Abstract
Objective
To evaluate the predictive value of abdominal aortic calcium (AAC) for incident cardiovascular disease (CVD) independent of coronary artery calcium (CAC).
Approach and Results
We evaluated the association of AAC with CVD in 1974 men and women aged 45 to 84 years randomly selected from the Multi-Ethnic Study of Atherosclerosis participants who had complete AAC and CAC data from computed tomographic scans. AAC and CAC were each divided into following 3 percentile categories: 0 to 50th, 51st to 75th, and 76th to 100th. During a mean of 5.5 years of follow-up, there were 50 hard coronary heart disease events, 83 hard CVD events, 30 fatal CVD events, and 105 total deaths. In multivariable-adjusted Cox models including both AAC and CAC, comparing the fourth quartile with the ≤50th percentile, AAC and CAC were each significantly and independently predictive of hard coronary heart disease and hard CVD, with hazard ratios ranging from 2.4 to 4.4. For CVD mortality, the hazard ratio was highly significant for the fourth quartile of AAC, 5.9 (P=0.01), whereas the association for the fourth quartile of CAC (hazard ratio, 2.1) was not significant. For total mortality, the fourth quartile hazard ratio for AAC was 2.7 (P=0.001), and for CAC, it was 1.9, P=0.04. Area under the receiver operating characteristic curve analyses showed improvement for both AAC and CAC separately, although improvement was greater with CAC for hard coronary heart disease and hard CVD, and greater with AAC for CVD mortality and total mortality. Sensitivity analyses defining AAC and CAC as continuous variables mirrored these results.
Conclusions
AAC and CAC predicted hard coronary heart disease and hard CVD events independent of one another. Only AAC was independently related to CVD mortality, and AAC showed a stronger association than CAC with total mortality.
Keywords: aortic diseases, calcium, cardiovascular diseases, diagnostic imaging, epidemiology
The standard methodology for predicting cardiovascular disease (CVD) risk has used risk scores consisting of traditional risk factors1,2 and, in some cases, novel markers as well.3 The General Framingham Risk Score is a risk score used for combined CVD end points.2 To improve CVD risk prediction beyond such risk scores, several subclinical CVD measures have been investigated. Data from the Multi-Ethnic Study of Atherosclerosis (MESA) have demonstrated the incremental value of several subclinical CVD measures, including the ankle-brachial index,4 carotid intimal medial thickness by ultrasound,5 endothelial function,6 and coronary artery calcium (CAC).7 The incremental value of these measures ranges from modest (endothelial function) to high (CAC). However, among these, only CAC uses ionizing radiation. The independent value of CAC as a risk marker has now been confirmed in multiple studies.8 In addition, CAC has been shown to substantially improve classification of risk status in the MESA.9 Calcified atherosclerosis in the abdominal aorta (abdominal aortic calcium or AAC) measured in plain lumbar radiographs has also been shown to predict incident CVD.10–13 A recent meta-analysis of 10 studies concluded that the association of AAC with CVD was graded and consistent.13 However, minimal data exist for the relation of AAC, quantified by computed tomography, with CVD outcomes. In addition, whether AAC predicts CVD independent of CAC is unclear. We report here the association of AAC with cardiovascular morbidity and mortality in the MESA, when CAC was simultaneously considered in CVD risk prediction.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Table 1 shows the distribution of CVD risk factors, including those used in the General Framingham Risk Score, across the 3 categories of AAC and 3 categories of CAC, and event rates for each of the 4 end points. The Agatston score ranges are given for each of the categories and illustrate the much higher scores for AAC versus CAC, and the skewed distribution of both AAC and CAC. At higher levels of AAC, participants were older, more likely to be white, and had higher blood pressure, and were more likely to be on hypertensive therapy. At higher levels of CAC, these trends were similarly present along with a greater male percentage, lower high-density lipoprotein-cholesterol, and a higher prevalence of diabetes mellitus. Statin use did not differ significantly by level of AAC or CAC. For both hard coronary heart disease (CHD) and hard CVD, incidence rates per 1000 person-years were 1.1 to 3.0 in the CAC and AAC 0 to 50th percentile categories, but increased to 11.1 and 12.1 for hard CHD in the fourth quartile of CAC and AAC, respectively, and 17.1 and 19.2 for hard CVD in the fourth quartile of CAC and AAC, respectively. Rates in the third quartiles were intermediate and higher for CAC than for AAC. For both CVD mortality and total mortality, higher rates were present for the fourth quartile of AAC, 7.0 and 21.3, respectively, compared with CAC, 5.5 and 18.0, respectively.
Table 1.
Ethnicity, Risk Factors for the General Framingham Risk Score, and Events by Categories of AAC and CAC
| Risk Factors | AAC
|
P Value Test for Trend | CAC
|
P Value Test for Trend | ||||
|---|---|---|---|---|---|---|---|---|
| 0–50th Percentile | 51st–75th Percentile | 76th–100th Percentile | 0–50th Percentile | 51st–75th Percentile | 76th–100th Percentile | |||
| Agatston score range | 0–241 | 242–1437 | 1438–20 952 | 0–9 | 9–136 | 136–4508 | ||
| n | 983 | 491 | 492 | 983 | 491 | 492 | ||
| Age, mean (SD), y | 60.4 (8.5) | 66.5 (8.8) | 71.4 (8.2) | <0.01 | 60.9 (8.7) | 67.0 (9.3) | 69.9 (8.7) | <0.01 |
| Male, n (%) | 459 (46.7) | 278 (56.6) | 253 (51.4) | 0.22 | 399 (40.6) | 264 (53.8) | 327 (66.5) | <0.01 |
| Non-Hispanic white, n (%) | 324 (33.0) | 204 (41.6) | 262 (53.3) | <0.01 | 336 (34.2) | 200 (40.7) | 254 (51.3) | <0.01 |
| Black, n (%) | 246 (25.0) | 95 (19.4) | 70 (14.2) | <0.01 | 252 (25.6) | 84 (17.1) | 75 (15.2) | <0.01 |
| Hispanic, n (%) | 271 (27.6) | 127 (25.9) | 108 (22.0) | 0.02 | 258 (25.3) | 136 (27.7) | 112 (22.8) | 0.10 |
| Chinese, n (%) | 142 (14.5) | 65 (13.2) | 52 (10.6) | 0.04 | 137 (13.9) | 71 (14.5) | 51 (10.4) | 0.04 |
| Total cholesterol, mean (SD), mg/dL | 187.1 (35.4) | 183.7 (34.3) | 185.9 (37.6) | 0.73 | 185.8 (34.7) | 188.0 (35.7) | 184.2 (37.6) | 0.25 |
| HDL-C, mean (SD), mg/dL | 52.7 (15.4) | 50.2 (14.6) | 50.6 (15.2) | 0.03 | 52.6 (15.4) | 51.0 (15.0) | 49.9 (14.8) | <0.01 |
| Systolic BP, mean (SD), mm Hg | 119.7 (19.9) | 127.3 (21.5) | 129.4 (20.1) | <0.01 | 120.6 (20.4) | 126.6 (20.4) | 128.2 (21.0) | <0.01 |
| BP treatment, n (%) | 321 (32.7) | 237 (48.3) | 301 (61.2) | <0.01 | 327 (33.3) | 237 (48.3) | 295 (60.0) | <0.01 |
| Current smoker, n (%) | 94 (9.6) | 70 (14.3) | 56 (11.4) | 0.50 | 121 (12.3) | 52 (10.6) | 47 (9.6) | 0.15 |
| Diabetes mellitus, n (%) | 65 (6.6) | 49 (10.0) | 43 (8.7) | 0.26 | 64 (6.5) | 42 (8.6) | 51 (10.4) | 0.02 |
| Statins, n (%) | 234 (23.8) | 94 (19.1) | 135 (27.4) | 0.29 | 232 (23.6) | 115 (23.4) | 116 (23.6) | 0.98 |
| Events at follow-up | ||||||||
| Hard CHD*, per 1000 person-years | 1.8 (10) | 3.9 (10) | 12.1 (30) | 1.1 (6) | 6.5 (17) | 11.1 (27) | ||
| Hard CVD, per 1000 person-years† | 3.0 (16) | 7.9 (20) | 19.2 (47) | 2.4 (13) | 11.2 (29) | 17.1 (41) | ||
| CVD mortality, per 1000 person-years‡ | 0.5 (3) | 2.9 (8) | 7.0 (19) | 0.7 (4) | 3.9 (11) | 5.5 (15) | ||
| Total mortality, per 1000 person-years | 4.4 (25) | 8.0 (22) | 21.3 (58) | 4.2 (24) | 11.4 (32) | 18.0 (49) | ||
AAC indicates abdominal aortic calcium; BP, blood pressure; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease; and HDL-C, high-density lipoprotein-cholesterol.
Hard CHD indicates myocardial infarction, resuscitated cardiac arrest, and coronary heart disease death.
Hard CVD indicates myocardial infarction, resuscitated cardiac arrest, stroke, coronary heart disease death, and stroke death.
CVD mortality indicates death from atherosclerotic coronary heart disease, stroke, atherosclerotic disease other than coronary disease or stroke, or other cardiovascular disease not defined.
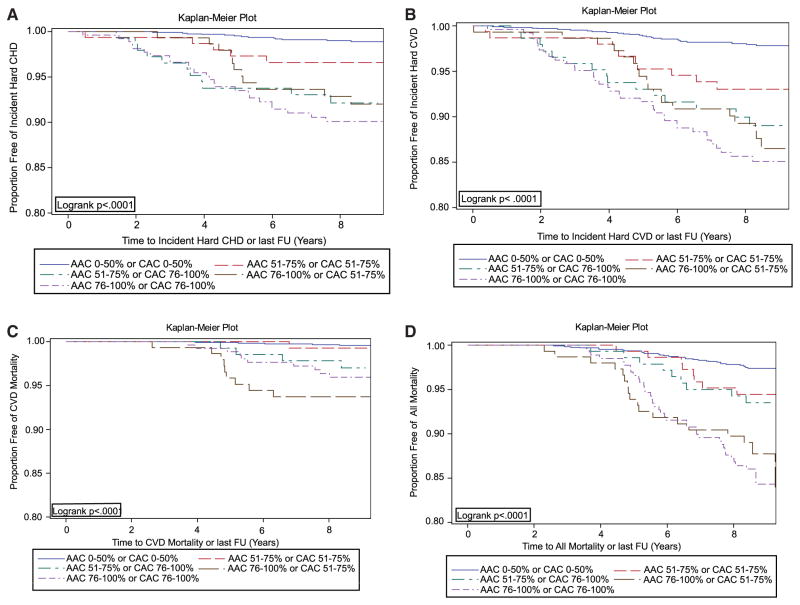
Of the 9 possible combined AAC/CAC categories (3 AAC categories multiplied by 3 CAC categories in Table 1), event rates were uniformly low in the 5 categories with either AAC or CAC ≤50th percentile, constituting 63% of the cohort. Thus, these categories were combined as the reference group for the Kaplan–Meier curves, and the remaining 4 groups were considered individually. Thirteen percent of the cohort was in the group with both AAC and CAC in the highest quartile, whereas in the remaining 3 groups, each contained 8% of the cohort. Figure A shows the rates for hard CHD, and Figure B shows the rates for hard CVD. The highest rates for both hard CHD and hard CVD occurred when both AAC and CAC were in the highest quartile, with the next highest rates for either AAC or CAC alone in the highest quartile, with intermediate rates when AAC and CAC were both in the third quartile. The overall log-rank P value was <0.0001. Figure C shows the results for CVD mortality. Here, mortality was greatest for the 2 groups with AAC in the fourth quartile, and the group with only CAC in the fourth quartile showed intermediate risk, log-rank P value of <0.0001. Figure D shows the results for total mortality, where similarly mortality was highest with AAC in the fourth quartile, but with only CAC in the fourth quartile an intermediate risk was present, log-rank P value of <0.0001.
Figure.
Kaplan–Meier curves for abdominal aortic calcium (AAC) and coronary artery calcium (CAC) categories and time to (A) a coronary heart disease (CHD) event, (B) a cardiovascular disease (CVD) event, (C) a CVD death, and (D) all mortality.
Table 2 shows the General Framingham Risk Score and ethnicity-adjusted Cox models. Models first show results for the AAC and CAC categories alone, and then to explore the independence of AAC and CAC, a model with AAC and CAC additionally adjusted for each other. Table 2 shows that for CHD, there were significant associations for the fourth quartiles of AAC and CAC, with hazard ratios (HRs) of 4.1 and 6.1, respectively, and that with mutual adjustment both HRs were attenuated but remained significant, with the HR for CAC (4.4) higher than that for AAC (2.4). For CVD, there were significant associations for the fourth quartiles of AAC and CAC, with HRs of 4.0 and 4.2, respectively, and with mutual adjustment both HRs were attenuated but remained significant, with the HR for AAC (2.7) similar to that for CAC (2.9). HRs were also elevated for the third quartile, but significant only for CAC. For CVD mortality, the fourth quartile of AAC showed a strong hazard, HR=7.8, with some attenuation after mutual adjustment, HR=5.9, P=0.01, whereas the fourth quartile of CAC showed no significant association after mutual adjustment, HR=2.1, P=NS. For total mortality, the HR for the fourth quartile of AAC after mutual adjustment was 2.7, P<0.001, whereas the HR for the fourth quartile of CAC was 1.9, P=0.04.
Table 2.
Cox Models for Hard CHD, Hard CVD, CVD Mortality, and Total Mortality for Categorical Definition of AAC and CAC, Adjusted for the General Framingham Risk Score and Ethnicity
| Hard CHD | Hard CVD | CVD Mortality | Total Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Events | 50 | 83 | 30 | 105 | ||||
| Total | 1930 | 1930 | 1966 | 1966 | ||||
| Percentile categories | HR | P Value | HR | P Value | HR | P Value | HR | P Value |
| AAC only, percentile | ||||||||
| 0–50th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| 51st–75th | 1.49 | 0.383 | 1.87 | 0.069 | 3.77 | 0.054 | 1.43 | 0.232 |
| >75th | 4.06 | <0.001 | 4.00 | <0.001 | 7.83 | 0.002 | 3.51 | <0.001 |
| CAC only, percentile | ||||||||
| 0–50th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| 51st–75th | 4.74 | 0.001 | 3.67 | <0.001 | 4.03 | 0.019 | 2.28 | 0.003 |
| >75th | 6.14 | <0.001 | 4.21 | <0.001 | 3.92 | 0.021 | 2.79 | <0.001 |
| AAC and CAC, percentile | ||||||||
| AAC 0–50th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| AAC 51st–75th | 1.08 | 0.875 | 1.46 | 0.279 | 3.11 | 0.104 | 1.23 | 0.506 |
| AAC >75th | 2.38 | 0.038 | 2.66 | 0.003 | 5.89 | 0.009 | 2.71 | <0.001 |
| CAC 0–50th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| CAC 51st–75th | 3.91 | 0.006 | 2.92 | 0.003 | 2.75 | 0.096 | 1.82 | 0.037 |
| CAC >75th | 4.35 | 0.004 | 2.90 | 0.003 | 2.10 | 0.231 | 1.85 | 0.036 |
AAC indicates abdominal aortic calcium; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease; and HR, hazard ratio.
Models exploring potential effect modification by sex or ethnicity showed nonsignificant interaction terms, and there was no significant interaction between the AAC and CAC categories. Tests for nonproportional hazards across AAC and CAC categories using Schoenfeld residuals14 were all nonsignificant.
Table 3 shows the receiver operating characteristic curve (area under the curve or AUC) analyses. Both AAC and CAC increased the AUC for both hard CHD and hard CVD, but the result was significant only for CAC, and when considered together, AAC added little to CAC. For CVD mortality, the results were reversed with only AAC significant and addition of CAC adding little to AAC. For total mortality, the increase for AAC was greater than CAC, but both were significant, and the highest AUC occurred when both were in the model.
Table 3.
AUC for Each of the 4 Outcomes With Addition of Categorical Definitions of AAC and CAC to the Base Model*
| Percentile Categories | CHD Hard
|
CVD Hard
|
CVD Mortality
|
Total Mortality
|
||||
|---|---|---|---|---|---|---|---|---|
| AUC | P Value* | AUC | P Value* | AUC | P Value* | AUC | P Value* | |
| Base model† | 0.749 | NA | 0.749 | NA | 0.768 | NA | 0.716 | NA |
| AAC categories | 0.772 | 0.210 | 0.772 | 0.074 | 0.816 | 0.037 | 0.753 | 0.015 |
| CAC categories | 0.807 | <0.001 | 0.793 | <0.001 | 0.802 | 0.090 | 0.743 | 0.037 |
| Both | 0.805 | 0.007 | 0.796 | 0.002 | 0.821 | 0.053 | 0.762 | 0.008 |
AAC indicates abdominal aortic calcium; AUC, area under the curve; CAC, coronary artery calcium; CHD, coronary heart disease; and CVD, cardiovascular disease.
Vs base model.
Adjusted for the General Framingham Risk Score and ethnicity.
The results of sensitivity analyses using log-transformed continuous measures of AAC and CAC, ln(AAC+1) and ln(CAC+1), mirrored these results. In models containing both variables, for ln(AAC+1), the HR per SD for CHD was 1.5 (P=0.11), for CVD 1.5 (P=0.02), for CVD mortality 3.3 (P≤0.01), and for total mortality 1.8 (P≤0.01). For ln(CAC+1), the HRs were 1.9 (P≤0.01), 1.5 (P=0.02), 1.2 (P=0.54), and 1.3 (P=0.04), respectively. These results confirm the somewhat stronger predictive power of CAC for hard CHD and the much stronger predictive power of AAC for CVD mortality and total mortality.
Following the recommendation of the recently published American College of Cardiology/the American Heart Association guideline on the assessment of cardiovascular risk to inform treatment decisions in patients considered uncertain by quantitative risk assessment,15 we compared a CAC cut point of 300 Agatston units (the 85th percentile in these data) with the same percentile cut point for AAC (2754). The results are shown in Table 4. With both AAC and CAC in the model, AAC was highly significantly associated for each of the 4 outcomes, with HRs ranging from 2.33 to 3.92, whereas CAC was not significantly associated with any of the 4 outcomes, with HRs ranging from 1.22 to 1.53.
Table 4.
Cox Models for Hard CHD, Hard CVD, CVD Mortality, and Total Mortality for ≥85th Percentile of AAC (2754) and CAC (300), Adjusted for the General Framingham Risk Score and Ethnicity
| Hard CHD | Hard CVD | CVD Mortality | Total Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Events | 50 | 83 | 30 | 105 | ||||
| Total | 1930 | 1930 | 1966 | 1966 | ||||
| Percentile categories | HR | P Value | HR | P Value | HR | P Value | HR | P Value |
| AAC only, percentile | ||||||||
| 0–84th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| ≥85th | 4.34 | <0.001 | 2.69 | <0.001 | 2.53 | 0.021 | 2.79 | <0.001 |
| CAC only, percentile | ||||||||
| 0–84th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| ≥85th | 2.15 | 0.014 | 1.98 | 0.005 | 1.67 | 0.198 | 1.65 | 0.026 |
| AAC and CAC, percentile | ||||||||
| AAC 0–84th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| AAC ≥85th | 3.92 | <0.001 | 2.37 | <0.001 | 2.33 | 0.046 | 2.62 | <0.001 |
| CAC 0–84th | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| CAC ≥85th | 1.42 | 0.288 | 1.53 | 0.095 | 1.29 | 0.552 | 1.22 | 0.392 |
AAC indicates abdominal aortic calcium; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease; and HR, hazard ratio.
Discussion
Among subclinical CVD measures, CAC has shown the largest HRs for subsequent CVD events.7,8 In the MESA, participants with CAC scores in the top quartile (Agatston scores >100) had an ≈7-fold risk of a major coronary event during the first 5 years of follow-up.7 However, using relatively crude measures of AAC from standard lumbar radiographs, AAC has also been reported to be strongly associated with future CVD events.10–13 We have shown in the MESA that CAC was moderately correlated with AAC in participants with at least some AAC and CAC, with a Spearman correlation coefficient of 0.38 (P<0.0001).16 Here, each calcium measure attenuated the other somewhat, but the fourth quartile for both measures remained independently significant in predicting hard CHD and hard CVD. However, only AAC was predictive of CVD mortality. CAC showed higher AUC values for hard CHD and hard CVD, but AAC showed higher AUCs for CVD mortality and total mortality. There are no prior reports available that have directly evaluated the prognostic value of AAC measured by computed tomography for CVD events with adjustment for CAC and the General Framingham Risk Score simultaneously.
Several other subclinical CVD measures, including the ankle-brachial index,4,17,18 carotid intimal medial thickness,5,19 endothelial function,6 subclavian stenosis,20 and thoracic aortic calcium21–25 have been reported to show independent predictive value for incident CVD events. Most of these measures have been reported to improve the area under the receiver operating characteristic curve. However, subclinical measures of CVD are correlated with each other,26,27 some highly so, and in evaluating a given subclinical CVD measure many reports have not adjusted for other correlated subclinical CVD measures, leaving the independence of the reported findings in doubt.
For CVD mortality, the independent HR for the fourth quartile of AAC was 5.9 versus 2.1 for CAC. For total mortality, the independent HR for AAC was 2.7, compared with 1.9 for CAC. CAC has been reported to be associated with both CVD and total mortality previously.21,24 However, these associations have been of variable strength. The reason for the discrepancy between hard CHD and hard CVD, and fatal outcomes here is unclear. AAC is more common, occurs earlier, is better correlated with risk factors than CAC, and seems to reflect atherosclerotic burden beyond CAC.16 A possibility is that the strong mortality association for AAC reflects the known significance of the total atherosclerotic burden for mortality beyond the extent of coronary disease per se.17–21 Of interest, a recent report from the Framingham study showed that AAC, but not CAC, was significantly associated with total mortality.28
Prior studies that have compared other subclinical CVD measures with CAC within the same cohort have not reported another subclinical measure equal to or superior to CAC in predicting CVD outcomes. Based on the data presented here, AAC could improve risk prediction. People below the 50th percentile for either measure had a low risk of events. These data suggest that AAC would add to CAC in predicting hard CVD and that AAC would be the stronger subclinical CVD measure for improving CVD mortality and total mortality risk prediction.
In the analyses of AAC and CAC in Table 4 where the CAC cut point of 300 recommended in the recent American College of Cardiology/the American Heart Association guideline was compared with the percentile equivalent for AAC, AAC was significant for each of the 4 end points, whereas CAC showed no significant association for any of the end points.
Strengths of this study include standardized and validated protocols in a multiethnic cohort, careful measurement of potentially confounding variables, and follow-up in a cohort free of CVD at baseline. Limitations include the modest number of CVD deaths (30), although the difference in the predictive value of AAC versus CAC for CVD mortality was marked. The cohort studied here was an ≈30% subset of the full MESA cohort. However, they were randomly selected and thus representative of the larger MESA cohort. To allow comparable assessment of the extent of AAC in participants, our AAC measure was limited to the distal 8 cm of the abdominal aorta. This choice seems reasonable because previous studies have shown the highest concentration of AAC is near the bifurcation. The MESA cohort was not a random sample of the US population, but MESA was structured for multiethnic representation. Finally, given the sparse data on the predictive value of AAC independent of CAC, our results will need to be replicated by others.
In conclusion, in a multiethnic cohort, both AAC and CAC were independently predictive for hard CHD and CVD. For CVD mortality, only AAC was independently predictive. The apparent predictive value of CAC for mortality was attenuated when AAC was added to the model. Given guidelines for screening for CAC in asymptomatic intermediate-risk people29 and the independent value of AAC in these data, further research should consider whether the added clinical use of AAC beyond CAC might warrant recommendations for CVD risk assessment.
Supplementary Material
Significance.
In previous studies of subclinical atherosclerosis measures, CAC has shown the strongest independent relationship to CVD events and has similarly shown the greatest increase in CVD risk prediction above standard CVD risk scores based on risk factors. This study is first to report a subclinical atherosclerosis measure, AAC, that shows a similar predictive value to CAC for hard CHD and hard CVD events, and a stronger association than CAC for CVD mortality. Importantly, the predictive values of AAC and CAC are independent and additive, such that overall risk prediction improved when both were measured simultaneously. These results, if confirmed elsewhere, suggest that future recommendations for CVD risk assessment should consider the added clinical utility of AAC measurement.
Acknowledgments
We thank Nova Rogers for assisting in article preparation. We thank the staff and participants of Multi-Ethnic Study of Atherosclerosis for their important contributions.
Sources of Funding
This work was supported by the National Institute of Health (HL72403) and the National Heart, Lung, and Blood Institute (N01-HC-95159 through N01-HC-95169 and K12HL83141).
Nonstandard Abbreviations and Acronyms
- AAC
abdominal aortic calcium
- AUC
area under the curve
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HR
hazard ratio
- MESA
Multi-Ethnic Study of Atherosclerosis
- ROC
receiver operating characteristic
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.114.303268/-/DC1.
Disclosures
N. Wong is a Consultant (significant) at Re-Engineering Healthcare, Inc. The other authors report no conflicts.
References
- 1.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 2.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Bonow RO, Brundage BH, et al. American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/ AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography); Society of Atherosclerosis Imaging and Prevention; Society of Cardiovascular Computed Tomography. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 9.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 12.Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, Wolf PA, O’Donnell CJ. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–331. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 14.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 15.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published online ahead of print November 12, 2013] [Accessed May 12, 2014.];Circulation. 2013 doi: 10.1161/01.cir.0000437741.48606.98. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437741.48606.98.full.pdf+html. [DOI]
- 16.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, Detrano R, Post W, Wong ND. Risk factor differences for aortic versus coronary calcified atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 18.Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboyans V, Criqui MH, McDermott MM, Allison MA, Denenberg JO, Shadman R, Fronek A. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007;49:1540–1545. doi: 10.1016/j.jacc.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–146. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic aortic calcification and coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong ND, Gransar H, Shaw L, Polk D, Moon JH, Miranda-Peats R, Hayes SW, Thomson LE, Rozanski A, Friedman JD, Berman DS. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:319–326. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs PC, Prokop M, van der Graaf Y, Gondrie MJ, Janssen KJ, de Koning HJ, Isgum I, van Klaveren RJ, Oudkerk M, van Ginneken B, Mali WP. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Santos RD, Rumberger JA, Budoff MJ, Shaw LJ, Orakzai SH, Berman D, Raggi P, Blumenthal RS, Nasir K. Thoracic aorta calcification detected by electron beam tomography predicts all-cause mortality. Atherosclerosis. 2010;209:131–135. doi: 10.1016/j.atherosclerosis.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, Criqui MH. The ankle brachial index and subclinical cardiac and carotid disease: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann U, Massaro JM, D’Agostino R, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. Circulation. 2013;128:A14097. doi: 10.1161/JAHA.115.003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland P, Alpert JS, Beller GA, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.