Abstract
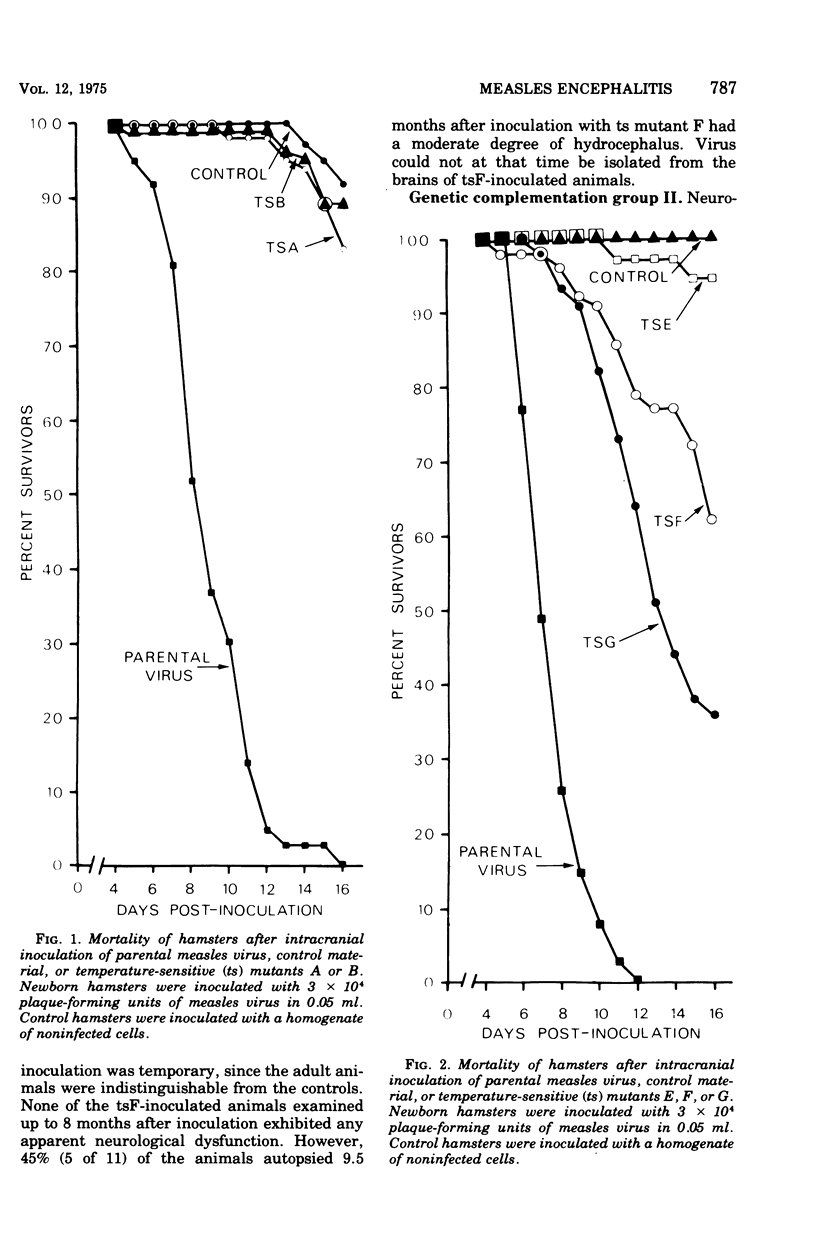
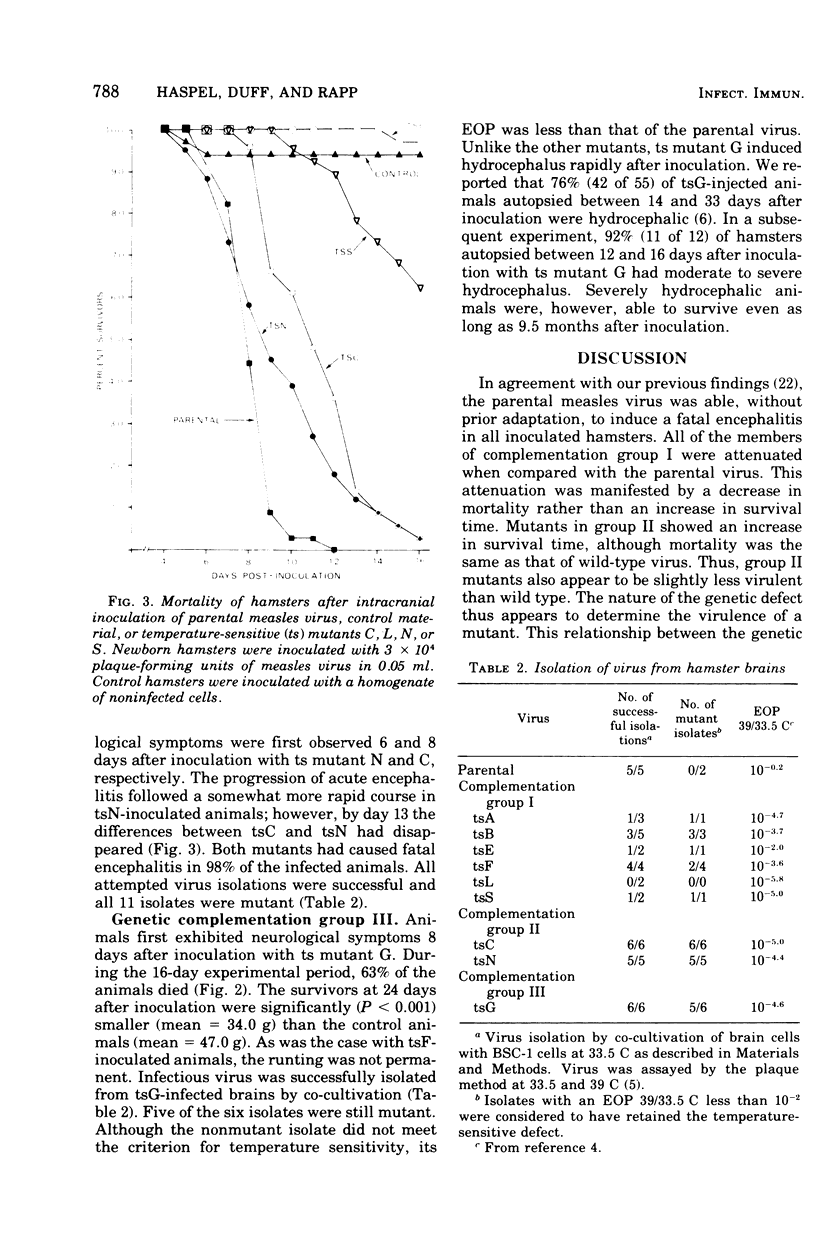
The encephalitogenic potential of nine temperature-sensitive mutants of measles virus was determined in newborn golden Syrian hamsters. The parental virus produced acute encephalitis without any prior adaptation. Six of the mutants were attenuated, two were virulent, and one was associated with hydrocephalus with acute onset. The attenuated mutants, blocked before measles virus antigen and ribonucleic acid synthesis at 39 C, were all members of one complementation group. The virulent temperature-sensitive mutants, defective in hemolysin antigen synthesis at 39 C, were members of a second complementation group. The hydrocephalus-inducing mutant was genetically distinct from the other mutants. The mechanism of attenuation most probably does not involve a temperature-induced inhibition of virus replication, but rather appears to be related to the partial defectiveness of the mutants under permissive conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P., Schumacher H. P. Neurotropic properties of measles virus in hamsters and mice. J Infect Dis. 1971 Jul;124(1):86–93. doi: 10.1093/infdis/124.1.86. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Castro A. E., Burnstein T. Adaptation to hamsters of neurotropic measles virus from subacute sclerosing panencephalitis. Nature. 1970 Feb 7;225(5232):554–555. doi: 10.1038/225554b0. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Isolation and preliminary characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Oct;16(4):1000–1009. doi: 10.1128/jvi.16.4.1000-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Ikegami N., Gomatos P. J. Temperature-sensitive conditional-lethal mutants of reovirus 3. I. Isolation and characterization. Virology. 1968 Nov;36(3):447–458. doi: 10.1016/0042-6822(68)90170-0. [DOI] [PubMed] [Google Scholar]
- Janda Z., Norrby E., Marusyk H. Neurotropism of measles virus variants in hamsters. J Infect Dis. 1971 Dec;124(6):553–564. doi: 10.1093/infdis/124.6.553. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. Subacute sclerosing panencephalitis. J Infect Dis. 1970 Feb;121(2):227–230. doi: 10.1093/infdis/121.2.227. [DOI] [PubMed] [Google Scholar]
- LABOCCETTA A. C., TORNAY A. S. MEASLES ENCEPHALITIS. REPORT OF 61 CASES. Am J Dis Child. 1964 Mar;107:247–255. [PubMed] [Google Scholar]
- Mills J., Chanock V., Chanock R. M. Temperature-sensitive mutants of influenza virus. I. Behavior in tissue culture and in experimental animals. J Infect Dis. 1971 Feb;123(2):145–157. doi: 10.1093/infdis/123.2.145. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V. Decreased reactivity of SSPE strains of measles virus with antibody. J Infect Dis. 1973 May;127(5):505–511. doi: 10.1093/infdis/127.5.505. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Neurotropic virus-host relationship alterations due to variation in viral genome as studied by electron microscopy. Am J Pathol. 1974 Apr;75(1):119–138. [PMC free article] [PubMed] [Google Scholar]
- Svobodová J., Tucková E., Vonka V. Linkage between reproductive capacity at increased temperature and neurotropic activity in A-NWS HONi (HON1) influenza virus. Infect Immun. 1974 Aug;10(2):400–401. doi: 10.1128/iai.10.2.400-401.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., BURNSTEIN T., ADAMS R. D. Histologic study of the encephalomyelitis produced in hamsters by a neurotropic strain of measles. J Neuropathol Exp Neurol. 1962 Jan;21:25–49. doi: 10.1097/00005072-196201000-00003. [DOI] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Encephalitis in newborn hamsters after intracerebral injection of attenuated human measles virus. Nature. 1970 Sep 26;227(5265):1347–1348. doi: 10.1038/2271347a0. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]
- Weiner L. P., Johnson R. T., Herndon R. M. Viral infections and demyelinating diseases. N Engl J Med. 1973 May 24;288(21):1103–1110. doi: 10.1056/NEJM197305242882106. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Woodend W. G., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus: in-vivo studies in hamsters. J Infect Dis. 1970 Dec;122(6):501–512. doi: 10.1093/infdis/122.6.501. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Käckell Y. M., Barbanti-Brodano G., Koprowski H., Lennette E. H. Subacute sclerosing panencephalitis: in-vitro characterization of viruses isolated from brain cells in culture. J Infect Dis. 1972 Jul;126(1):11–17. doi: 10.1093/infdis/126.1.11. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Müller D. Subacute sclerosing panencephalitis: a review. Curr Top Microbiol Immunol. 1972;57:1–38. doi: 10.1007/978-3-642-65297-4_1. [DOI] [PubMed] [Google Scholar]



