Abstract
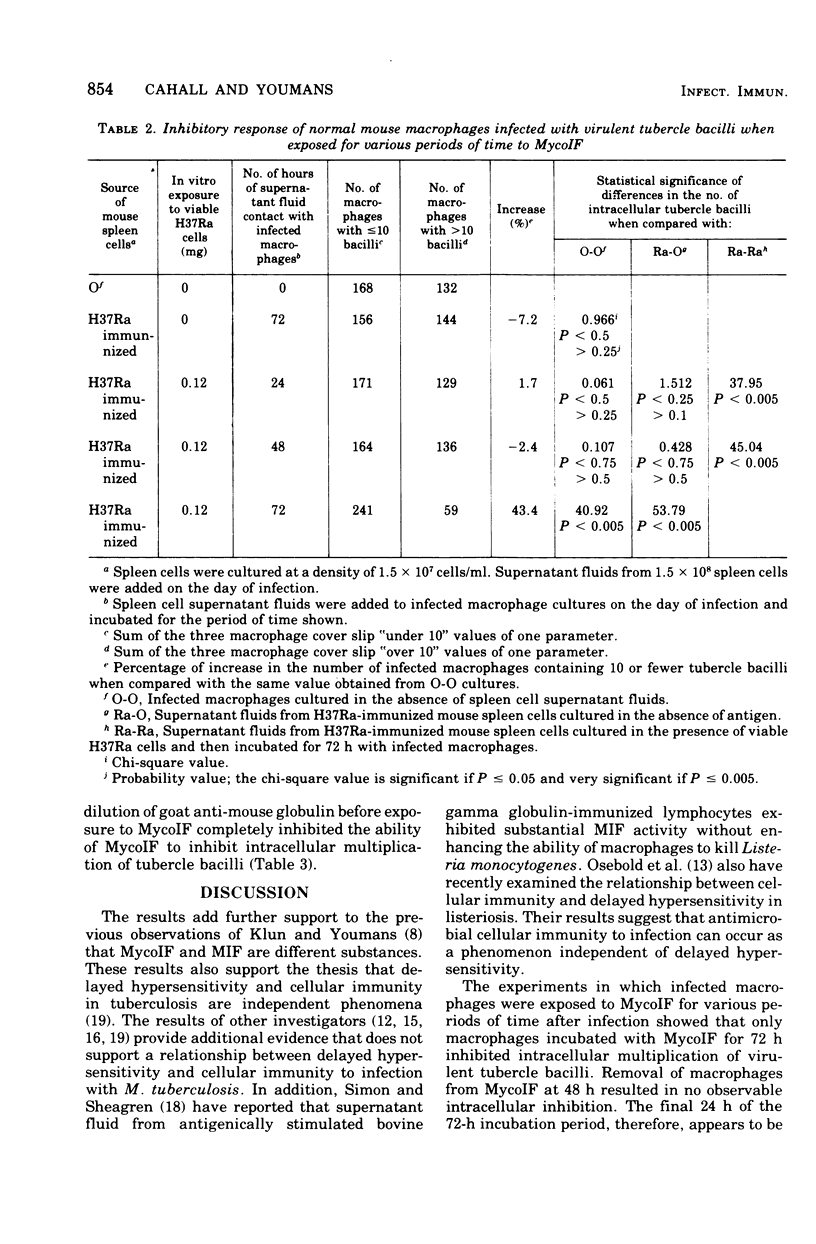
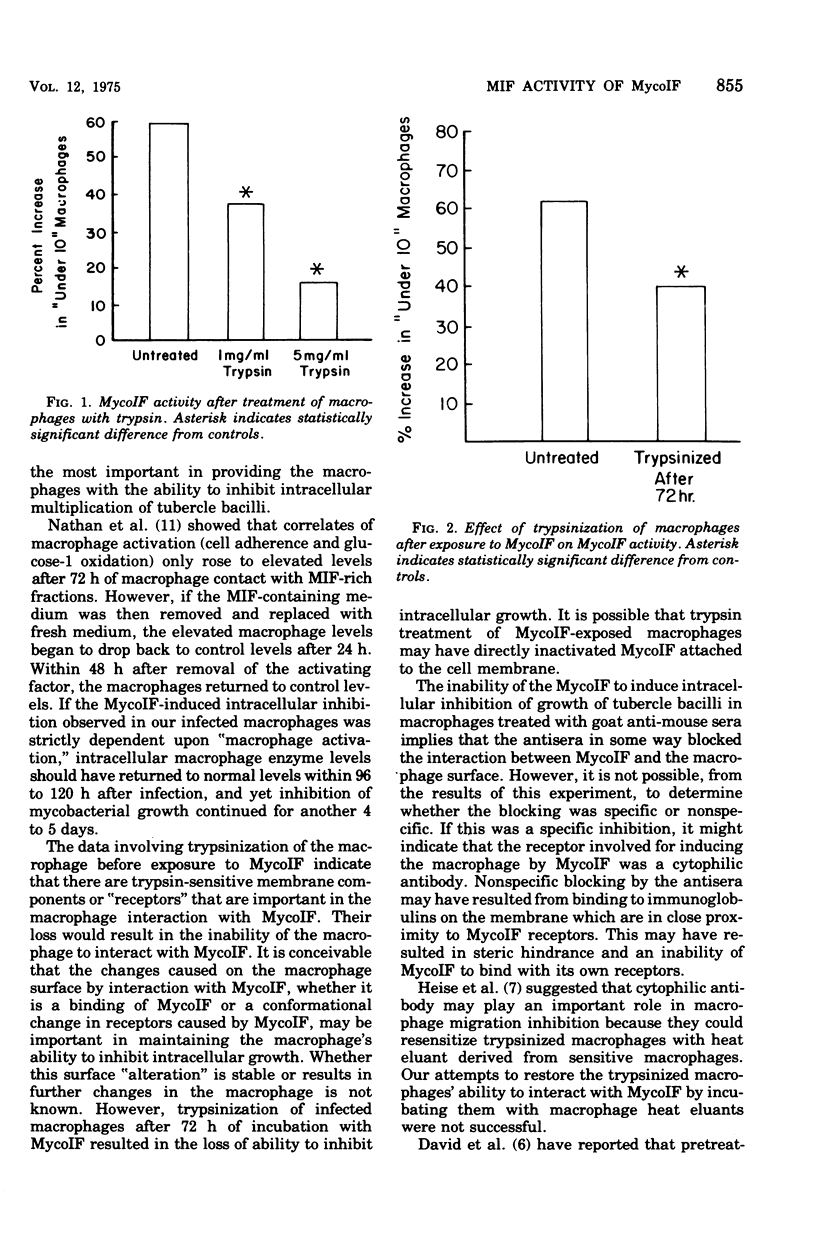
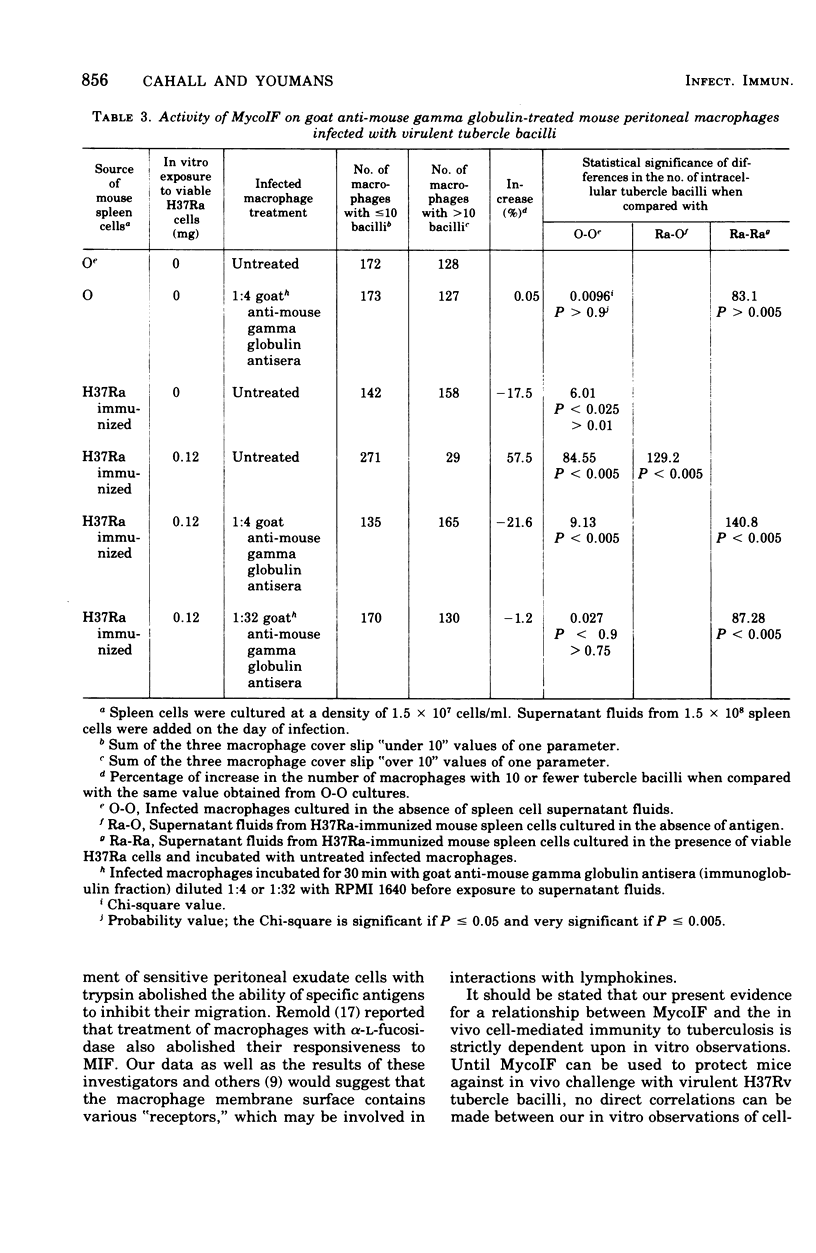
Mycobacterial growth inhibitory factor (MycoIF), which inhibits the intracellular multiplication of virulent tubercle bacilli within normal peritoneal macrophages in vitro, was tested for its ability to inhibit the migration of normal peritoneal exudate cells. The migration of peritoneal exudate cells was not inhibited by MycoIF. It was also shown that normal peritoneal macrophages infected with virulent Mycobacterium tuberculosis, strain H37Rv, required 72 h of incubation with spleen cell culture supernatant fluids containing MycoIF in order to inhibit intracellular bacillary multiplication. Treatment of infected macrophages with trypsin before their exposure to MycoIF abolished the ability of MycoIF to inhibit intracellular mutiplication of tubercle bacilli. Incubation of infected macrophages with goat anti-mouse globulin before their exposure to MycoIF also blocked the action of MycoIF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Molecular weight and other characteristics of mycobacterial growth inhibitory factor produced by spleen cells obtained from mice immunized with viable attenuated mycobacterial cells. Infect Immun. 1975 Oct;12(4):841–850. doi: 10.1128/iai.12.4.841-850.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- DAVID J. R., LAWRENCE H. S., THOMAS L. THE IN VITRO DESENSITIZATION OF SENSITIVE CELLS BY TRYPSIN. J Exp Med. 1964 Dec 1;120:1189–1200. doi: 10.1084/jem.120.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R., David R. R. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300–449. [PubMed] [Google Scholar]
- Heise E. R., Han S., Weiser R. S. In vitro studies on the mechanism of macrophage migration inhibition in tuberculin sensitivity. J Immunol. 1968 Nov;101(5):1004–1015. [PubMed] [Google Scholar]
- Klun C. L., Youmans G. P. The effect of lymphocyte supernatant fluids on the intracellular growth of virulent tubercle bacilli. J Reticuloendothel Soc. 1973 Mar;13(3):263–274. [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. The action of drugs on intracellular tubercle bacilli. J Pathol Bacteriol. 1952 Jul;64(3):429–446. doi: 10.1002/path.1700640302. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiburger R. G., Youmans G. P. Inhibition of migration of mouse macrophages by tuberculin-sensitive mouse lymphocytes and by mouse migration inhibitory factor. Infect Immun. 1973 Feb;7(2):190–195. doi: 10.1128/iai.7.2.190-195.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFFEL S. Chemical factors involved in the induction of infectious allergy. Experientia. 1950 Nov 15;6(11):410–419. doi: 10.1007/BF02150118. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Delayed-type hypersensitivity and immunity against aerogenic tuberculosis in guinea pigs. Infect Immun. 1974 May;9(5):815–820. doi: 10.1128/iai.9.5.815-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold H. G. Requirement for alpha-L-fucose on the macrophage membrane receptor for MIF. J Exp Med. 1973 Nov 1;138(5):1065–1076. doi: 10.1084/jem.138.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Feb;111(2):109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]



