Abstract
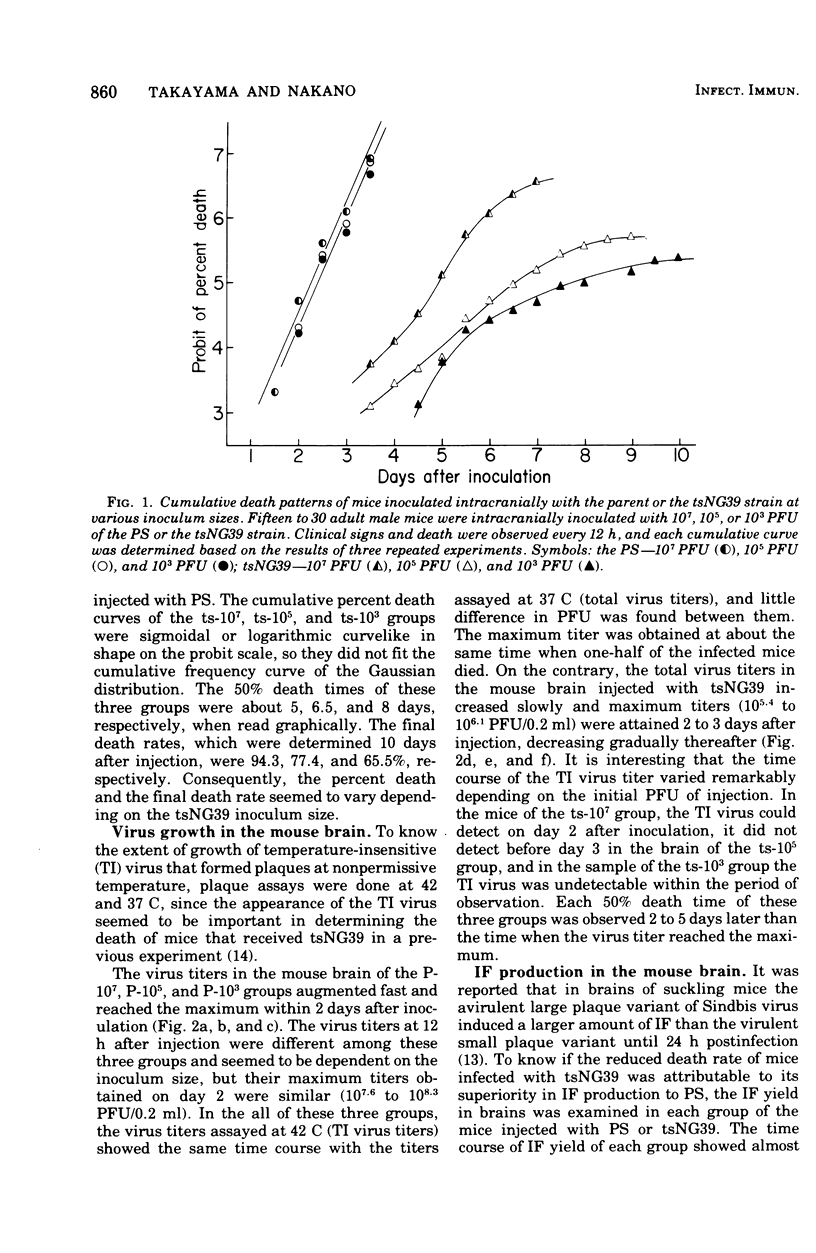
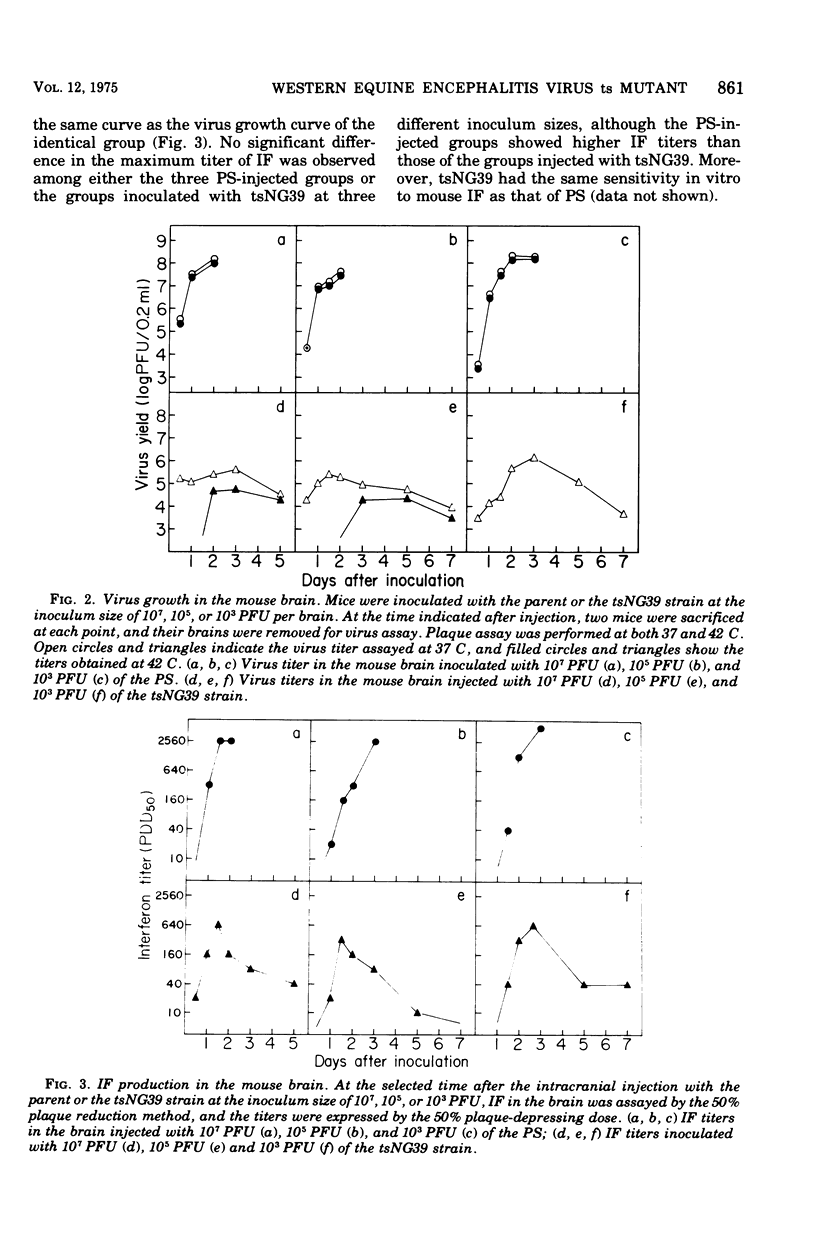
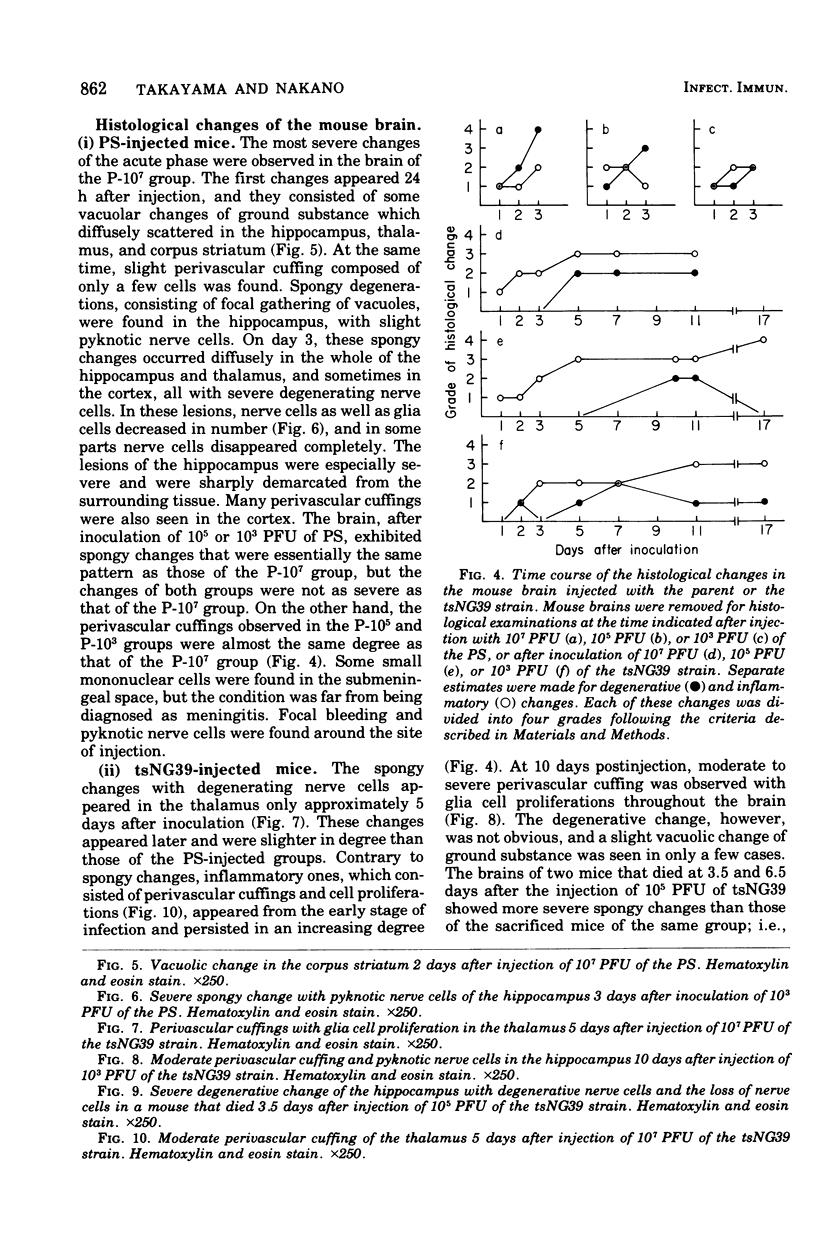
To know the pathogenicity of the chemically induced, temperature-sensitive (ts) mutant of western equine encephalitis virus, designated tsNG39, the lethality for mice injected with tsNG39, virus yield, interferon production, and histological changes in the brains of these mice were examined in parallel with those of mice inoculated with the parent strain (PS). All of the mice injected intracranially with PS died within 3.5 days after injection irrespective of the inoculum size of virus, whereas the lethality of the mice inoculated with tsNG39 varied from 94.3 to 65.5% among groups of mice and this variation seemed to be correlated with the inoculum size of virus rather than with the maximum virus titer in the brain. By histological examination, two types of changes in the brain were distinguished, inflammatory and degenerative ones. Inflammatory changes were more prominent in the brains injected with tsNG39 than in those receiving PS. Degenerative changes were dominant in the brains injected with PS, but they were slight in the earlier phase of infection by tsNG39 became prominent only later. The degree of degenerative change was well correlated with both the virus titer in the mouse brain and the death pattern of mice injected with PS or tsNG39. Since degenerative changes are thought to be caused by the direct effect of injected virus, these results indicated that the factor responsible for the low virulence of tsNG39 was the slow viral growth in the brain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin F. J., Scherer W. F. Studies of viral virulence. I. Growth and histopathology of virulent and attenuated strains of Venezuelan encephalitis virus in hamsters. Am J Pathol. 1971 Feb;62(2):195–210. [PMC free article] [PubMed] [Google Scholar]
- Campbell J. B., Colter J. S. Studies of three variants of Mengo encephalomyelitis virus. IV. Affinities for mouse tissues in vitro and in vivo. Virology. 1967 May;32(1):69–73. doi: 10.1016/0042-6822(67)90253-x. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Wisseman C. L., Jr The effect of hyperthermia on dengue virus infection of mice. Proc Soc Exp Biol Med. 1969 Feb;130(2):359–363. doi: 10.3181/00379727-130-33555. [DOI] [PubMed] [Google Scholar]
- GLEISER C. A., GOCHENOUR W. S., Jr, BERGE T. O., TIGERTT W. D. The comparative pathology of experimental Venezuelan equine encephalomyelitis infection in different animal hosts. J Infect Dis. 1962 Jan-Feb;110:80–97. doi: 10.1093/infdis/110.1.80. [DOI] [PubMed] [Google Scholar]
- Haahr S., Teisner B. The influence of different temperatures on mortality, virus multiplication and interferon production in adult mice infected with Coxsackie B1 virus. Arch Gesamte Virusforsch. 1973;42(3):273–277. doi: 10.1007/BF01265652. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Scherer W. F. Growth curves and clearance rates of virulent and benign Venezuelan encephalitis viruses in hamsters. Infect Immun. 1973 Sep;8(3):456–462. doi: 10.1128/iai.8.3.456-462.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn A., Schieffner A., Tinland R. Lack of correlation between production of interferon and protection of temperature in mice infected with Sindbis virus. Nature. 1967 Jul 1;215(5096):86–87. doi: 10.1038/215086a0. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., GIFFORD G. E. Studies on vaccinia virus plaque formation and its inhibition by interferon. III. A simplified plaque inhibition assay of interferon. Virology. 1963 Mar;19:302–309. doi: 10.1016/0042-6822(63)90068-0. [DOI] [PubMed] [Google Scholar]
- LWOFF A., LWOFF M. [On factors of viral growth and their role in the development of infection]. Ann Inst Pasteur (Paris) 1960 Feb;98:173–203. [PubMed] [Google Scholar]
- Rasmussen L. E., Armstrong J. A., Ho M. Interference mediated by a variant of Sindbis virus. I. Isolation of an avirulent variant and in vivo interference. J Infect Dis. 1973 Aug;128(2):156–162. doi: 10.1093/infdis/128.2.156. [DOI] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Virulence of a temperature-sensitive mutant of western equine encephalitis virus. Arch Gesamte Virusforsch. 1972;38(4):328–337. doi: 10.1007/BF01262823. [DOI] [PubMed] [Google Scholar]
- Takayama N. Further characterization of an attenuated western equine encephalitis virus: search for in vitro markers. Arch Gesamte Virusforsch. 1972;36(3):363–371. doi: 10.1007/BF01249867. [DOI] [PubMed] [Google Scholar]
- Takayama N., Simizu B. Japanese quail embryo cells as a host of group A arboviruses. Jpn J Microbiol. 1973 May;17(3):199–203. doi: 10.1111/j.1348-0421.1973.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Webb H. E., Smith C. E. Relation of immune response to development of central nervous system lesions in virus infections of man. Br Med J. 1966 Nov 12;2(5523):1179–1181. doi: 10.1136/bmj.2.5523.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebovitz E., Brown A. Interference among group A arboviruses. J Virol. 1968 Nov;2(11):1283–1289. doi: 10.21236/ad0844170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Carter G. B., Grant D. P. The persistence of louping ill virus in immunosuppressed guinea-pigs. Br J Exp Pathol. 1971 Aug;52(4):395–407. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Peacock S., Grant D. P., Batter-Hatton D. The pathogenesis of western equine encephalitis virus (W.E.E.) in adult hamsters with special reference to the long and short term effects on the C.N.S. of the attenuated clone 15 variant. Br J Exp Pathol. 1972 Feb;53(1):59–77. [PMC free article] [PubMed] [Google Scholar]








