Abstract
Background
Many blood biomarkers have a positive association with stroke outcome, but adding blood biomarkers to the National Institutes of Health Stroke Scale (NIHSS) did not significantly improve its discriminatory ability. We investigated the association of the CHA2DS2-VASc score with unfavourable functional outcome (defined as a 30-day modified Rankin Scale [mRS] ≥3) in patients presenting with acute ischemic stroke (AIS), and examined whether the addition of blood biomarkers (troponin I [TnI], fibrinogen, C-reactive protein [CRP]) affects the model discriminatory ability.
Methods
We conducted an observational single-centre study of consecutive patients with AIS. All patients were admitted to hospital within 24 hours from the neurological symptoms onset.
Results
Of 240 patients (mean age 70.0±8.9 years), unfavourable 30-day outcome occurred in 92 (38.3%). Patients with mRS≥3 were older and more likely to have atrial fibrillation or other comorbidities (all p<0.001). They had higher levels of CRP, fibrinogen, TnI and higher CHA2DS2-VASc and CHADS2 scores (all p<0.05). The adjusted CHA2DS2-VASc score had excellent predictive ability for poor stroke outcome (c-statistic 0.982;95%CI,0.964–1.000, p<0.001). Whilst CRP had the highest sensitivity (83.7%), cardiac TnI was the most specific (97.3%) for prediction of poor stroke outcome (cut-off: >0.09µg/L). Compared with each of these biomarkers, CHA2DS2-VASc score had significantly better predictive ability for poor stroke outcome (c-statistic for CRP, Fibrinogen and TnI was 0.853;95%CI,0.802–0.895, 0.848;95%CI,0.796–0.891, and 0.792;95%CI,0.736–0.842, all p<0.001, respectively, versus 0.932;95%CI,0.892–0.960, p<0.001 for the CHA2DS2-VASc, all p for the comparisons<0.01). There was no significant difference in the predictive ability of the CHA2DS2-VASc score vs. combinations of the CHA2DS2-VASc and TnI or TnI, fibrinogen and CRP (z statistic 0.369, p = 0.7119; integrated discrimination index 0.00801 and 0.00172, respectively, both p>0.05).
Conclusions
The CHA2DS2-VASc score alone reliably predicts 30-day unfavourable outcome of stroke. Adding blood biomarkers to the CHA2DS2-VASc score did not significantly increase the predictive ability of the model.
Introduction
The early prediction of death or disability following acute ischemic stroke (AIS) presently relies upon clinical variables such as age and stroke severity, as measured by the National Institutes of Health Stroke Scale (NIHSS) [1], [2]. These predictions are often broadly similar to the experienced stroke physicians clinical judgement [3], and continuous efforts are being made to improve the predictive ability of validated prognostic clinical variables by adding various biomarkers (whether blood, urine or imaging-based) to the original models based on clinical risk factors.
Many blood-based biomarkers have been extensively studied as potential predictors of poor stroke outcome. However, most of the associations were relatively weak and no single class of biomarkers had a stronger association than the others [4]. Nevertheless, the effect of cardiac biomarkers was consistent, and a number of studies found an increased mortality in stroke patients with elevated cardiac troponin I (TnI) [5]–[7] or troponin T (TnT) [8], [9]. Indeed, adding high-sensitivity TnT to several clinical variables including age and stroke severity resulted in incremental discrimination and reclassification of patients in one study [10], whilst another study showed that positive association of many biomarkers (including TnT) became statistically insignificant after adjustment for age and baseline NIHSS, and adding the N-Terminal pro-BNP or Interleukin-6 (the only statistically significant biomarkers after the adjustment) to age plus NIHSS made no significant difference to the model discriminatory ability [11].
A recent study showed that the CHADS2 and CHA2DS2-VASc scores, which were originally formulated for risk assessment of stroke in patients with atrial fibrillation (AF) [12], were good predictors of 5-year outcomes in non-AF patients with AIS [13]. The CHA2DS2-VASc score correlated well with stroke severity in AF patients [14], and was a multivariate predictor of 90-day stroke outcome, independently of baseline NIHSS values [15].
The aim of the present study was to investigate the association of the CHA2DS2-VASc score with poor short-term (30-day) functional outcome in patients with AIS, regardless of the heart rhythm, and to examine whether the addition of TnI affects the model discriminatory ability regarding the poor short-term outcome of AIS. We tested the hypothesis that the CHA2DS2-VASc score is significantly associated with poor short-term stroke outcome and that adding TnI improves the model predictive ability.
Materials and Methods
Patient selection and study design
An observational single-centre study of consecutive patients presenting with AIS who were admitted to hospital during 2010 was conducted in the University Clinical Centre Gracanica. All patients gave written informed consent, and the University Clinical Centre Gracanica review board approved the study.
All patients were admitted to hospital within 24 hours from the neurological symptoms onset. The diagnosis of AIS was established using the clinical evaluation and computed tomography (CT) of the brain within the first 24 hours of the event onset in all patients, and during hospitalization as needed. Patients with unclear timing of symptoms onset and those with haemorrhagic stroke or transient ischemic attack (TIA) were excluded (TIA was defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction) [16]. Patients with a history of prior myocardial infarction (MI) or coronary artery disease (CAD) documented by coronary angiography, as well as patients with symptoms and electrocardiographic (ECG) signs suggestive of transient myocardial ischemia or an acute coronary syndrome (ACS) were excluded from this study in an attempt to avoid the confounding interplay of elevated cardiac troponin, possible acute MI and stroke regarding the 30-day functional stroke outcome.
All patients underwent a detailed history and physical examination, blood and urine testing, 12-channel ECG recording, chest radiography and transthoracic echocardiography (TTE). A 24-hour ECG Holter monitoring was performed in all patients within the first three days of hospitalization. Cardiac diseases and non-cardiac disorders were noted in the presence of a detailed medical record or a self-reported history of the disease, or when standard diagnostic criteria were fulfilled at diagnostic evaluation. Patients with advanced mitral or aortic valve disease awaiting surgical valve repair were excluded from the study.
Blood biomarkers
Blood samples were taken upon admission to hospital and processed within 1 hour. In addition to routine biochemistry, C-reactive protein (CRP), cardiac TnI and D-dimer were measured in each patient. CRP was measured by latex-enhanced nephelometry with the use of high-sensitivity assays on the Behring Nephelometer II analyzer (Dade-Behring Diagnostics, Deerfield, IL) with a lower detection limit of 0.2 mg/L and interassay CVs of 5–9%. TnITnI and D-dimer were measured by ELFA (Enzyme Linked Fluorescent Assay) method (VIDAS TNI ULTRA and VIDAS D-Dimer Exclusion Assay, by BioMerieux Clinical Diagnostics), with a lower detection limit of 0.01µg/L and measurement range of 0.01–30µg/L (10% CV [Coefficient of Variation] point 0.11µg/L) for TnI, and a lower detection limit of ≤0.045µg/mL and measurement range of 0.045–10µg/mL (the clinical cut-off 0.5µg/mL) for D-dimer. In all patients, a creatinine clearance test was done on a 24-hour urine sample.
Stroke and bleeding risk scores
All stroke and bleeding scores were calculated on admission to hospital, according to the clinical status prior to acute stroke onset. The CHADS2 score was calculated by giving 1 point each for congestive heart failure, hypertension, age >75 years and diabetes, and 2 points for prior stroke or TIA, the CHA2DS2-VASc by giving 1 point each for congestive heart failure/left ventricular systolic dysfunction (left ventricular ejection fraction ≤40%), hypertension, diabetes, peripheral vascular disease (including prior MI or complex aortic plaque), age 65–74 years and female gender, and 2 points for prior stroke or TIA and for age ≥75 years, and the HAS-BLED by giving 1 point each for hypertension, abnormal renal function, abnormal liver function, prior stroke, labile INRs (International Normalized Ratio), age >65 years, concomitant drug (aspirin or non-steroidal anti-inflammatory drugs) or alcohol use [17].
Combined scores
Combined scores were formulated using the CHA2DS2-VASc score and TnI or CRP or fibrinogen or all three biomarkers, thus resulting in the CHA2DS2-VASc-T, CHA2DS2-VASc-CRP, CHA2DS2-VASc-F and CHA2DS2-VASc-CRPFT score, respectively.
Short-term stroke outcomes
In-hospital mortality was defined as death from any cause during the index hospitalization. A 30-day mRS was calculated as a measure of functional neurological outcome of AIS; the scale is graded from 0 to 6, with 0 for patients without any symptom, 3–5 for increasing disability and 6 for death, and a mRS of ≥3 signifies an unfavourable stroke outcome [18].
None of the patients received thrombolytic therapy, since it was not available in our centre during the study period. Nevertheless, symptomatic haemorrhagic transformation (sHT) of AIS, defined as a rapid significant clinical deterioration temporally related to HT documented by CT-scan or autopsy, was regarded as a serious adverse event in the evolution of AIS [19], [20].
Statistical analyses
Following a test of statistical normality, continuous variables are presented as mean (±SD), or with a skewed distribution as median with interquartile range (IQR, 25th to 75th quartile). Categorical variables are reported as counts with percentages. The Student t-test was used for comparison of continuous variables with normal distribution, and Mann-Whitney test for continuous variables with skewed distribution (Table 1). Differences in categorical variables were tested by Chi-square test. Since blood biomarkers' levels had skewed distribution, a logarithm transformation was used, and all logistic regression analyses were performed (and reported) in two ways: first, a biomarker was entered as a log transformed continuous independent variable and then, the analysis was performed with biomarker levels grouped to quartiles (the latter was done to facilitate a meaningful clinical interpretation of the results), Table 2 and 3. The CHADS2, CHA2DS2-VASc and HAS-BLED scores and all combined scores were entered as continuous variables in all models. Following unadjusted analyses (Table 4), the adjustment was done for each analysis as detailed in the Table 5 legend. Unfavourable 30-day functional outcome of AIS, including in-hospital death (i.e., a mRS of ≥3) was the dependent variable in all analyses.
Table 1. Baseline characteristic of patients with acute ischemic stroke according to favourable (mRS<3) or unfavourable 30-day functional stroke outcome (mRS≥3), including in-hospital death.
| A. Baseline parameters | All patients | mRS<3 | mRS≥3 | P |
| N = 240 | 148 (61.7) | 92 (38.3) | ||
| Age (years) | 70.0±8.9 | 67.2±8.1 | 74.5±8.4 | <0.001 |
| Males | 139 (57.9) | 77 (52.0) | 62 (67.4) | 0.022 |
| Body mass index | 29.2±2.3 | 29.2±2.2 | 29.1±2.4 | 0.737 |
| Systolic blood pressure (mmHg) | 150 (140–180) | 150 (140–170) | 150 (115–190) | 0.606 |
| Diastolic blood pressure (mmHg) | 90 (80–105) | 90 (80–105) | 90 (80–110) | 0.151 |
| Heart rate (beats per minute) | 80 (75–90) | 80 (75–90) | 90 (75–95) | 0.791 |
| Current smoker | 134 (55.8) | 83 (56.1) | 51 (55.4) | 1.000 |
| Excessive alcohol consumption | 36 (15.0) | 29 (19.6) | 7 (7.6) | 0.015 |
| NYHA class (mean) | 2.2±0.4 | 2.1±0.3 | 2.3±0.6 | <0.001 |
| NYHA class I-II | 202 (84.2) | 136 (91.9) | 66 (71.8) | <0.001 |
| NYHA class III-IV | 38 (20.2) | 12 (8.1) | 28 (28.2) | <0.001 |
| Comorbidities: | ||||
| Atrial fibrillation | 88 (36.7) | 35 (23.6) | 53 (57.6) | <0.001 |
| Hypertension | 178 (74.2) | 107 (72.3) | 71 (77.2) | 0.450 |
| Diabetes mellitus | 72 (30.0) | 28 (18.9) | 44 (47.8) | <0.001 |
| Heart failure | 18 (7.5) | 3 (2.0) | 15 (16.3) | <0.001 |
| Chronic kidney disease | 11 (4.6) | 6 (4.1) | 5 (5.4) | 0.753 |
| Prior stroke (any)/TIA | 24 (10.0) | 1 (0.7) | 23 (25.0) | <0.001 |
| Stroke and bleeding risk assessment scores* | ||||
| CHADS2 (mean) | 1.86±1.1 | 1.30±0.64 | 2.77±1.07 | <0.001 |
| median (IQR) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 3.0 (2.0–3.8) | |
| CHA2DS2-VASc (mean) | 3.64±1.66 | 2.70±1.10 | 5.14±1.25 | <0.001 |
| median (IQR) | 3.5 (3.0–5.0) | 3.0 (2.0–3.0) | 5.0 (4.0–6.0) | |
| HAS-BLED (mean) | 2.45±1.07 | 2.08±0.90 | 3.04±1.06 | <0.001 |
| median (IQR) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 3.0 (2.0–4.0) | |
| Echocardiographic parameters: | ||||
| Left atrial diameter (cm) | 4.28±0.64 | 4.08±0.53 | 4.61±0.67 | <0.001 |
| Left atrial volume index | 42.9±10.9 | 39.3±8.8 | 48.7±11.4 | <0.001 |
| emsp;Left ventricular ejection fraction | 56.3±8.9 | 58.7±5.6 | 52.4±11.5 | <0.001 |
| Left ventricular end-systolic diameter (cm) | 3.75±0.42 | 3.65±0.31 | 3.91±0.51 | <0.001 |
| Mitral annulus calcification | 62 (25.8) | 5 (3.4) | 57 (62.0) | <0.001 |
Values are presented as n (%) or mean ± standard deviation or median with interquartile range (IQR).
mRS, modified Rankin Scale (patients who died were assigned a mRS of 6); NYHA, New York Heart Association; TIA, transient ischemic attack; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA; CHA2DS2-VASc, congestive heart failure or left ventricular ejection fraction ≤40%, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA, vascular disease (including myocardial infarction and peripheral artery disease), age >65 years, female gender; HAS-BLED, hypertension, abnormal renal or liver function, prior stroke, labile INRs (International Normalized Ratio), elderly (>65 years), concomitant drugs or alcohol use; MAC, mitral annulus calcification; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 2. Baseline blood biomarkers levels in patients with acute ischemic stroke according to favourable (mRS<3) or unfavourable 30-day functional stroke outcome, including in-hospital death (mRS≥3).
| Blood biomarkers | All patients | mRS<3 | mRS≥3 | P |
| N = 240 | 148 (61.7) | 92 (38.3) | ||
| C-reactive protein, mean | 16.70±17.89 | 7.96±10.75 | 30.77±18.14 | <0.001 |
| C-reactive protein, median (IQR) | 4.0 (3.0–32.5) | 4.0 (3.0–4.0) | 35.0 (16.0–44.0) | <0.001 |
| Quartile 1: <3.0 | 18 (7.5) | 16 (10.8) | 2 (2.2) | |
| Quartile 2: 3.0–3.9 | 49 (20.4) | 44 (29.7) | 5 (5.4) | |
| Quartile 3: 4.0–32.4 | 113 (47.1) | 78 (52.7) | 35 (38.0) | |
| Quartile 4: ≥32.5 | 60 (25.0) | 10 (6.8) | 50 (54.3) | |
| Fibrinogen, mean | 4.15±2.12 | 3.25±1.17 | 5.61±2.47 | <0.001 |
| Fibrinogen, median (IQR) | 3.50 (2.90–5.00) | 3.15 (2.50–3.60) | 5.40 (3.90–6.80) | <0.001 |
| Quartile 1: <2.9 | 57 (23.8) | 51 (34.5) | 6 (6.5) | |
| Quartile 2: 2.9–3.4 | 51 (21.2) | 44 (29.7) | 7 (7.6) | |
| Quartile 3: 3.5–4.9 | 68 (28.3) | 44 (29.7) | 24 (26.1) | |
| Quartile 4: ≥5.0 | 64 (26.7) | 9 (6.1) | 55 (59.8) | |
| Cardiac TnI, mean | 0.217±0.453 | 0.06±0.10 | 0.468±0.649 | <0.001 |
| Cardiac TnI, median (IQR) | 0.06 (0.03–0.09) | 0.04 (0.02–0.07) | 0.20 (0.05–0.65) | <0.001 |
| Quartile 1: <0.03 | 47 (19.6) | 42 (28.4) | 5 (5.4) | |
| Quartile 2: 0.03–0.059 | 70 (29.2) | 51 (34.5) | 19 (20.7) | |
| Quartile 3: 0.06–0.089 | 49 (20.4) | 36 (24.3) | 13 (14.1) | |
| Quartile 4: ≥0.09 | 74 (30.8) | 19 (12.8) | 55 (59.8) | |
| D-dimer, mean | 0.61±0.85 | 0.45±0.68 | 0.87±1.02 | <0.001 |
| D-dimer, median (IQR) | 0.24 (0.18–0.80) | 0.22 (0. 17–0.32) | 0.50 (0.19–1.24) | <0.001 |
| Quartile 1: <0.180 | 56 (23.3) | 41 (27.7) | 15 (16.3) | |
| Quartile 2: 0.180–0.234 | 64 (26.7) | 41 (27.7) | 23 (25.0) | |
| Quartile 3: 0.235–0.799 | 58 (24.2) | 44 (29.7) | 14 (15.2) | |
| Quartile 4: ≥0.800 | 62 (25.8) | 22 (14.9) | 40 (43.5) | |
| WBC, mean | 8.21±1.68 | 7.99±1.76 | 8.51±1.47 | 0.008 |
| WBC, median (IQR) | 8.1 (7.0–9.0) | 7.8 (6.9–9.0) | 8.80 (7.70–9.68) | 0.072 |
| Quartile 1: <7.0 | 50 (20.8) | 38 (25.7) | 12 (13.0) | |
| Quartile 2: 7.0–8.0 | 69 (28.7) | 43 (29.1) | 26 (28.3) | |
| Quartile 3: 8.1–8.9 | 38 (15.8) | 23 (15.5) | 15 (16.3) | |
| Quartile 4: ≥9.0 | 83 (34.6) | 44 (29.7) | 39 (47.0) | |
| CrCl, mean | 55.35±7.60 | 56.36±7.35 | 53.73±7.77 | 0.009 |
| CrCl, median (IQR) | 56.00 (51.00–60.00) | 56.00 (53.25–60.00) | 55.00 (48.25–59.00) | 0.020 |
| Quartile 1: <51 | 58 (24.2) | 27 (18.2) | 31 (33.7) | |
| Quartile 2: 51–55 | 61 (25.4) | 36 (24.3) | 25 (27.2) | |
| Quartile 3: 56–59 | 52 (21.7) | 38 (25.7) | 14 (15.2) | |
| Quartile 4: ≥60 | 69 (28.7) | 47 (31.8) | 22 (23.9) | |
| Total cholesterol, mean | 5.71±1.17 | 5.57±1.09 | 5.91±1.26 | 0.025 |
| Total cholesterol, median (IQR) | 5.35 (5.00–6.50) | 5.20 (5.00–6.00) | 6.00 (5.00–6.80) | |
| HDL, mean | 1.04±0.46 | 1.02±0.31 | 1.07±0.63 | 0.416 |
| HDL, median (IQR) | 1.00 (0.90–1.00) | 1.00 (0.93–1.05) | 1.00 (0.90–1.00) | |
| LDL, mean | 3.95±1.17 | 3.83±1.06 | 4.14±1.31 | 0.043 |
| LDL, median (IQR) | 3.80 (3.20–4.70) | 3.60 (3.20–4.36) | 4.40 (3.30–4.98) | |
| Hematocrit, mean | 0.41±0.07 | 0.42±0.06 | 0.40±0.07 | 0.040 |
| Haematocrit, median (IQR) | 0.41 (0.38–0.45) | 0.42 (0.39–0.45) | 0.40 (0.34–0.46) | |
| Haemoglobin, mean | 134.13±18.71 | 135.03±19.63 | 132.68±17.13 | 0.084 |
| Haemoglobin, median (IQR) | 135 (127–145.75) | 136.50 (129–146) | 134 (125.50–145) |
Values are presented as n (%) or mean ± standard deviation or median with interquartile range (IQR). C-reactive protein is given in mg/L, fibrinogen in g/L, TnI in μg/L and D-dimer in μg/mL.
mRS, modified Rankin Scale (patients who died were assigned a mRS of 6); WBC, white blood cell count; CrCl, creatinine clearance; HDL, high density lipoprotein; LDL, low density lipoprotein.
Table 3. Crude associations of biomarkers and the CHADS2, CHA2DS2-VASc and HAS-BLED score with unfavourable 30-day outcome of ischemic stroke, predictive ability of each biomarker or score, and pairwise comparisons of each biomarker and stroke risk score predictive ability.
| Predictive ability | Pairwise comparison | ||||||||
| OR | 95%CI | P | c-statistic | 95%CI | P | z-statistic | P | ||
| Biomarker | |||||||||
| 1 | CRP | 3.9 | 2.9–5.3 | <0.001 | 0.853 | 0.802–0.895 | <0.001 | 0.335 (1 vs. 2) | 0.7390 |
| quartiles | 5.8 | 3.5–9.5 | <0.001 | 0.795 | 0.738–0.844 | <0.001 | 1.678 (1 vs. 3) | 0.0934 | |
| 4.616 (1 vs. 4) | <0.001 | ||||||||
| 5.641 (1 vs. 5) | <0.001 | ||||||||
| 6.081 (1 vs. 6) | <0.001 | ||||||||
| 2 | Fibrinogen | 61.7 | 21.1–180.7 | <0.001 | 0.848 | 0.796–0.891 | <0.001 | 1.536 (2 vs. 3) | 0.1246 |
| quartiles | 4.2 | 1.9–6.0) | <0.001 | 0.835 | 0.781–0.879 | <0.001 | 4.312 (2 vs. 4) | <0.001 | |
| 5.530 (2 vs. 5) | <0.001 | ||||||||
| 5.549 (2 vs. 6) | <0.001 | ||||||||
| 3 | TnI | 3.2 | 2.3–4.4 | <0.001 | 0.792 | 0.736–0.842 | <0.001 | 3.249 (3 vs. 4) | 0.0012 |
| quartiles | 2.7 | 2.0–3.7 | <0.001 | 0.767 | 0.705–0.830 | <0.001 | 3.441 (3 vs. 5) | 0.0006 | |
| 3.847 (3 vs. 6) | 0.0001 | ||||||||
| 4 | D-dimer | 1.9 | 1.4–2.5 | <0.001 | 0.642 | 0.578–0.703 | <0.001 | 0.371 (4 vs. 5) | 0.7109 |
| quartiles | 1.6 | 1.2–2.0 | <0.001 | 0.636 | 0.572–0.697 | <0.001 | 0.626 (4 vs. 6) | 0.5313 | |
| 5 | WBC | 1.2 | 1.1–1.5 | 0.010 | 0.622 | 0.558–0.684 | 0.001 | 0.236 (5 vs. 6) | 0.8134 |
| quartiles | 1.3 | 1.1–1.7 | <0.001 | 0.593 | 0.528–0.656 | 0.009 | |||
| 6 | CrCl | 0.09 | 0.04–0.59 | 0.012 | 0.610 | 0.545–0.672 | 0.004 | ||
| quartiles | 0.7 | 0.5–0.7 | 0.007 | 0.601 | 0.536–0.664 | 0.006 | |||
| Stroke risk score | |||||||||
| 7 | CHA2DS2-VASc | 7.9 | 4.6–13.4 | <0.001 | 0.932 | 0.892–0.960 | <0.001 | 4.265 (7 vs. 8) | <0.001 |
| 8 | CHADS2 | 10.8 | 5.6–21.0 | <0.001 | 0.872 | 0.823–0.912 | <0.001 | 3.834 (8 vs. 9) | <0.001 |
| 9 | HAS-BLED | 2.8 | 2.0–3.8 | <0.001 | 0.750 | 0.690–0.804 | <0.001 | 5.674 (7 vs. 9) | <0.001 |
| z-statistic | P | ||||||||
| CHA2DS2- | CHA2DS2-VASc vs. CRP | 2.963 | 0.0030 | ||||||
| VASc score vs. biomarkers | CHA2DS2-VASc vs. Fibrinogen | 2.961 | 0.0031 | ||||||
| CHA2DS2-VASc vs. TnI | 3.977 | 0.0001 | |||||||
OR, Odds Ratio; CI, Confidence Interval.
Only biomarkers with p<0.01 regarding unfavourable 30-day functional stroke outcome are shown.
CRP, C-reactive protein; WBC, white blood cell count; CrCl, creatinine clearance; CHA2DS2-VASc, congestive heart failure or left ventricular ejection fraction ≤40%, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA, vascular disease (including myocardial infarction and peripheral artery disease), age >65 years, female gender (1 point each, age ≥75 years and prior stroke/TIA 2 points each); CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA (1 point each, prior stroke/TIA 2 points); HAS-BLED, hypertension, abnormal renal or liver function, prior stroke, labile INRs (International Normalized Ratio), elderly (>65 years), concomitant drugs or alcohol use.
Table 4. Crude associations of the CHA2DS2-VASc score and combinations of the score plus C-reactive protein, fibrinogen or TnI, or the CHA2DS2-VASc score plus all three biomarkers with unfavourable 30-day functional outcome of ischemic stroke and pairwise comparisons of the scores.
| Score | OR | 95%CI | P | c-statistic | 95%CI | P | z-statistic | P | |
| 1 | CHA2DS2-VASc | 7.9 | 4.6–13.4 | <0.001 | 0.932 | 0.892–0.960 | <0.001 | 2.268 (2 vs. 1) | 0.0233 |
| 2 | CHA2DS2-VASc-CRP | 5.9 | 3.8–9.3 | <0.001 | 0.948 | 0.911–0.972 | <0.001 | 2.020 (3 vs. 1) | 0.0434 |
| 3 | CHA2DS2-VASc-F | 6.0 | 3.8–9.5 | <0.001 | 0.946 | 0.909–0.971 | <0.001 | 3.274 (4 vs. 1) | 0.0011 |
| 4 | CHA2DS2-VASc-T | 10.0 | 5.3–18.9 | <0.001 | 0.955 | 0.921–0.978 | <0.001 | 1.947 (5 vs. 1) | 0.0515 |
| 5 | CHA2DS2-VASc-CRFT | 3.6 | 2.6–4.8 | <0.001 | 0.953 | 0.917–0.976 | <0.001 | 1.514 (3 vs. 2) | 0.1299 |
| 1.242 (4 vs. 2) | 0.2144 | ||||||||
| 0.995 (5 vs. 2) | 0.3195 | ||||||||
| 1.433 (4 vs. 3) | 0.1517 | ||||||||
| 1.267 (5 vs. 3) | 0.2053 | ||||||||
| 0.427 (5 vs. 4) | 0.6692 |
CHA2DS2-VASc, congestive heart failure or left ventricular ejection fraction ≤40%, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA, vascular disease (including myocardial infarction and peripheral artery disease), age >65 years, female gender (1 point each, age ≥75 years and prior stroke/TIA 2 points each); CHA2DS2-VASc-CRP, 1 additional point if C-reactive protein was >4 mg/L; CHA2DS2-VASc-F, 1 additional point if fibrinogen was >3.7 g/L; CHA2DS2-VASc-T, 1 additional point if TnI was >0.09µg/L; CHA2DS2-VASc-CRPFT, 1 additional point for each of three biomarkers if above the correspondent cut-off level.
Table 5. The adjusted associations of biomarkers, ‘classic’ scores and ‘composed’ scores with unfavourable 30-day functional outcome of ischemic stroke, with model discriminatory ability and goodness of model fit, and pairwise comparison of each model.
| Adjusted analysis | Model discriminatory ability | Model fit | Pairwise comparisons of the ROC curves | ||||||
| Variable | OR (95% CI) | P | c-statistic (95% CI) | P | Hosmer-Lemeshow test (HL) | z-statistic | P | IDI | |
| Nagelkerke R square (NR2) | P | ||||||||
| 0.00801 | |||||||||
| 1 | TnI (quartiles) | 1.8 (1.1–3.2) | 0.036 | 0.957 (0.931–0.983) | <0.001 | HL 0.036; NR2 0.786 | 2.576 (3 vs. 1) | 0.0100 | (3 vs. 4) |
| 2 | Fibrinogen (quartiles) | 2.2 (1.8–4.1) | 0.013 | 0.957 (0.931–0.983) | <0.001 | HL 0.036; NR2 0.786 | 2.576 (3 vs. 2) | 0.0100 | P = 0.15460 |
| 3 | CHA2DS2-VASc | 14.1 (3.4–59.5) | <0.001 | 0.982 (0.964–1.000) | <0.001 | HL <0.001; NR2 0.870 | 0.369 (4,5 vs. 3) | 0.7119 | 0.00172 |
| 4 | CHA2DS2-VASc-T | 22.5 (3.1–162.9) | 0.002 | 0.983 (0.963–1.000) | <0.001 | HL <0.001; NR2 0.877 | 2.588 (4 vs. 1,2) | 0.0097 | (3 vs. 5) |
| 5 | CHA2DS2-VASc-CRPFT | 7.7 (1.8–32.7) | 0.006 | 0.983 (0.963–1.000) | <0.001 | HL <0.001; NR2 0.877 | 2.588 (5 vs. 1,2) | 0.0097 | P = 0.61428 |
OR, Odds Ratio; CI, Confidence Interval; ROC, Receiver Operating Characteristic; IDI – integrated discrimination index.
Only biomarkers or scores with significant association with unfavourable 30-day functional stroke outcome following adjustment are shown.
Cardiac TnI and fibrinogen adjusted for other biomarkers (white blood cell count, haemoglobin, haematocrit, C-reactive protein, D-dimer, creatinine clearance, total cholesterol, high-density lipoprotein and low-density lipoprotein), age, gender, body mass index, smoking status, alcohol consumption, NYHA class, atrial fibrillation, history of hypertension, heart failure, diabetes mellitus, chronic kidney disease, prior stroke/TIA, left atrial volume, left ventricular ejection fraction, the presence of mitral annular calcification and symptomatic haemorrhagic transformation of ischemic stroke.
The CHA2DS2-VASc score adjusted for CHADS2 and HAS-BLED score, all biomarkers, gender, body mass index, smoking status, alcohol consumption, NYHA class, atrial fibrillation, chronic kidney disease, left atrial volume, left ventricular ejection fraction, the presence of mitral annular calcification and symptomatic haemorrhagic transformation of ischemic stroke.
The CHA2DS2-VASc-T and CHA2DS2-VASc-CRPFT scores adjusted for all blood biomarkers, gender, body mass index, smoking status, alcohol consumption, NYHA class, atrial fibrillation, chronic kidney disease, left atrial volume, left ventricular ejection fraction, the presence of mitral annulus calcification and symptomatic haemorrhagic transformation of ischemic stroke.
The c-statistic, a measure of the area under the receiver-operator characteristic (ROC) curve, was used to quantify the predictive validity of each biomarker, the CHADS2, CHA2DS2-VASc and HAS-BLED and the combined scores, and tested the hypothesis that these models performed significantly better than chance (indicated by a c-statistic ≥0.5). In addition to model discriminatory ability (the ROC curve analysis), model calibration of each adjusted model was tested by the Hosmer-Lemeshow goodness-of-fit test, which assesses whether the observed event rates match expected event rates in subgroups of the model population. Explanatory power was tested using the pseudo- R 2 statistic according to the “Nagelkerke R2 ” to assess the degree to which the model explained the variance of the binary outcome. Pairwise comparisons of the ROC curves were performed using the approach of DeLong, DeLong and Clarke-Pearson [21] and the MedCalc statistical software – version 12.7.0.0. Finally, using the Stata 11 software, we calculated the integrated discrimination index (IDI), which is equivalent to the difference in discrimination slopes [22]. All other statistical analyses were performed using SPSS 20.0 software package (SPSS Inc., Chicago, Illinois). A value of P <0.05 was considered statistically significant in all analyses.
Results
Of 273 patients with AIS, 33 were excluded from this study because of the history of CAD or an acute MI during the hospitalization for AIS. These patients were not significantly older as compared to those without clinically evident CAD (73.2±10.1 vs. 70.0±8.9 years, respectively, p = 0.064), but they had higher CHADS2 and CHA2DS2-VASc scores (2.82±1.21 vs. 1.86±1.10 and 4.91±1.65 vs. 3.64±1.66, respectively, both p<0.001) and mean NYHA class (2.6±0.8 vs. 2.2±0.4, p<0.001), lower CrCl (50.9±9.3 vs. 55.4±7.6, p = 0.002) and higher CRP levels (27.3±16.6±16.7±17.9, p = 0.001), whilst there was no significant difference in the mean TnI levels at baseline (0.32±0.35 vs. 0.22±0.45, p = 0.266) or the prevalence of AF (18 [54.5%] vs. 88 [36.7%], p = 0.057). An unfavourable 30-day stroke outcome occurred more frequently in the CAD patients (28 [84.8] vs. 92 [38.3]%, p<0.001).
Of 240 patients included in this study (Table 1), unfavourable 30-day functional stroke outcome occurred in 92 patients (38.3%), including in-hospital death in 21 patients (8.8%). Patients with mRS≥3 were older, more often males and more likely to have AF, diabetes mellitus, HF, prior stroke/TIA, mitral annulus calcification, larger left atrial size or lower left ventricular ejection fraction (all p<0.001). They were more often prescribed diuretics and digoxin, and less frequently received angiotensin-converting enzyme inhibitors (all p<0.05).
Blood biomarkers
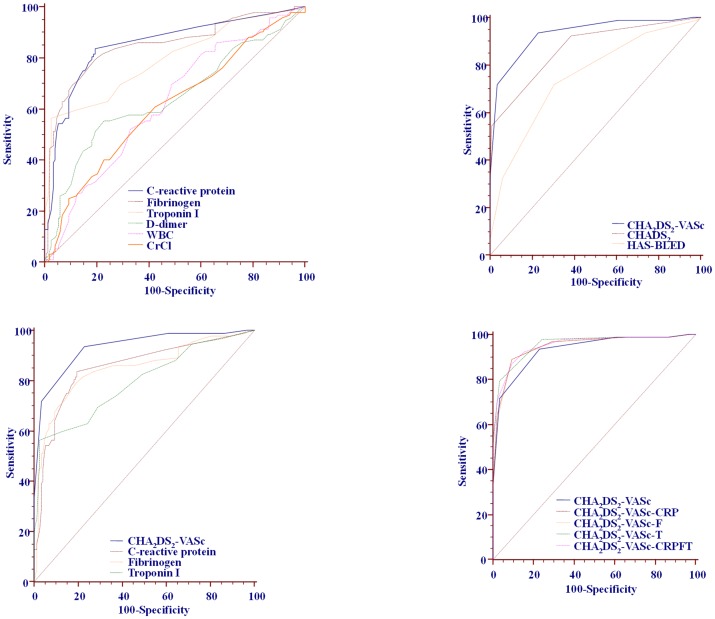
Patients with 30-day mRS≥3 had higher levels of CRP, fibrinogen, TnI, D-dimer, total cholesterol and LDL, higher white blood cell count, and lower hematocrit and CrCl (all p<0.05, Table 2]. Significant associations of blood biomarkers with unfavourable 30-day stroke outcome, predictive ability of each biomarker and pairwise comparisons of biomarker predictive abilities are shown in Table 3 and Figure 1 (upper left panel). CRP, fibrinogen and TnI had significantly better predictive ability compared with D-dimer, CrCl or total cholesterol (all p<0.001), whilst there were no significant differences among the first three biomarkers (Table 3 and Figure 1).
Figure 1. Receiver-operator characteristic analyses of the biomarkers and stroke risk scores predictive ability for unfavourable 30-day outcome of ischemic stroke and pairwise comparisons (Table 2).
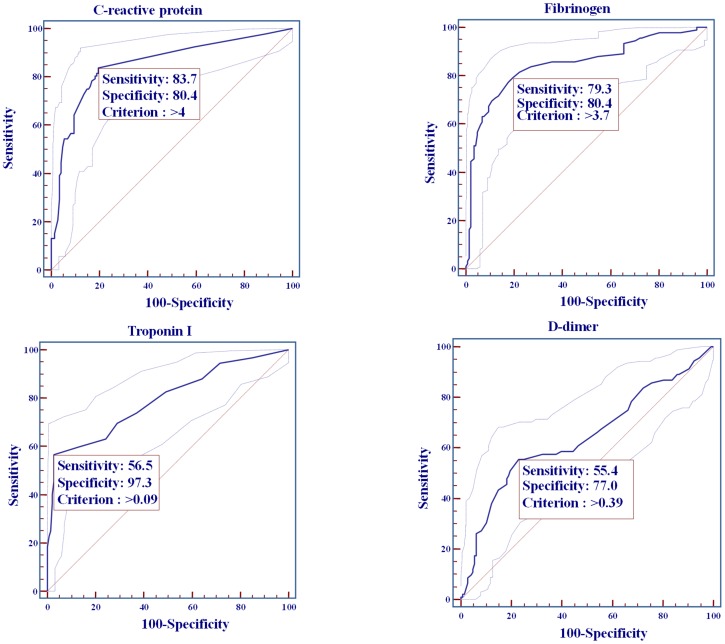
Sensitivity, specificity and cut-off values for cardiac CRP, fibrinogen, TnI and D-dimer are shown in Figure 2. Whilst CRP had the highest sensitivity (83.7%), cardiac TnI was the most specific (97.3%) for prediction of unfavourable short-term stroke outcome, with cut-off value of >0.09µg/L (Figure 2).
Figure 2. C-reactive protein, fibrinogen, TnI and D-dimer sensitivity, specificity and associated criterion for unfavourable 30-day outcome of ischemic stroke.
CHA2DS2-VASc, CHADS2 and HAS-BLED scores
Patients with unfavourable short-term functional stroke outcome had higher values of all three scores, compared with patients with mRS<3. The crude association of CHA2DS2-VASc, CHADS2 and HAS-BLED scores with unfavourable 30-day outcome, predictive ability of each score and pairwise comparison of the scores predictive abilities are shown in Table 3 and Figure 1 (upper right panel). The CHA2DS2-VASc score had significantly better predictive ability for unfavourable short-term stroke outcome than the CHADS2 or HAS-BLED score (both p<0.001) [Table 3 and Figure 1]. Within the CHA2DS2-VASc score, age, diabetes mellitus and prior stroke or TIA were significantly associated with unfavourable 30-day outcome (Odds Ratio [OR] 1.2; 95% Confidence Interval [CI] 1.1–1.2, OR 6.3; 95%CI 2.8–14.0 and OR 47.7; 95%CI 5.2–439.0, respectively, all p<0.001).
The CHA2DS2-VASc score had better sensitivity and specificity than the CHADS2 or HAS-BLED score (93.5% vs. 92.4% vs. 71.7% and 77.0% vs. 61.5% vs. 69.6%, respectively, all p<0.05) for unfavourable short-term functional outcome of AIS.
CHA2DS2VASc-score versus CRP, fibrinogen or cardiac TnI
Pairwise comparisons of predictive abilities of the CHA2DS2-VASc score versus CRP, fibrinogen or TnI are shown in Table 3 and Figure 1 (lower left panel). Compared with each of these biomarkers, CHA2DS2-VASc score had significantly better predictive ability for unfavourable 30-day functional stroke outcome (all p<0.01).
Combined scores (CHA2DS2VASc plus CRP or fibrinogen or TnI and CHA2DS2VASc plus all three biomarkers)
As above, the cut-off values for increased risk of unfavourable stroke outcome in our cohort were determined for each of three biomarkers, and 1 point was added to the CHA2DS2-VASc score if the level of a given biomarker in a given patient exceeded the cut-off value.
On the unadjusted analyses, all combined scores (Table 4) were significantly associated with unfavourable 30-day functional stroke outcome (all p<0.001), and the combination of CHA2DS2-VASc with TnI had the best predictive ability (c-statistic 0.955; 95%CI,0.921–0.978, p<0.001). All unadjusted combined scores were slightly better than unadjusted CHA2DS2-VASc alone (all p≤0.05). The combination of CHA2DS2-VASc with all three biomarkers (CHA2DS2-VASc-CRPFT) had similar predictive ability as each of the combinations of CHA2DS2-VASc score with a single biomarker (all p≥0.05) [Table 4 and Figure 1 (lower right panel)].
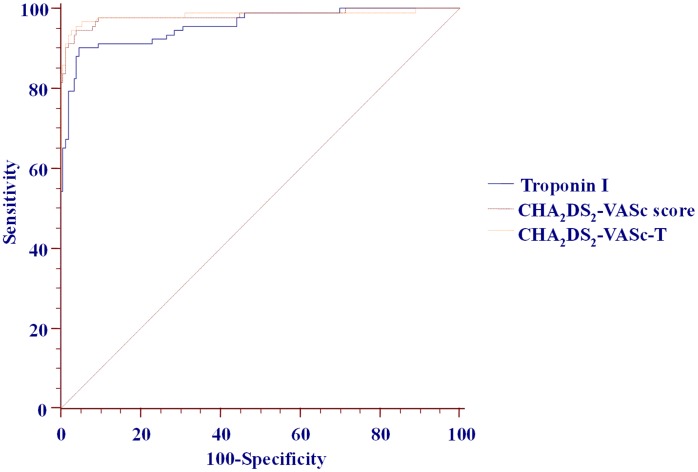
Following adjustment (see Table 5 legend), only TnI, fibrinogen, CHA2DS2-VASc, CHA2DS2-VASc-T and CHA2DS2-VASc-CRPFT score remained significantly associated with unfavourable 30-day stroke outcome (Table 5). The pairwise comparisons of their predictive abilities are shown in Table 5 and Figure 3. Adjusted CHA2DS2-VASc and CHA2DS2-VASc-T (or CHA2DS2-VASc-CRPFT) score models had significantly better predictive ability than TnI alone, whilst there was no significant difference between adjusted CHA2DS2-VASc vs. CHA2DS2-VASc-T score (or the CHA2DS2-VASc-CRPFT) models (all p>0.05), with statistically insignificant discrimination improvement when adding TnI only or CRP plus fibrinogen plus TnI to the ‘classic’ CHA2DS2-VASc score (Table 5).
Figure 3. Pairwise comparison of Receiver-operator characteristic of fully adjusted TnI, ‘classic’ and composed scores for unfavourable 30-day functional outcome of ischemic stroke.
Analyses including patients with clinically evident CAD
Analyses of the whole AIS cohort (n = 273) including patients with clinically evident CAD (n = 33) yielded essentially identical results. Patients with unfavourable short-term functional stroke outcome had higher values of all three scores, compared with patients with mRS<3 (mean CHADS2 of 2.83±1.10 vs. 1.31±0.64, mean CHA2DS2-VASc of 5.17±1.31 vs. 2.71±1.10 and mean HAS-BLED score of 3.06±1.06 vs. 2.08±0.91, respectively, all p<0.001).
All 3 scores were significantly associated with unfavourable 30-day stroke outcome on the univariate analyses (CHADS2: OR 11.9, 95%CI 6.3–22.4; CHA2DS2-VASc: OR 7.6, 95%CI 4.7–12.2; HAS-BLED: OR 2.8, 95%CI 2.1–3.8, all p<0.001), whilst only the CHA2DS2-VASc was significantly associated with unfavourable 30-day stroke outcome in the multivariable analysis including all 3 scores (OR 7.6, 95%CI 4.7–12.2). All 3 scores had a good predictive ability for unfavourable short-term stroke outcome (CHADS2: c-statistic 0.882, 95%CI 0.841–0.922; CHA2DS2-VASc: c-statistic 0.932, 95%CI 0.91–0.962; HAS-BLED: c-statistic 0.754, 95%CI 0.696–0.812, all p<0.001), but the CHA2DS2-VASc score had significantly better predictive ability compared with the CHADS2 or HAS-BLED score (z-statistic 4.256 and 5.648, respectively, both p<0.001). Within the CHA2DS2-VASc score, age, diabetes mellitus, prior stroke or TIA and CAD were significantly associated with unfavourable short-term stroke outcome (OR [95%CI]: 1.2 [1.1–1.2], 5.3 [2.5–11.1], 43.9 [5.0–384.3] and 8.5 [2.3–31.5], respectively, all p ≤0.001). Other analyses with the biomarkers and combined scores also yielded essentially identical results as shown for the AIS cohort without clinically overt CAD (data not shown).
Discussion
In the present study, we show that the CHA2DS2-VASc score reliably predicts a short-term, 30-day unfavourable outcome of AIS (regardless of the presence or absence of AF), and that adding the high-sensitivity TnI (or fibrinogen or CRP, or all three biomarkers) to CHA2DS2-VASc score does not further improve the prediction. These findings could be of clinical relevance, as the pre-stroke CHA2DS2-VASc score can be easily calculated in most circumstances, thus offering a simplified, quick and reliable initial risk assessment in the acute stroke setting.
The CHA2DS2-VASc score has been originally developed to refine the assessment of stroke risk in patients with non-valvular AF [12], and was subsequently validated in a number of AF cohorts [23]–[27]. Recently, it was tested in a non-AF cohort and was comparably successful for stroke prediction in that cohort as in AF patients [28]. In addition, many recent studies showed that the CHA2DS2-VASc score was associated with poor clinical outcomes, including mortality, in various cohorts of cardiovascular patients [29]. Furthermore, the CHA2DS2-VASc score has been shown to correlate well with stroke severity in AF patients [14], and to be a multivariable predictor of 90-day stroke outcome, independently of baseline NIHSS values [15]. A recent study found that the CHADS2 and CHA2DS2-VASc scores also were good predictors of 5-year outcomes in non-AF patients with AIS [13]. However, the association of the CHADS2 and CHA2DS2-VASc scores with 30-day outcome of AIS has not been previously investigated prospectively [30], [31].
Our findings suggest that the CHA2DS2-VASc score reliably predicts poor 30-day stroke outcome, with significantly better predictive ability compared to the CHADS2 score. Indeed, on the logistic regression analysis which accounted for blood biomarkers, the presence of AF and a number of clinical/echocardiographic parameters, the model with CHA2DS2-VASc score had an excellent predictive ability for poor short-term stroke outcome (c-statistic of 0.982). Indeed, even the unadjusted CHA2DS2-VASc score predictive ability was very good, with c-statistic of 0.932.
Increasing body of evidence shows that a number of blood biomarkers are significantly associated with various clinical events [4], [11], [32], [33], [34], [35]. For example, a recent large biomarker substudy of the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy) showed that elevated TnI and NT-proBNP were independently related to increased risks of stroke and mortality and significantly improved risk prediction in AF patients beyond currently used clinical tools (i.e., the CHADS2 and CHA2DS2-VASc scores) [36]. However, the substudy had not investigated the association of biomarkers with stroke outcomes in patients who suffered AIS during follow-up. A recent systematic review of diagnostic and prognostic role of blood biomarkers in AIS highlighted blood glucose and fibrinogen levels as the most consistent predictors of poor functional outcome of stroke [33]. Nonetheless, studies on biomarkers generally suffer from several shortcomings, including relatively small number of patients, variable analytical techniques and interpretation of results (which may limit the reproducibility), different study population risk profiles, etc. Indeed, a recent study of 270 patients with AIS or TIA, which investigated the prognostic role of 18 blood biomarkers (including glucose, fibrinogen, troponin T, CRP, D-dimer, leukocytes, etc.) for 90-day functional outcome, found that on adjusted analyses only Interleukin-6 and NT-proBNP were significantly related to poor 90-day outcome, but with no significant improvement in predictive ability when added to age plus NIHSS model [11].
In our study, adjusted TnI and fibrinogen were significantly related to 30-day poor functional outcome of stroke. However, upon adjustment for other biomarkers and clinical/echocardiographic variables, the CHA2DS2-VASc-F score (i.e., the ‘classic’ score plus fibrinogen) was no longer significantly associated with 30-day poor outcome, in contrast to the CHA2DS2-VASc-T score (i.e., the ‘classic’ score plus TnI). Cardiac troponin (a protein involved in the heart muscle contraction) is a well established indicator of myocardial damage, and elevations in troponin levels have been observed in patients with acute MI, stable CAD, HF, renal failure, pulmonary embolism, and even in elderly apparently healthy individuals [32], [33], [37]–[42]. In addition, it has been shown that more than half AF patients have detectable levels of TnI [36]. Importantly, troponin has been uniformly associated with worse outcomes and increased mortality in all these cohorts, independent of traditional major coronary risk factors [32], [33], [36]–[39].
Increased troponin T levels have been reported in 5–34% of patients with AIS [43], and the elevation of troponin T was associated with stroke severity, insular cortex lesions, short- and long-term clinical outcome and increased risk of mortality, thus indicating prognostic significance of increased troponin T in AIS [43]–[47]. TnI increase in the AIS patients may be caused by the coincident ACS with myocardial necrosis or by a neurogenic cardiac damage due to autonomic imbalance in the acute stroke setting [48], [49]. It is not always possible to elucidate the exact cause of elevated TnI an individual patient with AIS, and an ongoing study will try to prospectively determine the frequency and possible etiology of troponin elevation in a large cohort of AIS patients, using coronary angiography [49]. In our study, all patients with known CAD and those with clinical, electrocardiographic and/or echocardiographic findings suggesting myocardial ischemia or acute MI at baseline (or during the subsequent 4 weeks) were excluded, in an effort to eliminate the impact of possible acute MI on short-term outcome of acute stroke (nonetheless, we are aware of the possibility that some of our patients could still suffer an unrecognized MI).
Overall, our findings suggest that regardless of the significant association of high-sensitivity TnI and fibrinogen with short-term functional outcome of AIS, these biomarkers do not significantly improve the excellent predictive ability of the CHA2DS2-VASc score alone. Hence, a routine measurement of cardiac TnI in patients presenting with AIS does not seem justified unless an ACS is suspected. Given the relatively small size of our study, these findings (and their potential implications for treatment decisions) need further evaluation in larger cohorts with acute ischemic stroke.
Study limitations
This was a single centre study performed in a university hospital and our findings might not be fully reflective of a high volume centre setting [50], although a 8.8% in-hospital mortality in our study is comparable to other reports [50], [51]. Nonetheless, our findings should be interpreted with some caution, given the relatively small number of participants in our study. Since the baseline NIHSS was not available for majority of our patients, the results were not adjusted for stroke severity. The NIHSS has been shown to be an important predictor of mortality in AIS [1], [52], but less than 50% of hospitals participating in the Get With The Guidelines – Stroke regularly reported patient NIHSS scores in a recent study [48], and the CHA2DS2-VASc score has been shown previously to be a good predictor of stroke outcome in a model which included NIHSS [15]. In addition, although all patients in our study underwent a 24-hour Holter monitoring, there is still a possibility of undiagnosed AF, which could affect the outcomes. Finally, the observational design of our study does not exclude a possibility of patient selection bias and residual confounding, although we have prospectively included consecutive patients presenting with AIS during one calendar year.
Conclusion
High-sensitivity TnI, fibrinogen and the CHA2DS2-VASc score predict 30-day unfavourable functional outcome of AIS regardless of the presence or absence of AF and other cardiovascular comorbidities or risk factors. The CHA2DS2-VASc score has better predictive ability for stroke outcome than TnI or fibrinogen, and adding these biomarkers to the CHA2DS2-VASc score does not further improve the prediction of poor 30-day stroke outcome in the absence of clinically evident CAD. The use of CHA2DS2-VASc score could facilitate a simplified, quick and reliable initial risk assessment of patients presenting with AIS.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Weimar C, Konig IR, Kraywinkel K, Ziegler A (2004) Diener HC; German Stroke Study Collaboration (2004) Age and National Institutes of Health Stroke Scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 2. Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, et al. (2010) Risk score for in-hospital ischemic stroke mortality derived and validated within the Get WithThe Guidelines– Stroke Program. Circulation 122: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 3. Counsell C, Dennis M, McDowall M (2004) Predicting functional outcome in acute stroke: comparison of a simple six variable model with other predictive systems and informal clinical prediction. J Neurol Neurosurg Psychiatry 75: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whiteley W, Chong WL, Sengupta A, Sandercock P (2009) Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke 40: e380–e389. [DOI] [PubMed] [Google Scholar]
- 5. Dixit S, Castle M, Velu RP, Swisher L, Hodge C, et al. (2000) Cardiac involvement in patients with acute neurologic disease: confirmation with cardiac TnI. Arch Intern Med 160: 3153–3158. [DOI] [PubMed] [Google Scholar]
- 6. Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G (2004) Serum cardiac TnI in acute stroke is related to serum cortisol and TNF-alpha. Cerebrovasc Dis 18: 194–199. [DOI] [PubMed] [Google Scholar]
- 7. Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, et al. (2005) Prognostic significance of admission levels of TnI in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 76: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James P, Ellis CJ, Whitlock RM, McNeil AR, Henley J, et al. (2000) Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ 320: 1502–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jensen JK, Kristensen SR, Bak S, Atar D, Høilund-Carlsen PF, et al. (2007) Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol 99: 108–112. [DOI] [PubMed] [Google Scholar]
- 10. Faiz KW, Thommessen B, Einvik G, Omland T, Ronning OM (2014) Prognostic value of high-sensitivity cardiac troponin T in acute ischemic stroke. J Stroke Cerebrovasc Dis 23 241: 248. [DOI] [PubMed] [Google Scholar]
- 11. Whiteley W, Wardlaw J, Dennis M, Lowe G, Rumley A, et al. (2012) The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke 43: 86–91. [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 13. Ntaios G, Lip GY, Makaritsis K, Papavasileiou V, Vemmou A, et al. (2013) CHADS2, CHA2S2DS2-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology 80: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 14. Deguchi I, Hayashi T, Ohe Y, Kato Y, Nagoya H, et al. (2013) The CHA(2)DS(2)-VASc Score Reflects Clinical Outcomes in Nonvalvular Atrial Fibrillation Patients with an Initial Cardioembolic Stroke. J Stroke Cerebrovasc Dis 22: e343–e346. [DOI] [PubMed] [Google Scholar]
- 15. Giralt-Steinhauer E, Cuadrado-Godia E, Ois Á, Jiménez-Conde J, Rodríguez-Campello A, et al. (2012) CHA(2)DS(2)-VASc score and prognosis in ischemic strokes with atrial fibrillation. J Neurol 259: 745–751. [DOI] [PubMed] [Google Scholar]
- 16. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, et al. (2009) Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 40: 2276–2293. [DOI] [PubMed] [Google Scholar]
- 17. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, et al. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation *Developed with the special contribution of the European Heart Rhythm Association. Europace 14: 1385–1413. [DOI] [PubMed] [Google Scholar]
- 18. Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 19. The Multicentre Acute Stroke Trial–Italy Group (MAST-I) (1995) Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Lancet 346: 1509–1514. [PubMed] [Google Scholar]
- 20. The Multicenter Acute Stroke Trial–Europe (MAST-E) (1996) Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med 335: 145–150. [DOI] [PubMed] [Google Scholar]
- 21. DeLong R, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845. [PubMed] [Google Scholar]
- 22. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Comments on Integrated discrimination and net reclassification improvements – practical advice. Statist Med 27: 207–212. [Google Scholar]
- 23.Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, et al.. (2011) Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 342–d124. [DOI] [PMC free article] [PubMed]
- 24. Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GYH (2011) A comparison of risk stratification schemes for stroke in 79 884 atrial fibrillation patients in general practice. J Thromb Haemost 9: 39–48. [DOI] [PubMed] [Google Scholar]
- 25. Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY (2012) The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost 107: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 26. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, et al. (2012) Reliable identification of “truly low” thromboembolic risk in patients initially diagnosed with “lone” atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol 5: 319–326. [DOI] [PubMed] [Google Scholar]
- 27. Lip GY (2013) Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J 34: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 28. Lip GY, Lin HJ, Chien KL, Hsu HC, Su TC, et al. (2013) Comparative assessment of published atrial fibrillation stroke risk stratification schemes for predicting stroke, in a non-atrial fibrillation population: The Chin-Shan Community Cohort Study. Int J Cardiol 168: 414–419. [DOI] [PubMed] [Google Scholar]
- 29. Paoletti Perini A, Bartolini S, Pieragnoli P, Ricciardi G, Perrotta L, et al. (2014) CHADS2 and CHA2DS2-VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. Europace 16: 71–80. [DOI] [PubMed] [Google Scholar]
- 30. Tu HTH, Campbell BCV, Meretoja A, Churilov L, Lees KR, et al. (2013) Pre-stroke CHADS 2 and CHA2DS2 - VASc scores are useful in stratifying three-month outcomes in patients with and without atrial fibrillation. Cerebrovasc Dis 36: 273–280. [DOI] [PubMed] [Google Scholar]
- 31. Lip GYH (2013) Using the CHADS 2 and CHA2DS2 -VASc Scores for Stroke Risk Prediction as well as the Identification of Stroke Outcomes and Cardiac Complications in Patients with and without Atrial Fibrillation. Cerebrovasc Dis 36: 281–282. [DOI] [PubMed] [Google Scholar]
- 32. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L (2013) Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 34: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 33. Hasan N, McColqan P, Edwards RJ, Sharma P (2012) Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol 74: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salat D, Penalba A, García-Berrocoso T, Campos-Martorell M, Flores A, et al. (2013) Immunological biomarkers improve the accuracy of clinical risk models of infection in the acute phase of ischemic stroke. Cerebrovasc Dis 35: 220–227. [DOI] [PubMed] [Google Scholar]
- 35. Montaner J, García-Berrocoso T, Mendioroz M, Palacios M, Perea-Gainza M, et al. (2012) Brain natriuretic peptide is associated with worsening and mortality in acute stroke patients but adds no prognostic value to clinical predictors of outcome. Cerebrovasc Dis 34: 240–245. [DOI] [PubMed] [Google Scholar]
- 36. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, et al. (2012) Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation. A randomized evaluation of long-term anticoagulation therapy (RE-LY) substudy. Circulation 125: 1605–1616. [DOI] [PubMed] [Google Scholar]
- 37. Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, et al. (1996) Cardiac-specific TnI levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 335: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 38. Horwich TB, Patel J, MacLellan WR, Fonarow GC (2003) Cardiac TnI is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 108: 833–838. [DOI] [PubMed] [Google Scholar]
- 39. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, et al. (2009) A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 361: 2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeremias A, Gibson CM (2005) Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 142: 786–791. [DOI] [PubMed] [Google Scholar]
- 41. Agewall S, Giannitsis E, Jernberg T, Katus H (2011) Troponin elevation in coronary vs. non-coronary disease. Eur Heart J 32: 404–411. [DOI] [PubMed] [Google Scholar]
- 42. Freda BJ, Tang WHW, van Lente F, Peacock WF, Francis GS (2002) Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol 40: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 43. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P (2009) Elevated troponin after stroke: a systematic review. Cerebrovasc Dis 28: 220–226. [DOI] [PubMed] [Google Scholar]
- 44. James P, Ellis CJ, Whitlock RM, McNeil AR, Henley J, et al. (2000) Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ 320: 1502–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, et al. (2005) Prognostic significance of admission levels of TnI in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatr 76: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jensen JK, Kristensen SR, Bak S, Atar D, Høilund-Carlsen PF, et al. (2007) Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol 99: 108–112. [DOI] [PubMed] [Google Scholar]
- 47. Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, et al. (2006) Neuroanatomic correlates of strokerelated myocardial injury. Neurology 66: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 48. Mahajan VS, Jarolim P (2011) How to interpret elevated cardiac troponin levels. Circulation 124: 2350–2354. [DOI] [PubMed] [Google Scholar]
- 49. Scheitz JF, Mochmann HC, Nolte CH, Haeusler KG, Audebert HJ, et al. (2011) Troponin elevation in acute ischemic stroke (TRELAS) - protocol of a prospective observational trial. BMC Neurology 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, et al. (2012) Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA 308: 257–264. [DOI] [PubMed] [Google Scholar]
- 51. Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, et al. (2004) Predictors of in-hospital mortality and attributable risk of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med 164: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 52. Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, et al. (2010) Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With The Guidelines– Stroke Program. Circulation 122: 1496–1504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.