Abstract
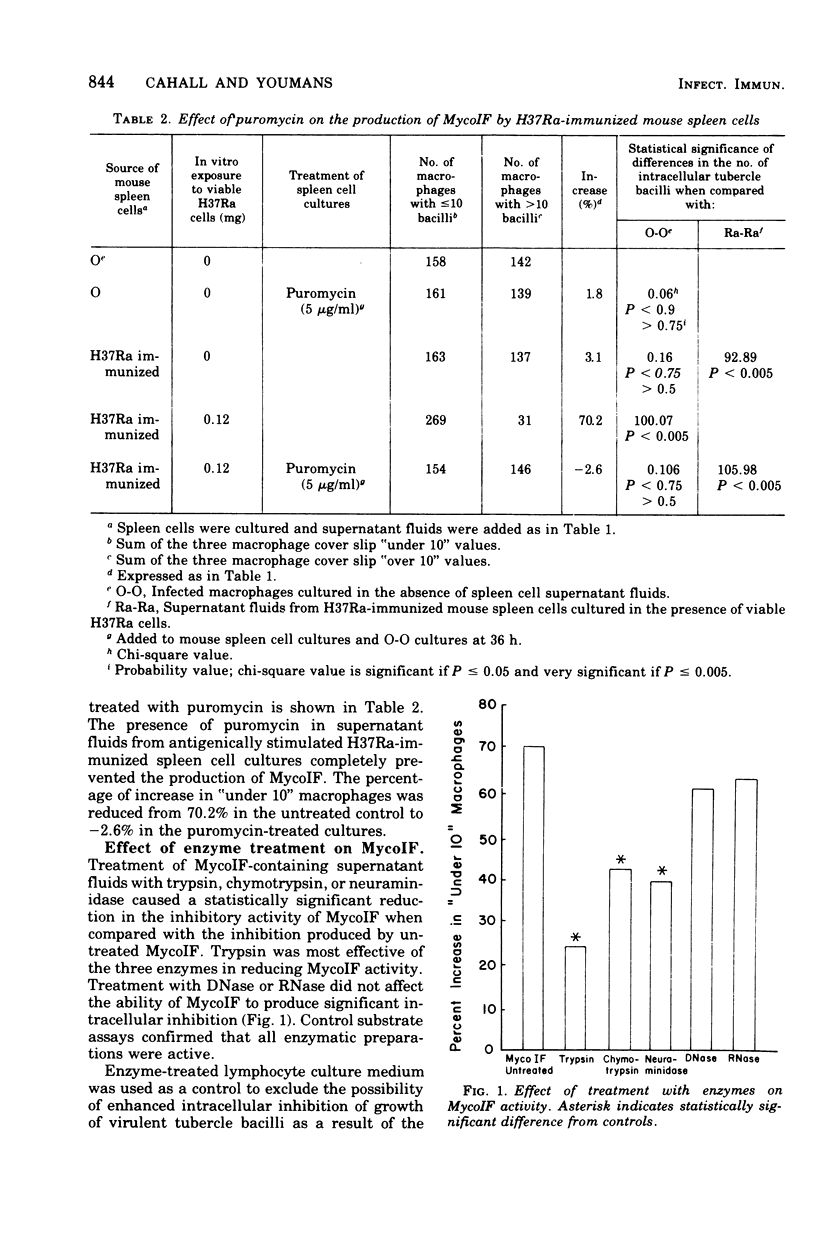
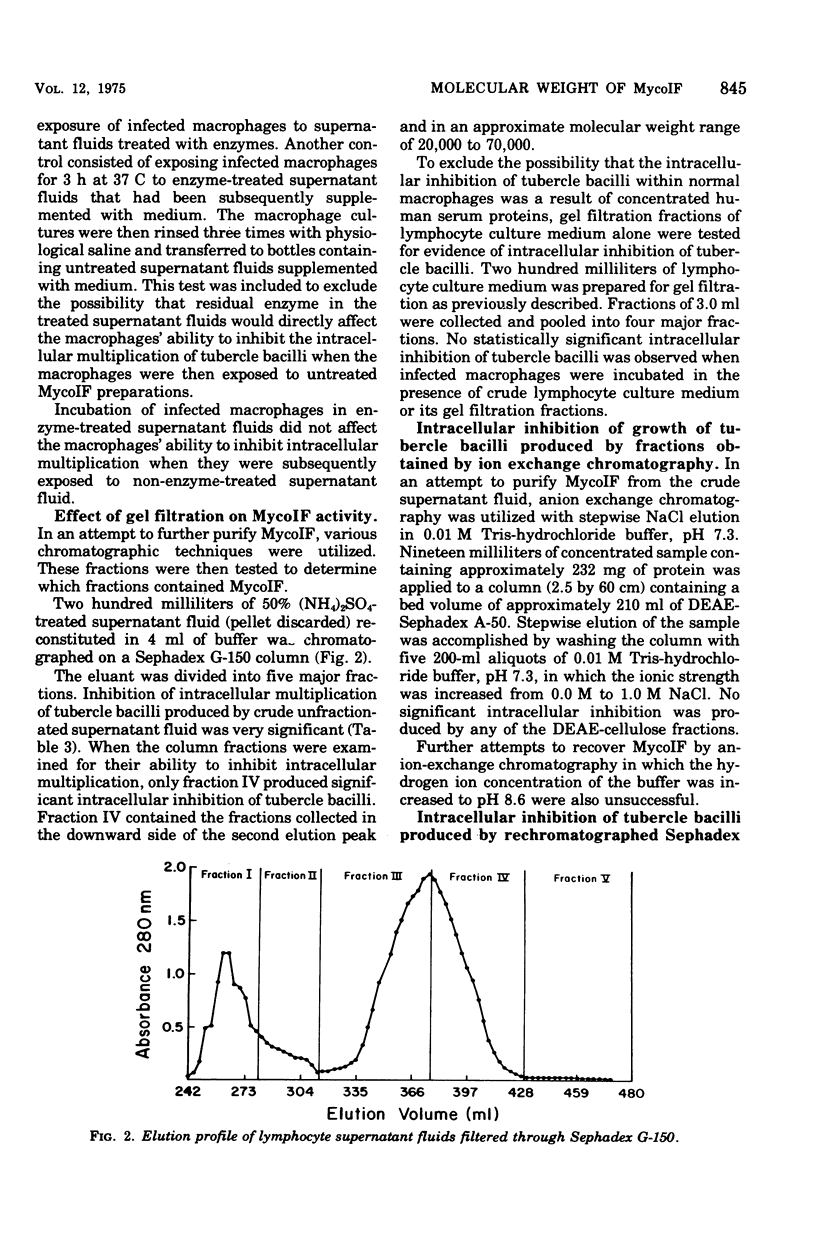
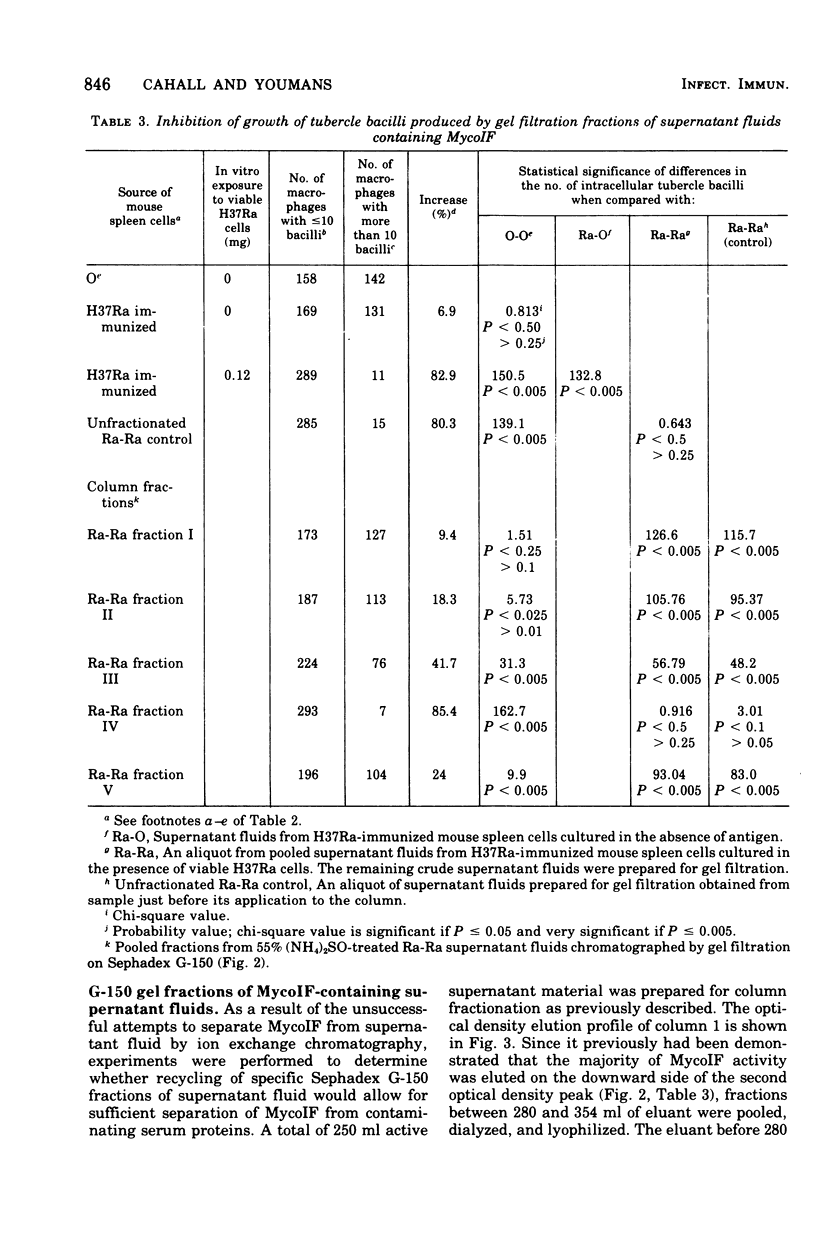
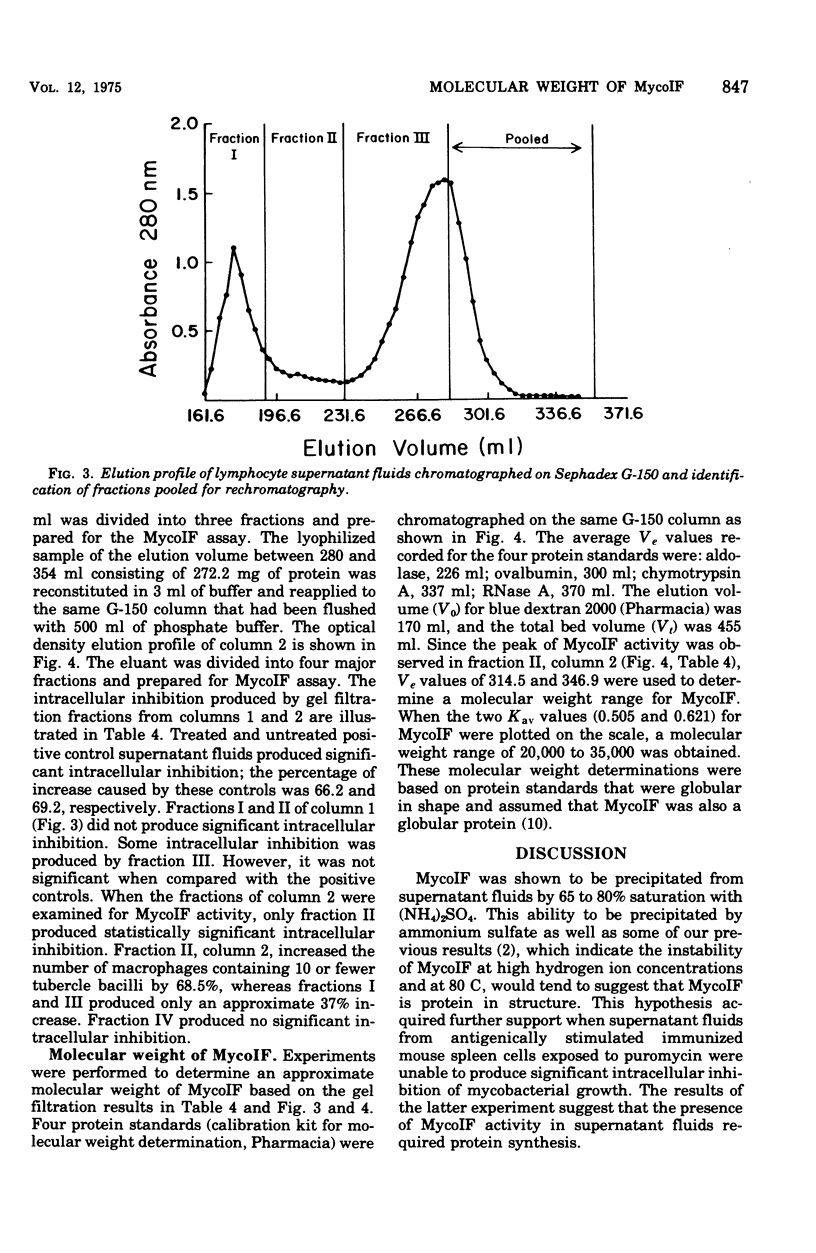
Exposure of mycobacterial growth inhibitory factor (MycoIF) to trypsin, chymotrypsin, or neuraminidase decrease its ability to produce intracellular inhibition of mycobacterial growth within macrophages, suggesting that MycoIF was a glycoprotein. MycoIF was unaffected by deoxyribonuclease or ribonuclease. Supernatant fluids from antigenically stimulated H37Ra-immunized mouse spleen cells exposed to puromycin were unable to produce significant intracellular inhibition. This indicated that the presence of MycoIF activity in supernatant fluids required protein synthesis. The filtration of MycoIF-containing supernatant fluids on Sephadex G-150 demonstrated that significant MycoIF activity appeared only in those fractions which eluted on the downward side of the serum albumin peak. Based on protein standards filtered through the Sephadex gel, the molecular weight of MycoIF was calculated to be between 20,000 and 35,000. These calculations assumed that MycoIF is a globular protein. Attempts to purify MycoIF by anion exchange chromatography (diethylaminoethylcellulose) was not successful.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Bennett B. Relation of the migration inhibitory factor (MIF) to delayed-type hypersensitivity reactions. Ann N Y Acad Sci. 1970 Feb 13;169(1):258–265. doi: 10.1111/j.1749-6632.1970.tb55994.x. [DOI] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Granger G. A. Lymphocyte in vitro cytotoxicity: characterization of mouse lymphotoxin. Cell Immunol. 1970 May;1(1):122–132. doi: 10.1016/0008-8749(70)90065-1. [DOI] [PubMed] [Google Scholar]
- Remold H. G., Katz A. B., Haber E., David J. R. Studies on migration inhibitory factor (MIF): recovery of MIF activity after purification by gel filtration and disc electrophoresis. Cell Immunol. 1970 May;1(1):133–145. doi: 10.1016/0008-8749(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Rosenau W., Goldberg M. L., Kunitomi G. Purification of human lymphotoxin. J Immunol. 1972 Oct;109(4):784–790. [PubMed] [Google Scholar]



