Abstract
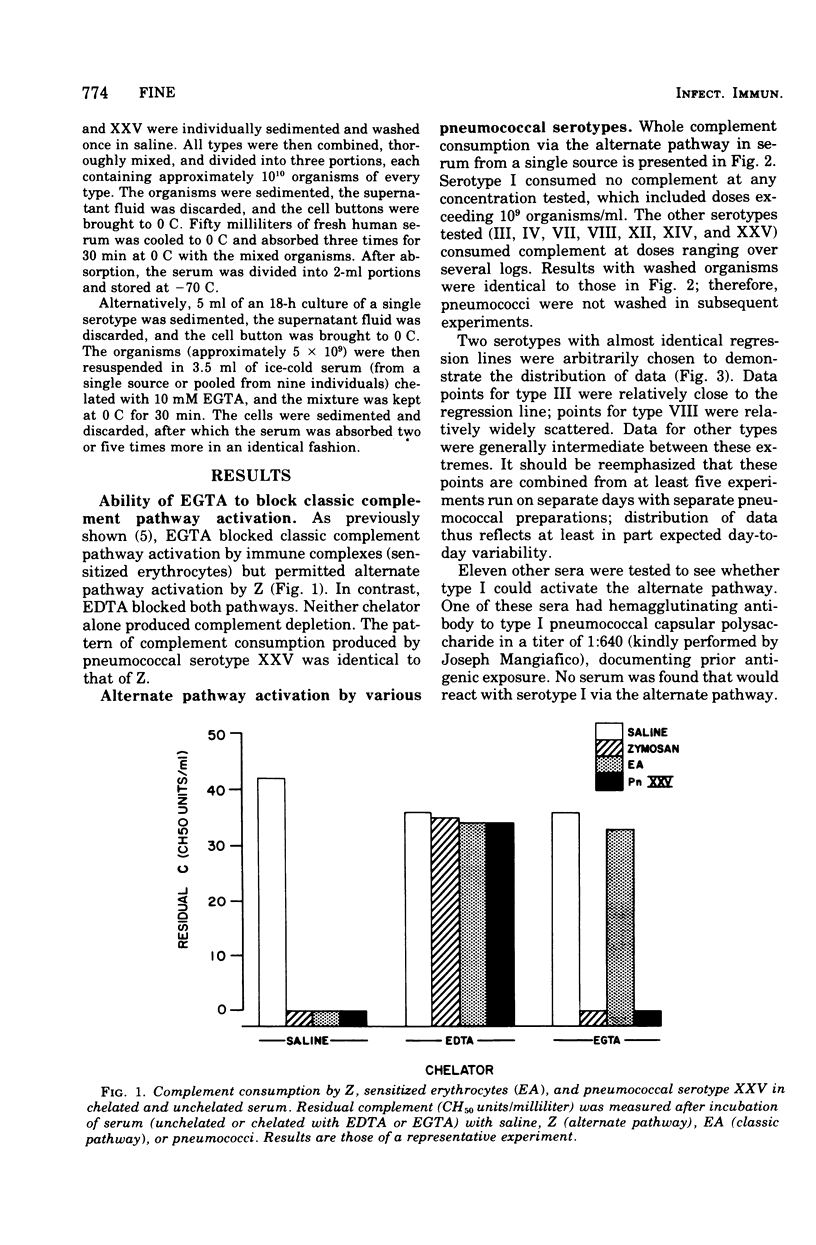
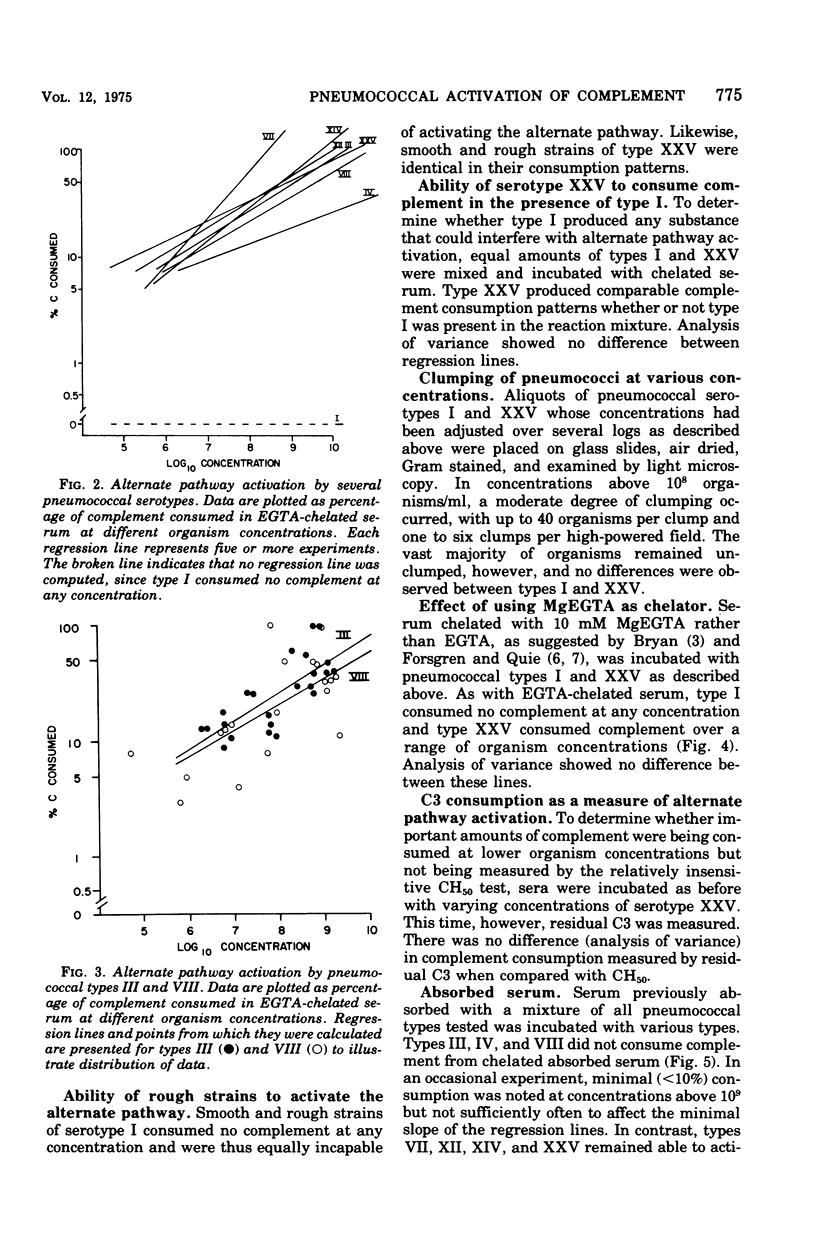
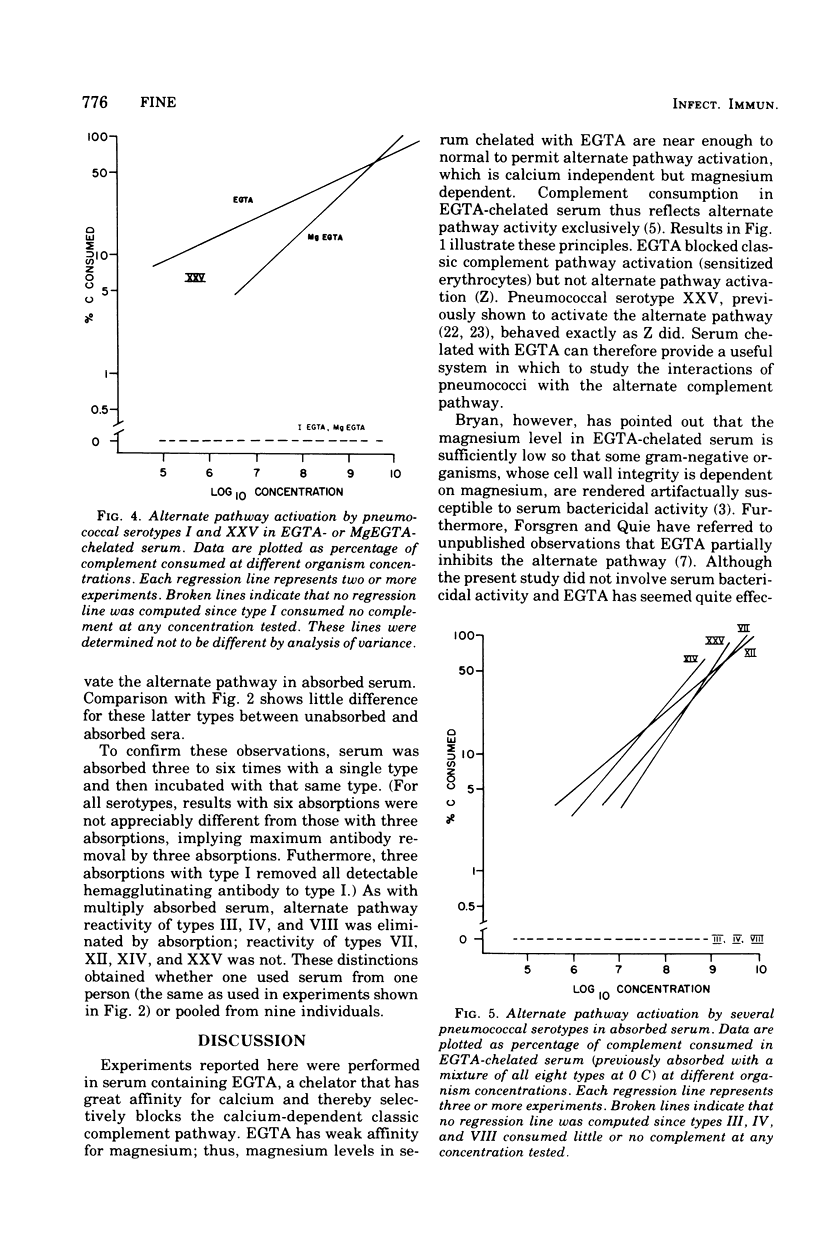
Opsonization of Streptococcus pneumoniae may be mediated by the alternate complement pathway. To study the importance of this interaction to human disease, complement consumption by pneumococci of various serotypes was measured in humwn serum chelated with ethyleneglycoltraacetic acid, a substance that blocks the classic but not the alternate complement pathwway. Serotype I, in contrast to all other types studied, lacked ability to consume complement in this system. The ability for serotypes III, IV, and VIII to activate the alternate pathway could be eliminated by prior serum absorption at O C with they type in question, a condition that would remove antibody but not complement. Types VII, XII, XIV, and XXV readily activated the alternate pathway in unabsorbed and absorbed sera. Differences could not be related to properties of the capsules. It was concluded that types I, III, IV, and VIII lack intrinsic ability to activate and thus be opsonized by the alternate complement pathway, although types III, IV, and VIII can do so in concert with specific antibody. The fact that these same types are especially prominent in human disease suggests that the ability to evade opsonization by the alternate complement pathway in pre-antibody phases of infection may be a virulence factor in pneumococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., GOLD J. PNEUMOCOCCAL BACTEREMIA WITH ESPECIAL REFERENCE TO BACTEREMIC PNEUMOCOCCAL PNEUMONIA. Ann Intern Med. 1964 May;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- Bryan C. S. Sensitization of E. coli to the serum bactericidal system and to lysozyme by ethyleneglycoltetraacetic acid. Proc Soc Exp Biol Med. 1974 Apr;145(4):1431–1433. doi: 10.3181/00379727-145-38028. [DOI] [PubMed] [Google Scholar]
- Day N. K., Gewurz H., Johannsen R., Finstad J., Good R. A. Complement and complement-like activity in lower vertebrates and invertebrates. J Exp Med. 1970 Nov;132(5):941–950. doi: 10.1084/jem.132.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974 Jun;26(6):1251–1256. [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., May J., Gaither T., Ellman L. In vitro studies of complement function in sera of C4-deficient guinea pigs. J Exp Med. 1971 Jul 1;134(1):176–187. doi: 10.1084/jem.134.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Austen K. F. Phylogeny and function of the complement system. Annu Rev Microbiol. 1971;25:309–332. doi: 10.1146/annurev.mi.25.100171.001521. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Jasin H. E. Human heat labile opsonins: evidence for their mediation via the alternate pathway of complement activation. J Immunol. 1972 Jul;109(1):26–31. [PubMed] [Google Scholar]
- Lund E. Types of pneumococci found in blood, spinal fluid and pleural exudate during a period of 15 years (1954-1969). Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(3):333–336. doi: 10.1111/j.1699-0463.1970.tb04311.x. [DOI] [PubMed] [Google Scholar]
- McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958 Feb;30(4):257–291. [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Kruss D. M., Wasil R. E., Metzger W. I. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974 Sep;134(3):505–510. [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Root R. K., Ellman L., Frank M. M. Bactericidal and opsonic properties of C4-deficient guinea pig serum. J Immunol. 1972 Sep;109(3):477–486. [PubMed] [Google Scholar]
- Williams R. C., Jr, Quie P. G. Opsonic activity of agammaglobulinemic human sera. J Immunol. 1971 Jan;106(1):51–55. [PubMed] [Google Scholar]
- Winkelstein J. A. Opsonins: their function, identity, and clinical significance. J Pediatr. 1973 May;82(5):747–753. doi: 10.1016/s0022-3476(73)80062-9. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974 May;112(5):1635–1642. [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S., Wood W. B., Jr Heat labile opsonins to Pneumococcus. 3. The participation of immunoglobulin and of the alternate pathway of C3 activation. J Immunol. 1972 Jun;108(6):1681–1689. [PubMed] [Google Scholar]



