylosing spondylitis [26]. Indeed, previous studies also confirmed an important role of IL23R gene in AS [27]. IL23R belongs to the hemopoietin receptor family for IL-23, a proinflammatory cytokine, and is involved in the production and differentiation of memory T-cells [27]. rs11209032 showed a significant correlation with AS in a UK case-control study and meta-analysis [28]. Another meta-analysis showed that rs11209032 was significantly associated with AS in European but not Asian populations [29]. Genetic differences between different ethnic groups may explain why rs11209032 had no statistical association with AS in a Taiwanese population. Interestingly, many studies reported that variations in IL23R were not associated with AS [15], [30] but were correlated with inflammatory bowel disease (IBD) which is clinically related to AS [31]. Our previous results also indicated that two susceptibility SNPs identified from Han Chinese GWAS study [9], HAPLN1-EDIL3 (rs4552569) and ANO6 (rs17095830), were not associated with AS severity but IBD in a Taiwanese population [18]. Further studies are needed to clarify the mechanism by which MHC and IL-23 involve the pathogenesis of AS and IBD.
By a systemic overview of genetic variations of the risk in three previous GWASs, we were better able to understand the high-risk polymorphisms in the Taiwanese population. A new susceptibility polymorphism at 2p15 (rs10865331) was associated with AS and disease severity. However, even with large-scale screening of candidate susceptibility loci, the exact pathological mechanism and interactions between AS genes, e.g., ERAP1 and HLA-B, are still unknown. Furthermore, examination of epigenetic factors and copy number variations are required, and functional studies that show how susceptible genes actually affect AS should be conducted. Combining all the functional causal alleles in Taiwanese populations may improve our understanding of the genetic basis of the disease and lead to novel treatment approaches.
Supporting Information
Supporting tables. Table S1, Comparison between HapMap CHB population and Taiwanese population. Table S2, Genotype and allele frequencies in controls and patients among HLA-B27 (+) with AS.
(DOCX)
Funding Statement
This work was supported by the funding from an Excellence for Cancer Research Center grant, Department of Health, Executive Yuan, Taiwan, R.O.C. (DOH102-TD-C-111-002) and grants from the National Science Council, Taiwan, ROC (NSC101-2628-B038-001-MY2; NSC101-2320-B038-029-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reveille JD (2011) Epidemiology of spondyloarthritis in North America. Am J Med Sci 341: 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zochling J, Smith EU (2010) Seronegative spondyloarthritis. Best Pract Res Clin Rheumatol 24: 747–756. [DOI] [PubMed] [Google Scholar]
- 3. Brown M, Laval S, Brophy S, Calin A (2000) Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Annals of the rheumatic diseases 59: 883–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown MA, Kennedy LG, Macgregor AJ, Darke C, Duncan E, et al. (1997) Susceptibility to ankylosing spondylitis in twins the role of genes, HLA, and the environment. Arthritis & Rheumatism 40: 1823–1828. [DOI] [PubMed] [Google Scholar]
- 5. Yang KL, Chen IH, Hsiao CK, Cherng JM, Yang KZ, et al. (2004) Polymorphism of HLA-B27 in Taiwanese Chinese. Tissue Antigens 63: 476–479. [DOI] [PubMed] [Google Scholar]
- 6. Wei JC, Tsai WC, Lin HS, Tsai CY, Chou CT (2004) HLA-B60 and B61 are strongly associated with ankylosing spondylitis in HLA-B27-negative Taiwan Chinese patients. Rheumatology (Oxford) 43: 839–842. [DOI] [PubMed] [Google Scholar]
- 7. Australo-Anglo-American Spondyloarthritis Consortium (TASC) (2010) Reveille JD, Sims AM, Danoy P, Evans DM, et al. (2010) Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 42: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, et al. (2011) Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet 43: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin Z, Bei JX, Shen M, Li Q, Liao Z, et al. (2012) A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat Genet 44: 73–77. [DOI] [PubMed] [Google Scholar]
- 10. Wei JC, Yen JH, Juo SH, Chen WC, Wang YS, et al. (2011) Association of ORAI1 haplotypes with the risk of HLA-B27 positive ankylosing spondylitis. PLoS One 6: e20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei JC, Hung KS, Hsu YW, Wong RH, Huang CH, et al. (2012) Genetic Polymorphisms of Stromal Interaction Molecule 1 Associated with the Erythrocyte Sedimentation Rate and C-Reactive Protein in HLA-B27 Positive Ankylosing Spondylitis Patients. PLoS One 7: e49698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC) (2007) Burton PR, Clayton DG, Cardon LR, et al. (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39: 1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Zhang X, Wang Y (2012) ANTXR2 and IL-1R2 polymorphisms are not associated with ankylosing spondylitis in Chinese Han population. Rheumatol Int 32: 15–19. [DOI] [PubMed] [Google Scholar]
- 14. Bang SY, Kim TH, Lee B, Kwon E, Choi SH, et al. (2011) Genetic studies of ankylosing spondylitis in Koreans confirm associations with ERAP1 and 2p15 reported in white patients. J Rheumatol 38: 322–324. [DOI] [PubMed] [Google Scholar]
- 15. Davidson SI, Liu Y, Danoy PA, Wu X, Thomas GP, et al. (2011) Association of STAT3 and TNFRSF1A with ankylosing spondylitis in Han Chinese. Ann Rheum Dis 70: 289–292. [DOI] [PubMed] [Google Scholar]
- 16. Chen R, Yao L, Meng T, Xu W (2012) The association between seven ERAP1 polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis involving 8,530 cases and 12,449 controls. Rheumatol Int 32: 909–914. [DOI] [PubMed] [Google Scholar]
- 17. Wang CM, Ho HH, Chang SW, Wu YJ, Lin JC, et al. (2012) ERAP1 genetic variations associated with HLA-B27 interaction and disease severity of syndesmophytes formation in Taiwanese ankylosing spondylitis. Arthritis Res Ther 14: R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei JC, Hsu YW, Hung KS, Wong RH, Huang CH, et al. (2013) Association study of polymorphisms rs4552569 and rs17095830 and the risk of ankylosing spondylitis in a Taiwanese population. PLoS One 8: e52801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou CT, Tsai YF, Liu J, Wei JC, Liao TS, et al. (2001) The detection of the HLA-B27 antigen by immunomagnetic separation and enzyme-linked immunosorbent assay-comparison with a flow cytometric procedure. J Immunol Methods 255: 15–22. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez A, Szczypiorska M, Juanola X, Bartolome N, Gratacos J, et al. (2010) Association of the intergenic single-nucleotide polymorphism rs10865331 (2p15) with ankylosing spondylitis in a Spanish population. J Rheumatol 37: 2345–2347. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki T, Ikari K, Yano K, Inoue E, Toyama Y, et al. (2013) PADI4 and HLA-DRB1 Are Genetic Risks for Radiographic Progression in RA Patients, Independent of ACPA Status: Results from the IORRA Cohort Study. PLoS One 8: e61045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenny EE, Pe'er I, Karban A, Ozelius L, Mitchell AA, et al. (2012) A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci. PLoS Genet 8: e1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, et al. (2011) Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet 43: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cui X, Rouhani FN, Hawari F, Levine SJ (2003) Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J Immunol 171: 6814–6819. [DOI] [PubMed] [Google Scholar]
- 25. Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G (2010) From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev 233: 181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, et al. (2013) Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 45: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trinchieri G, Pflanz S, Kastelein RA (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19: 641–644. [DOI] [PubMed] [Google Scholar]
- 28. Karaderi T, Harvey D, Farrar C, Appleton LH, Stone MA, et al. (2009) Association between the interleukin 23 receptor and ankylosing spondylitis is confirmed by a new UK case-control study and meta-analysis of published series. Rheumatology (Oxford) 48: 386–389. [DOI] [PubMed] [Google Scholar]
- 29. Lee YH, Choi SJ, Ji JD, Song GG (2012) Associations between interleukin-23R polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis. Inflamm Res 61: 143–149. [DOI] [PubMed] [Google Scholar]
- 30. Davidson SI, Wu X, Liu Y, Wei M, Danoy PA, et al. (2009) Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum 60: 3263–3268. [DOI] [PubMed] [Google Scholar]
- 31. Tremelling M, Cummings F, Fisher SA, Mansfield J, Gwilliam R, et al. (2007) IL23R variation determines susceptibility but not disease phenotype in inflammatory bowel disease. Gastroenterology 132: 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting tables. Table S1, Comparison between HapMap CHB population and Taiwanese population. Table S2, Genotype and allele frequencies in controls and patients among HLA-B27 (+) with AS.
(DOCX)