Abstract
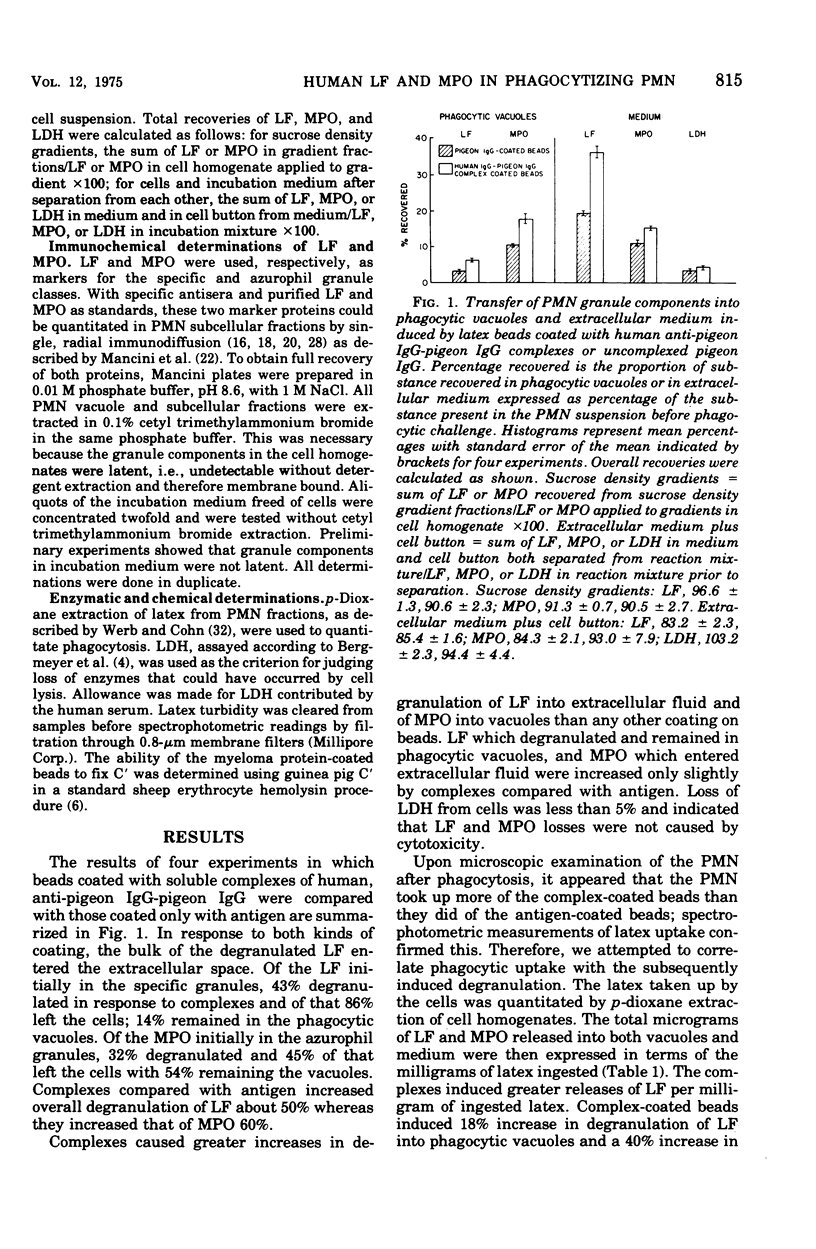
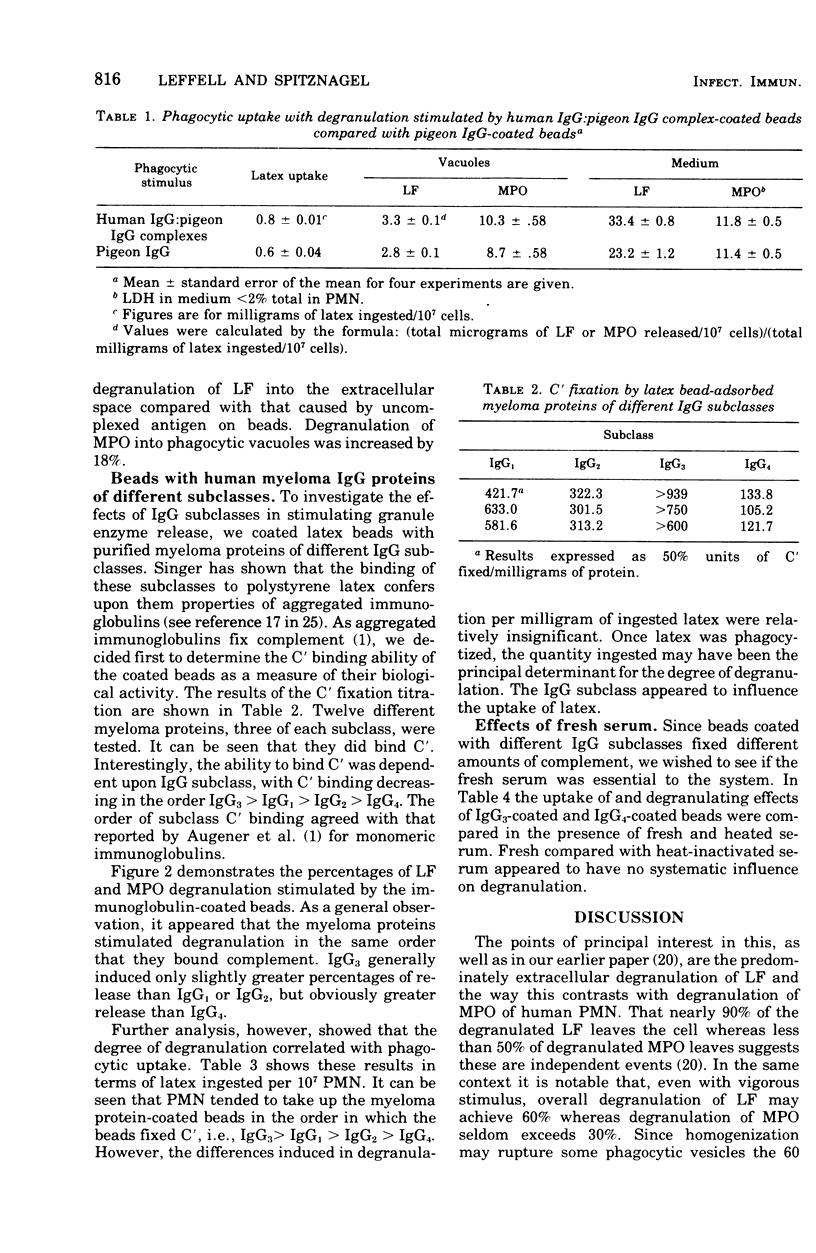
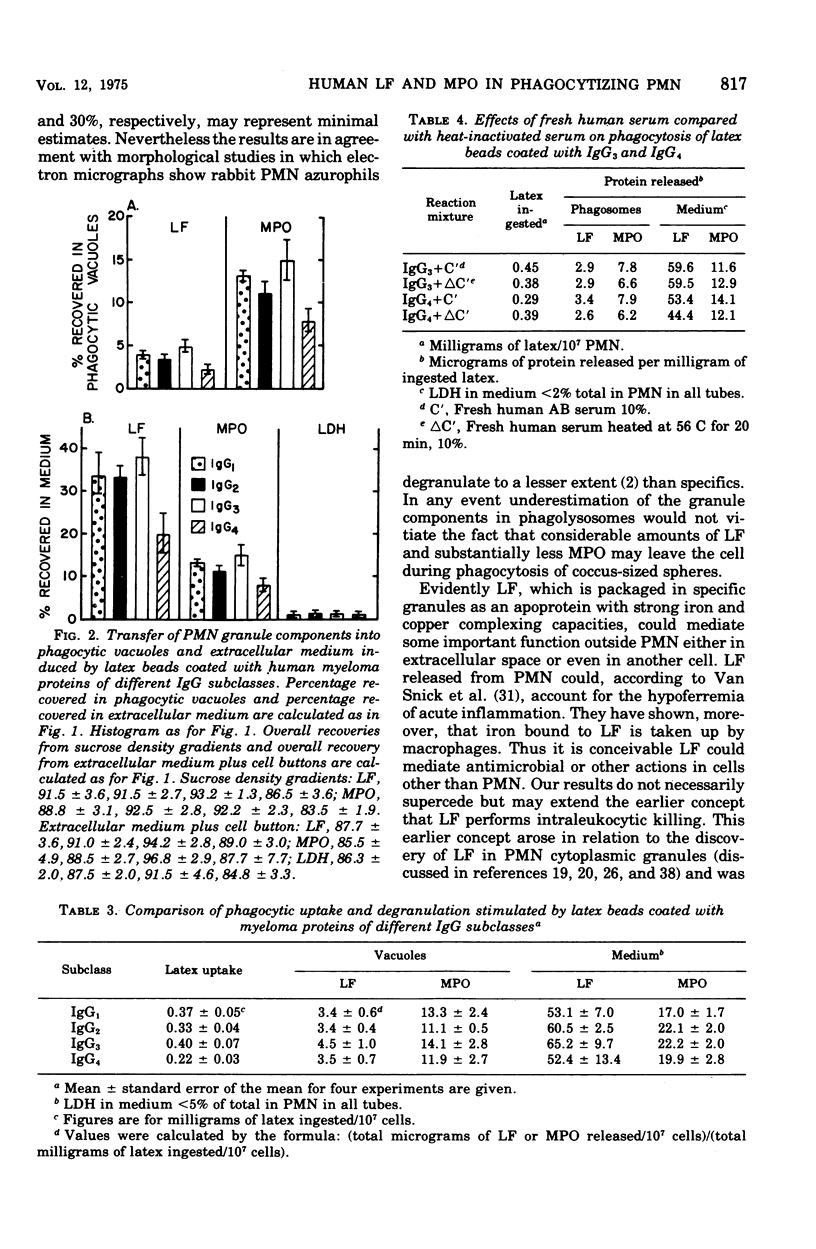
Human neutrophils (PMN) degranulated in response to soluble human immune complexes and to myeloma proteins, including subclasses of immunoglobulin G (IgG)1, IgG2, IgG3, and IgG4 coated on 1.09-mum latex beads. Immunochemical measurement of lactoferrin (LF) from specific granules and myeloperoxidas (MPO) from azurophil granules showed that both classes of granule degranulated. Beads with soluble complexes of human anti-pigeon IgG-normal pigeon IgG, prepared from serum of a patient with pigeon breeders disease, induced significantly greater degranulation than did pigeon IgG-coated beads. Up to 40% of LF in the PMN degranulated during phagocytic challenge and 86% of that entered the extracellular fluid. Twenty to 30% of the MPO degranulated, but less than 50% of that entered the extracellular fluid. The degranulated LF and MPO which remained in the PMN were recovered from phagocytic vacuoles. Beads coated with purified human myeloma proteins (12 different ones, three of each subclass) induced degranulation in the order IgG3 greater than IgG1 greater than IgG2 greater than IgG4; however, these differences were found to be a function of the amount of latex ingested. Thus, the amount of degranulation was dependent more on the opsonizing capacity of the immunoglobulins rather than on their intrinsic capacities for inducing degranulation. Degranulation of both LF and MPO in response to IgG subclasses followed patterns similar to those caused by soluble immune complexes, and IgG3 coated on beads caused degranulation equal to that caused by human complex-coated beads. Degranulation to IgG3 and IgG4 was uninfluenced by fresh compared with heat-inactivated human AB serum. This was true although IgG3 beads fixed greater than sixfold more complement than did IgG4 beads. Evidently human IgG subclasses enhance phagocytosis and degranulation of human PMN. The overwhelmingly extracellular degranulation of LF in response to various bead coating suggest that it subserves a major protion of it role outside PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augener W., Grey H. M., Cooper N. R., Müller-Eberhard H. J. The reaction of monomeric and aggregated immunoglobulins with C1. Immunochemistry. 1971 Nov;8(11):1011–1020. doi: 10.1016/0019-2791(71)90489-7. [DOI] [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Karnovsky M. L., Karnovsky M. J. Cytochemical demonstration of hydrogen peroxide in polymorphonuclear leukocyte phagosomes. J Cell Biol. 1975 Jan;64(1):254–260. doi: 10.1083/jcb.64.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., White J. G., Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974 Mar 22;248(446):347–348. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- Gladstone G. P. The effect of iron and haematin on the killing of staphylococci by rabbit polymorphonuclear leucocytes. Contrib Microbiol Immunol. 1973;1:222–243. [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Hawkins D., Peeters S. The response of polymorphonuclear leukocytes to immune complexes in vitro. Lab Invest. 1971 Jun;24(6):483–491. [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Kvarstein B. The effect of temperature, mabolic inhibitors, andEDTA on pgocytosis of polystyrene latex particles by human leucocytes. Scand J Clin Lab Invest. 1969 Oct;24(3):271–277. doi: 10.3109/00365516909080162. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Intracellular and extracellular degranulation of human polymorphonuclear azurophil and specific granules induced by immune complexes. Infect Immun. 1974 Dec;10(6):1241–1249. doi: 10.1128/iai.10.6.1241-1249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Disorders of phagocyte function. Curr Probl Pediatr. 1972 Sep;2(11):3–53. doi: 10.1016/s0045-9380(72)80004-5. [DOI] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Pruzanski W., Ranadive N. S. Release of intracellular constituents from rabbit polymorphonuclear leukocytes exposed to soluble and insoluble immune complexes. Int Arch Allergy Appl Immunol. 1972;43(2):182–195. doi: 10.1159/000230836. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Singer J. M. The binding of immune globulins and other proteins by polystyrene latex particles. J Reticuloendothel Soc. 1966 May;3(1):29–40. [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]



