Abstract
Opioid dependence is a growing public health problem. Maintenance on the antagonist naltrexone for clinic or office-based treatment of opioid dependence is plagued by high rates of relapse. This paper identifies critical determinants of lapses to opioid use during naltrexone maintenance. Time retained in treatment was examined as a function of whether lapses to opioid use occurred while adherent to naltrexone (blocked use), or after having missed naltrexone doses (unblocked). Method: Participants (N=83) met DSM-IV criteria for opioid dependence and identified a significant other willing to participate in their treatment. Following inpatient detoxification, participants were enrolled in a 26-week outpatient course of therapy and naltrexone maintenance. Results: Patients with unblocked use had a very high rate of dropout (10% retained at 6 months), dropout usually occurring within two weeks after unblocked use. Patients with only blocked use had less dropout (33% retained at 6 months). However, episodes of blocked use were often followed by unblocked use and dropout. Conclusions: During naltrexone maintenance for opioid dependence unblocked opioid use calls for immediate intervention, such as detoxification or switching to the partial agonist buprenorphine. Episodes of blocked use warrant increased clinical attention, such as direct observation of naltrexone ingestion, increased dose, or increased intensity of treatment contact. Maintenance on oral naltrexone is a fragile treatment because it is so easily undermined by episodes of opioid use while non-compliant. New long-acting injectable or implantable formulations of naltrexone may address this limitation and should be investigated for treatment of opioid dependence.
1. Introduction
Opioid dependence is a significant public health problem, exacerbated by falling heroin prices and rapidly rising rates of prescription opioid abuse. Only two office-based medication treatments for opioid dependence exist: buprenorphine, an opioid partial agonist, and naltrexone, an antagonist. Naltrexone has been underutilized because it requires detoxification from opioids before it can be started, and adherence has generally been poor. Its effectiveness can be improved by combining naltrexone with behavioral treatments that focus on adherence (Preston et al. 1999; Carroll et al. 2001; Fals-Stewart & O'Farrell 2003; Nunes et al. 2006; Sullivan et al. 2006a). Further, naltrexone has potential advantages for office-based practice. It lacks abuse potential, it might reduce concurrent alcohol or cocaine use (Anton et al. 2006, Oslin et al. 1999, Schmitz et al. 2001), and it is an alternative for patients who refuse agonist treatment.
A critical challenge in naltrexone maintenance for opioid dependence is the management of episodes of opioid use. While taking naltrexone, effects of opioids are blocked, which should facilitate extinction of opioid-seeking behavior. However, if a patient uses opioids a few days after stopping oral naltrexone, opioid effects are not blocked and there is a of risk relapse and even overdose because tolerance is lowered. Hence, much is at stake when a naltrexone-treated patient presents with resumed opioid use. Yet, the literature contains little evidence to guide clinical practice at this critical juncture. We therefore examined the clinical course of lapses and relapses to opioid use in a series of opioid-dependent patients receiving naltrexone maintenance plus behavioral therapy, focusing on two groups of patients, those who used opioids while on naltrexone (blocked) and those who used opioids after having stopped naltrexone (unblocked).
2. Method
2.1 Participants
The sample consisted of 83 individuals meeting DSM-IV criteria for opioid dependence, who completed an inpatient detoxification and naltrexone induction and were subsequently treated with Behavioral Naltrexone Therapy (BNT) in one of two clinical trials described previously (Rothenberg et al., 2002; Nunes et al., 2006). To be eligible, participants had to have a significant other willing to engage in the treatment. These studies were approved by the New York State Psychiatric Institute Institutional Review Board (IRB), and all participants gave written informed consent. Of 553 individuals screened for participation, 159 were eligible and consented, 116 completed detoxification, and 83 were assigned to BNT.
2.2 Procedures
BNT consists of two weekly sessions across 26 weeks, one individual session and one network session with the significant other, with the overall aim of securing compliance with naltrexone and abstinence (Rothenberg et al. 2002, Nunes et al. 2006). Relapse prevention sessions included teaching coping skills (e.g. managing cravings and urges, and adherence strategies), building a support network, and administering a voucher incentive regimen. Network sessions focused on naltrexone monitoring and medication compliance. At each visit, urine was tested for opioids and by visual assessment with UV light for fluorescence, demonstrating adherence with riboflavin-labeled naltrexone.
At the outset, naltrexone was administered at the clinic (e.g. 100mg Monday, 100mg Wednesday, 150mg Friday), and transitioned to home-based administration, monitored by the significant other. Participants compliant with naltrexone and opioid-negative after two weeks began home-based administration of naltrexone, observed by the monitor. In the event of a lapse to opioid use, both individual and network sessions focused on identifying triggers for use, other coping strategies and naltrexone adherence. Clinic based naltrexone administration could be resumed. However, participants who provided opioid-positive urine specimens that failed to fluoresce (indicating they had not taken naltrexone) were considered to have had one or more episodes of unblocked use. Intensive efforts were made to try to restart such patients on naltrexone. A naloxone challenge was offered to patients who reported no opioid use in the past 24 hours, as this was deemed the minimum period of abstinence needed to “pass” a challenge by not exhibiting precipitated withdrawal. Individuals were not eligible to resume naltrexone until they had passed a naloxone challenge, demonstrating their ability to tolerate the longer-acting antagonist. Patients showing minimal withdrawal symptoms in response to the challenge were restarted on partial doses of naltrexone, to be followed by a full dose as soon as withdrawal symptoms subsided. Participants were removed if there was clinical evidence of relapse to opioid dependence and were referred to other treatment programs for detoxification or methadone maintenance.
2.3 Data Analysis
Weeks retained in treatment was the principal outcome measure since dropout is usually an indicator of treatment failure and relapse in this population. Time-to-dropout was examined using Kaplan Meier survival curves and the log-rank test.
3. Results
The sample (N=83) included 24% women and was 57% Caucasian, 28% Hispanic, 13% African-American; mean age was 35 years (range 20-57). The sample was divided into three groups: 1) blocked users (11%; 9/83), defined as those whose episodes of opioid use occurred only while taking naltrexone (simultaneous urine positive for opioids and also positive for riboflavin fluorescence); 2) unblocked users (59%; 49/83), who had one or more episodes of opioid use while not taking naltrexone (urine opioid positive, but riboflavin negative; and 3) nonusers (30%, 25/83), who had no opioid positive urines during treatment.
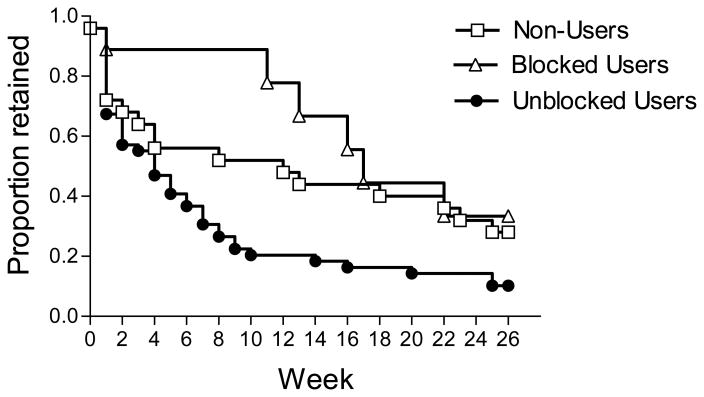
Figure 1 shows the survival curves for weeks retained in treatment for the blocked use, unblocked use, and non-user groups. The groups differ significantly in treatment retention (Log Rank = 7.78, df = 2, p=.02), with blocked users showing the best retention, unblocked users the worst, and non-users showing intermediate retention. The shape of the retention curves, and details of clinical course in the three groups are also of interest.
Figure 1. Weeks Retained in Naltrexone Maintenance, by Opioid Use Group Type.
Unblocked users demonstrated an early steep decline in retention. Blocked users showed better retention for the first 3 months, then experienced progressive dropout; only 33% were retained at 6 months. Non-users had an intermediate course. This group likely included a mixture of patients who never used and those who dropped out before opioid-positive urine could be measured.
The unblocked use group shows a rapid drop in retention over the early weeks of treatment, with only 10% (5/49) of patients completing 26 weeks. Patients in the unblocked group were retained in treatment only a median of 1.0 week (range 0-25) after using heroin, and only 39% (19/49) were retained for more than two weeks after first unblocked use. Clinically, the period after unblocked use consisted of efforts, usually futile, to help patients sustain abstinence for long enough to pass a naloxone challenge, and safely restart naltrexone. Of 49 unblocked users, only 28 participants underwent a naloxone challenge (many having refused), and 17 actually resumed naltrexone. Those who resumed naltrexone were retained in treatment only a median of 2.0 (range 0-14) additional weeks. Of interest as well, 25% (12/49) of the unblocked group began using opioids while taking naltrexone, before stopping naltrexone and converting to unblocked use (median of 2.0 episodes of blocked use preceding unblocked use, range 1-15). By comparison, those who remained blocked users had a median of only 1.0 episode of blocked use (range 1-6).
The blocked use group showed better retention: 89% (8/9) at10 weeks, although after 10 weeks there is a decrease in retention, with 33% (3/9) ultimately completing 26 weeks. Patients in the blocked group were retained a median of 12.0 weeks (range 0-24) beyond the first opioid-positive urine. By contrast, blocked users who became unblocked were retained a median of 3.5 (range 0-14) weeks after initial opioid use. And only 8% of blocked-to-unblocked users completed treatment.
The non-user group shows a steep initial decrease in retention, similar to the early reduction observed in the unblocked group, followed by a flattening of the retention curve, ultimately achieving similar 26-week retention (28%, 7/25) to that of the unblocked group.
4. Discussion
These findings on the clinical course following episodes of opioid use during naltrexone maintenance suggest two main conclusions. The first is that unblocked use, that is use of opioids after having stopped taking naltrexone, is a catastrophic event within naltrexone maintenance therapy, almost always leading to full relapse to opioid dependence. Despite intensive clinical interventions, the vast majority of patients who used after having stopped oral naltrexone dropped out of treatment within several weeks, and few were able to resume naltrexone in a sustained fashion. For most of these patients, dependence on opioids was re-established very rapidly, within days, after which detoxification and resumption of naltrexone on an outpatient basis was difficult. It also seems likely that the early dropoff in retention in the non-user group represents patients who used opioids unblocked, i.e. having discontinued naltrexone, but never returned to the clinic to be tested. In addition to the deleterious effects of relapse itself, relapse after having stopped naltrexone, like relapse after a detoxification, is a time of high risk for opioid overdose because of lack of tolerance (Ritter 2002, White & Irvine 1999, Miotto et al. 1997).
We therefore recommend two specific options in response to an incident of unblocked use of opioids after having stopped naltrexone: (1) Admit the patient for inpatient detoxification and stabilization, or (2) Start a course of buprenorphine-naloxone. Our experience with patients at this juncture is that many will decline admission or referral to a methadone maintenance program, and will ask for the opportunity to restart naltrexone on an outpatient basis. Buprenorphine-naloxone maintenance can be easily initiated in the office or clinic setting, is of equivalent efficacy to methadone maintenance, and may be more acceptable to patients in this situation. A brief effort to restart naltrexone on an outpatient basis, perhaps using a buprenorphine taper as a bridge, may be warranted in a particularly reliable, well-motivated patient with no history of overdose, or perhaps with a history of successful outpatient opioid detoxifications in the past. At the outset of treatment, before naltrexone induction, it may he helpful to orient patients to the range of rescue strategies and treatment options available in case of relapse, and that failure of a course of naltrexone maintenance does not need to translate into failure of the larger treatment effort.
Our second main conclusion is that repeated episodes of blocked use may be danger signs during naltrexone maintenance. On the one hand, our patients who “tested” the blockade by using opiates while complying with naltrexone showed the best initial rates of treatment retention, exceeding that of non-users during the first 3 months of treatment. This finding suggests that the experience of finding opiates non-rewarding may, as theorized, promote extinction and reduce risk of subsequent opiate use. On the other hand, for many patients unblocked use was preceded by episodes of blocked use. Further, the fall-off in treatment retention observed after week 10 in the blocked use group (see Figure 1) may well reflect subsequent episodes of unblocked use that were not measured. Thus, recurrent blocked use by a patient warrants clinical attention and intensified efforts to ensure continued full compliance with naltrexone. These could include direct observation of pill ingestion (either by clinical staff or by significant others at home), increasing the naltrexone dose to provide longer-lasting blockade, and increasing the frequency or intensity of treatment.
The present study has several strengths. It included a broad sample of patients seeking detoxification from opiates and thus may offer wider generalizability compared to studies of professionals or probationers with strong external motivations to adhere to treatment (Washton et al. 1984, Cornish et al. 1997). Participants in the present study were closely monitored prospectively for drug use and naltrexone compliance. Nonetheless one limitation is that our findings are subject to uncertainty in measurement, since urine toxicology and riboflavin are of limited sensitivity in detecting opioid use and naltrexone compliance (e.g. patients ingesting vitamins containing riboflavin may be subject to false positive readings).
Taken together, the findings may be interpreted as reflecting the fragility of treatment of opioid dependence with oral naltrexone. After missing just a few days of pill-taking, the blockade of opioid effects wears off, and risk of relapse is high, after which naltrexone is not easily resumed. New injectable (Comer et al. 2006, Sullivan et al. 2006b), or implantable (Hulse et al. 2005, Waal et al. 2006) formulations of naltrexone with durations of opioid blockade of a month or more may overcome this limitation and should be a focus of future investigation for treatment of opioid dependence.
Acknowledgments
This study was supported by NIH grants DA00433 and DA10746. Dr. Nunes has consulted for Cephalon/Alkermes, and Dr. Comer is a consultant for Grunenthal GmbH, Johnson & Johnson Pharmaceutical Research and Development, L.L.C., Shering-Plough Corporation, and Avigen Pharmaceuticals, Inc. Contributions by the staff of the Substance Treatment and Research Service (STARS) of Columbia University are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadi J, Ahmadi K, Ohaeri J. Controlled, randomized trial in maintenance treatment of intravenous buprenorphine dependence with naltrexone, methadone or buprenorphine: a novel study. Eur J Clin Invest. 2003;33(9):824–9. doi: 10.1046/j.1365-2362.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58(8):755–61. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O'Brien CP. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treatment. 1997;14:529–534. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. J Consult Clin Psychol. 2003;71(3):432–42. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- Gerra G, Fantoma A, Zaimovic A. Naltrexone and buprenorphine combination in the treatment of opioid dependence. J Psychopharmacol. 2006;20(6):806–14. doi: 10.1177/0269881106060835. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Tait RJ, Comer SD, Sullivan MA, Jacobs IG, Arnold-Reed D. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol Depend. 2005;79(3):351–7. doi: 10.1016/j.drugalcdep.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend. 1997;45(1-2):131–4. doi: 10.1016/s0376-8716(97)01348-3. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral nalrexone for opioid dependence: A ceiling on effectiveness. American Journal of Drug and Alcohol Abuse. 2006;32(4):503–17. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O'Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16:163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54(2):127–35. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Ritter AJ. Naltrexone in the treatment of heroin dependence: relationship with depression and risk of overdose. Aust N Z J Psychiatry. 2002;36(2):224–8. doi: 10.1046/j.1440-1614.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J Subst Abuse Treat. 2002;23(4):351–60. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse treatment for cocaine-dependent patients, Addict. Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Rothenberg JL, Vosburg SK, Church SH, Feldman SJ, Epstein EM, Kleber HD, Nunes EV. Predictors of retention in naltrexone maintenance for opioid dependence: analysis of a stage I trial. Am J Addictions. 2006a;15(2):150–9. doi: 10.1080/10550490500528464. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006b;189(1):37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Waal H, Frogopsahl G, Olsen L, Christophersen AS, Morland J. Naltrexone implants – duration, tolerability and clinical usefulness. A pilot study. Eur Addict Res. 2006;12(3):138–44. doi: 10.1159/000092115. [DOI] [PubMed] [Google Scholar]
- White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94(7):961–72. [PubMed] [Google Scholar]