Abstract
IMPORTANCE
Small studies have implicated the association of specific autoantibodies with morphea subtype or severity, but no large-scale studies have been conducted. This prospective case-control study confirmed the presence of antinuclear antibodies (ANAs) and other autoantibodies in morphea but found they are of limited significance.
OBJECTIVE
To determine the prevalence of ANAs, extractable nuclear antigens such as antihistone antibodies (AHAs), and anti–single-stranded DNA antibodies (ssDNA abs) in patients with morphea vs a healthy control population and their association with clinical measures of morphea severity.
DESIGN, SETTING, AND PARTICIPANTS
Nested case-control study, conducted at the University of Texas Southwestern Medical Center, Dallas, and University of Texas Health Science Center, Houston. Study participants included individuals enrolled in the Morphea in Adults and Children (MAC) cohort and Scleroderma Family Registry and DNA Repository.
MAIN OUTCOMES AND MEASURES
Prevalence of ANAs, AHAs, ssDNA abs in patients with morphea vs matched controls and association of the presence of autoantibodies with clinical indicators of morphea severity.
RESULTS
The prevalence of ANAs, AHAs, and ssDNA abs in patients with morphea was 34%, 12%, and 8%, respectively. Antinuclear antibodies and AHAs, but not ssDNA abs, were present more frequently in cases than in controls. There was no difference in ANA prevalence among morphea subtypes. Among patients with linear morphea, the presence of autoantibodies was associated with clinical indicators of severe morphea including functional limitation (ssDNA ab, P = .005; and AHA, P = .006), extensive body surface area involvement (ssDNA ab, P = .01; and ANA, P = .005), and higher skin scores (ANA, P = .004). The presence of autoantibodies was not associated with clinical measures of morphea activity.
CONCLUSIONS AND RELEVANCE
Our results demonstrate that ANAs and AHAs are more prevalent among patients with morphea but are of limited clinical utility except in linear morphea, where their presence, although infrequent, is associated with greater lesion burden and functional impairment.
Morphea, also known as localized scleroderma, is characterized by excessive collagen deposition that results in sclerosis of the dermis and sometimes subcutaneous tissue. Morphea causes significant morbidity due to associated functional and cosmetic impairment, reduced quality of life, and rarely, internal manifestations.1,2 While the pathophysiologic mechanism of morphea is poorly described, it is considered an autoimmune disease, at least partially because of the reported autoantibody associations. Several studies have also reported an association between autoantibodies and disease activity and severity, especially anti–single-stranded DNA antibody (ssDNA ab) in linear morphea.3–7 However, these studies are limited by lack of controls, small sample size, variable definition of morphea subtypes, different criteria for defining disease activity and/or severity, and the use of different autoantibody assays and cutoff titers. As a result, the prevalence of autoantibodies in morphea remains uncertain, as does the nature of the association between these autoantibodies and disease activity and severity. Nonetheless, our own cross-sectional survey of dermatologists and rheumatologists practicing in the United States revealed that 15% to 47% order ANA testing in the evaluation of their patients with morphea.8
The present study, referred to as the Morphea in Adults and Children (MAC) cohort, was designed to examine demographic, clinical, antibody, and autoimmune features in a carefully phenotyped cohort of adults and children with morphea (Table 1 outlines subtype classifications). By studying patients in a prospective nested case-control fashion (the third study undertaken in this cohort, thus the inclusion of the Roman numeral III in the title), we aimed to define the prevalence and clinical significance of autoantibodies in morphea. Specifically, we determined the prevalence of antinuclear antibodies (ANAs), antibodies to extractable nuclear antigens (SS-A, SS-B, Smith, Scl-70, ribo-nucleoprotein [RNP]), RNA-polymerase 3 (RNA–pol 3), single-stranded DNA antibodies (ssDNA abs), and antihistone antibodies (AHAs) among patients with morphea compared with healthy, age-matched controls, hypothesizing that patients with morphea would have a higher prevalence of these autoantibodies. We also examined the association of these autoantibodies with validated measures of disease activity and severity, hypothesizing that the presence of autoantibodies would be associated with greater disease activity and severity.
Table 1.
Classification of Morphea Subtypes in the Morphea in Adults and Children Cohorta
| Morphea Subtype | Modifiers | Clinical |
|---|---|---|
| Plaque | Superficial | Single or multiple oval to round lesions limited to epidermis and dermis |
| Deep | Single or multiple oval to round lesions involving subcutaneous tissue, fascia, or muscle | |
| Linear | Trunk/limbs | Linear lesions involving dermis, subcutis, or deeper tissues; primary lesion may involve subcutis or deeper |
| Head | En coup de sabre, progressive hemifacial atrophy, linear lesions of the face (may involve underlying bone) | |
| Generalized | ||
| Coalescent plaque | NA | ≥4 Plaques in at least 2 of 7 anatomic sites |
| Pansclerotic | NA | Circumferential involvement of majority of body surface area (sparing fingertips and toes), affecting skin, subcutaneous tissue, muscle, or bone; no internal organ involvement |
| Mixed | NA | Combination of any of the above subtypes: eg, linear-circumscribed |
Abbreviation: NA, not applicable.
Adapted with permission from Current Opinion in Rheumatology.9
Methods
Study Participants
Patients With Morphea
The MAC cohort comprises 251 adults (age, ≥18 years at enrollment) and children (age ≤17 years at enrollment). All patients or guardians provided written consent for inclusion in this study, which was approved by the University of Texas (UT) Southwestern Medical Center institutional review board. The study protocol and informed consent were in compliance with Declaration of Helsinki Principles. Criteria for inclusion in the study reported herein included eligibility for enrollment in the MAC cohort (the details of eligibility have been reported previously).10
The MAC cohort was designed to capture prevalent and incident cases of morphea. Patients were recruited from within the UT Southwestern Medical Center system, encompassing 2 dedicated pediatric care facilities, a county hospital, and a faculty-based practice. In addition, patients were routinely enrolled through regional and national referrals from private practitioners (dermatologists and rheumatologists, both pediatric and adult). This represents a conscious effort to enroll patients of varied disease severity, subtypes, and socioeconomic backgrounds. After patients (or guardians) signed consent, all data were abstracted using a comprehensive clinical report form designed prior to the study, including demographic, clinical, medical history, and family history data. Patients with morphea onset before age 18 years were classified as having childhood-onset disease. Medical records were obtained and reviewed for confirmation of patient-reported findings, particularly for confirmation of systemic manifestations and concomitant autoimmune disorders At the time of enrollment, all patients were examined by 1 examiner with expertise in morphea (H.J.), who assigned each patient 1 of 5 clinical subtypes as defined by the criteria of Laxer and Zulian9 (Table 1). Serum samples for immunologic studies were obtained from patients at the time of enrollment. Patients were excluded from the present study for the following reasons: if the morphea subtype was indeterminate or if insufficient clinical information or serum sample was present for analysis.
Control Subjects
Autoantibody data (ANA, Smith, RNP, Ro or SS-A, La or SS-B, topoisomerase, and RNA-pol 3) and serum samples for auto-antibody determination (ssDNA and histone) were obtained from a pool of more than 1800 unrelated healthy subjects recruited for the Scleroderma Family Registry and DNA Repository. All subjects provided written informed consent. Controls were age-, sex-, and race-matched in a 3.5:1 ratio for ANA testing (controls, n = 651) and in a 1:1 ratio for ssDNA and antihistone testing (controls, n = 149).
Assessment of Disease Severity and Activity
All patients with morphea were examined by 1 dermatologist (H.J.) at the time of enrollment and assessed for predetermined clinical outcome measures of disease severity and activity. Functional limitation was defined as having at least 1 of the following conditions: (1) limited joint mobility (clinically appreciable limited range of motion of a joint secondary to skin and subcutaneous tissue involvement, but not due to abnormality of the joint itself and or contracture) or (2) limb-length discrepancy.
The total number of body sites affected in each patient was recorded. Extensive body surface area (BSA) was defined as having lesions of morphea involving 3 or more body sites, determined by dividing the skin surface into 14 sites as previously described.5 Patients were scored by the same investigator (H.J.) using the modified Rodnan Skin Score (mRSS)11 and the newly validated clinical outcome measure, the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT).12 Because the LoSCAT was not validated at the time of the inception of the cohort, patients enrolled before 2008 were only assessed with the mRSS. Although the mRSS is not validated in morphea, it was selected because its components offer an assessment of disease severity based on body sites affected and the degree of skin thickening or hardening, and it was used in prior studies of morphea (owing to a lack of any validated outcome measures in morphea). After 2008, MAC patients were assessed with both the mRSS (for continuity with initial assessments) and the newly validated LoSCAT. The LoSCAT scoring system is divided into the Localized Scleroderma Skin Severity Index (LoSSI) and the Localized Scleroderma Skin Damage Index (LoSDI).12 The LoSDI has some overlapping content with the mRSS in that the assessment of skin thickness is scored identically to the mRSS. The Physician Global Assessment of Activity (PGA-A) and of Damage (PGA-D) were scored as part of the LoSSI and LoSDI, respectively. The validation of these measures has been previously reported.12
Autoantibody Testing
Sera was isolated from whole blood from patients and controls at the time of enrollment and stored at −80°C. Levels of ANAs for all subjects were determined using indirect immunofluorescence on HEp-2 cells (Antibodies Inc), using previously published methods.13 An ANA titer of 1:80 or greater was considered positive. Serum samples meeting the cutoff titer of 1:80 were serially diluted to 1:1280. Immunofluorescence patterns were classified as speckled, centromere, nucleolar, homogenous, and mitochondrial based on the interpretation of a single investigator (F.A.) for all samples (cases and controls). The serum samples that were positive for ANAs were further tested for the presence of antibodies to topoisomerase I, RNP, Smith, Ro or SS-A and La or SS-B by passive immunodiffusion against calf thymus extract using commercially available kits (Inova Diagnostics Inc).13 Antibodies to RNA-pol 3 were measured by an enzyme-linked immunosorbent assay (ELISA) kit from Inova Diagnostics Inc, performed in accordance with the manufacturer’s instructions. ssDNA abs and antihistone antibodies from cases and controls were assayed by ELISA kits (Orgentec Diagnostika) according to the manufacturer’s directions in a single laboratory (H.J.). Levels of ssDNA abs higher than 20 U/mL and AHA levels higher than 40 U/mL were considered positive, according to manufacturers’ specifications.
Statistical Analysis
To examine whether the proportion of autoantibodies (ANA, ssDNA ab, AHA, or combinations thereof) was different between patients with morphea and controls (or between morphea subtypes), the 2-tailed χ2 test or Fisher exact test was used. χ2 Tests and/or Fisher exact tests were used in a similar fashion to examine the relationship between the presence of autoantibodies and functional limitation and between the presence of autoantibodies and extensive BSA. Wilcoxon rank sum tests were used to examine if there were significant differences in mRSS, LoSSI, LoSDI, PGA-A, and PGA-D scores between patients with morphea with and without autoantibodies. Because mRSSs were available for all patients, analysis for associations between mRSS and autoantibodies was performed in 187 patients. An analysis of association between LoS-CAT scores and presence of autoantibodies was performed in patients enrolled from 2008 forward. In all cases, P < .05 was considered statistically significant. Bonferroni correction for comparison between morphea subtypes and autoantibodies (ANA, AHA, ssDNA ab) was applied when appropriate. SAS version 9.2 statistical software (SAS Institute Inc) was used for all analyses.
Results
Demographic Characteristics and Clinical Features
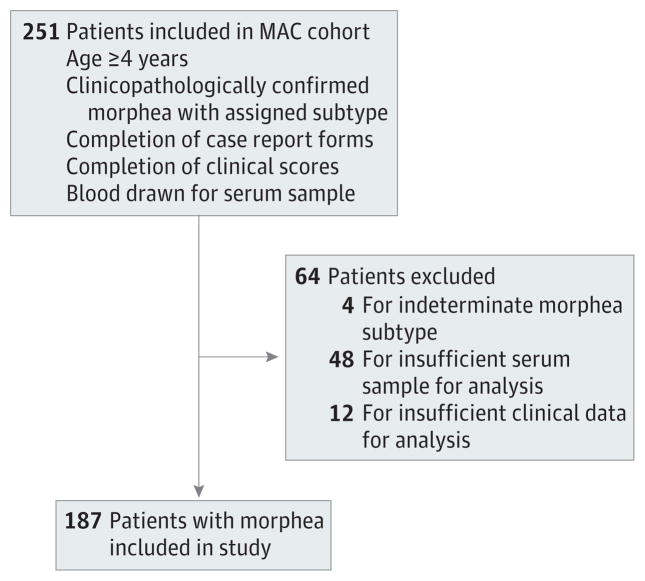
Of 251 patients in the registry, 64 were excluded because they did not meet inclusion criteria, leaving 187 patients for analysis. The details of this process are presented in Figure 1. The demographic features of study patients and controls are provided in detail in Table 2. In patients and controls, sex and ethnicity were similar, 110 (59%) had adult-onset morphea (mean [SD] age at onset, 45.3 [15.6] years), and 77 (41%) had childhood-onset morphea (mean [SD] age at onset, 10.1 [3.9] years). As previously reported, the linear subtype was predominant in patients with childhood-onset morphea (72%), while the majority of patients with adult-onset morphea had a generalized subtype (85%).
Figure 1. Algorithm Summarizing Patients Included in the Present Study.
MAC indicates Morphea in Adults and Children
Table 2.
Patient Demographics
| Characteristics | Total Group of Patients With Morphea (n = 187) | Controls (n = 651) |
|---|---|---|
| Ethnicity, No. (%) | ||
| White | 138 (74) | 493 (76) |
| Hispanic/Latino | 30 (16) | 92 (14) |
| Asian | 6 (3) | 11 (2) |
| African American | 6 (3) | 43 (7) |
| Other | 7 (4) | 12 (2) |
| Sex, No. (%) | ||
| Female | 153 (82) | 547 (84) |
| Male | 34 (18) | 104 (16) |
| Age at enrollment, mean (SD), ya | ||
| Adults | 46.2 (16.4) | 48.6 (15.5) |
| Children | 12.2 (3.12) | 11.33 (3.3) |
Age at enrollment is also the age at which a serum sample was obtained for analysis.
Autoantibody Prevalence
Table 3 highlights the prevalence of ANAs, ssDNA abs, and AHAs among patients with morphea and controls. The over-all prevalence among patients with morphea was 34% (63 of 187) for ANAs, 8% (15 of 187) for ssDNA abs, and 12% (22 of 187) for AHAs. When comparing the prevalence of antibodies among patients with morphea vs controls, the prevalence of ANAs was higher in the patients with morphea than in controls (34% [63 of 187] vs 11% [69 of 651]; P < .001), as was the prevalence of AHAs (12% [22 of 187] vs 2% [3 of 651]; P < .001). The prevalence of ssDNA abs was similar in cases and controls (8% [15 of 187] vs 7% [10 of 149]; P = .17). When comparing the prevalence of autoantibodies present in specific morphea subtypes with the controls, the only significant finding was an association between AHAs in linear morphea relative to controls (18% [15 of 85]; P = .02); however, this became statistically nonsignificant when adjusted for multiple comparisons (Bonferroni corrected value for α level is .0167).
Table 3.
Prevalence of Autoantibodies and Antinuclear Antibodies Patterns Among Morphea Subtypes and Age-, Sex-, and Race-Matched Controlsa
| Autoantibodies | Patients, No. (%)
|
P Value (vs Controls)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Morphea Subtype
|
Total Group of Patients With Morphea (n = 187) | Controlsb | Total Group of Patients With Morphea | Morphea Subtype
|
|||||
| Generalized (n = 73) | Linear (n = 85) | Plaque (n = 18) | Generalized | Linear | Plaque | ||||
| Positive ANA | 26 (36) | 28 (33) | 6 (33) | 63 (34) | 69 (11) | <.001 | NS | NS | NS |
|
| |||||||||
| Speckled | 21 (29) | 24 (28) | 4 (24) | 52 (28) | 56 (9) | <.001 | NS | NS | NS |
|
| |||||||||
| Positive | |||||||||
|
| |||||||||
| Anti-ssDNA | 3 (4) | 11 (13)c | 1 (6) | 15 (8) | 10 (7) | NS | NS | NS | NS |
|
| |||||||||
| AHA | 5 (7) | 15 (18)d | 1 (6) | 22 (12) | 3 (2) | <.001 | NS | .02e | NS |
Abbreviations: AHA, antihistone antibody; ANA, antinuclear antibody; anti-ssDNA, anti–single-stranded DNA; NS, nonsignificant.
Unless indicated, there were no other significant associations seen between autoantibodies and morphea subtypes.
For ANA, n = 651; for anti-ssDNA and antihistone, n = 149.
P = .06 for an association between anti-ssDNA and linear subtype (vs nonlinear subtypes).
P = .04 for an association between AHA and linear subtype (vs nonlinear subtypes).
Bonferroni correction for multiple comparisons would yield an α level of .0167. Because .0170 is greater than .0167, this value becomes nonsignificant with correction.
In addition to comparing the prevalence of autoantibodies between patients with morphea and controls, the prevalence of autoantibodies between the 3 most common morphea subtypes were compared to determine if an association exists between disease subtypes and a particular autoanti-body profile. Linear morphea was the only subtype in which specific autoantibodies were present with a higher frequency. They included AHAs and ssDNA abs but not ANAs. There were no associations seen in patients with generalized or plaque morphea. Antihistone antibodies were more frequently present in patients with linear morphea (18% [15 of 85]; P = .04) than in nonlinear subtypes (generalized, 5 of 73; and plaque, 1 of 18). Similarly, there was a trend toward an association of the presence of ssDNA abs in patients with linear morphea (13% [11 of 85]; P = .06) compared with nonlinear subtypes (generalized, 4% [3 of 73]; and plaque, 6% [1 of 18]), but this was not statistically significant. Five patients were simultaneously positive for ANA, AHA, and ssDNA ab and 7 were positive for AHA and ssDNA ab; all had linear morphea.
ANA Immunofluorescence Pattern
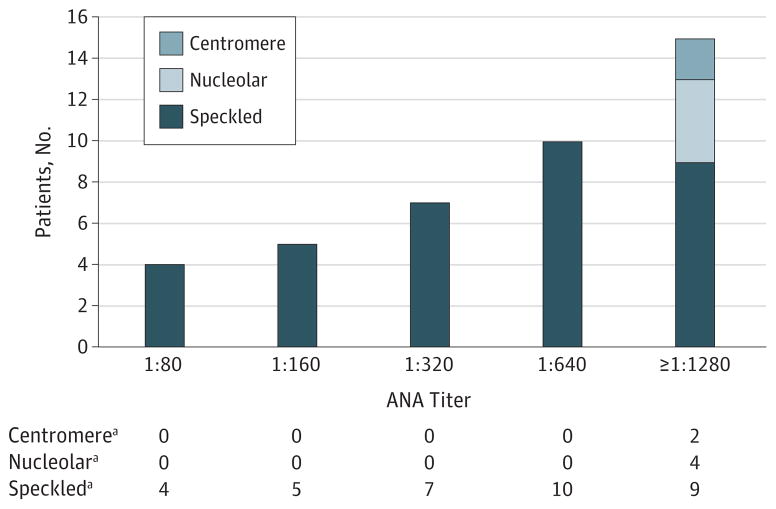
The speckled pattern on immunofluorescence of Hep-2 cells was predominant, as was observed in 81% (52 of 63) of patients with morphea positive for ANA. Other patterns were infrequently present in patients with morphea, including nucleolar, centromere, and cytoplasmic patterns, and were not more frequently present compared with controls. Serum samples that had ANA present at a cutoff titer of 1:80 were serially diluted up to a titer of 1:1280. Thirty-six samples had a titer of 1:160 or greater (Figure 2). Of the other autoantibodies tested (antibodies to Ro or SS-A, La or SS-B, Smith, RNP, Scl-70, and anti–RNA-pol 3), only 2 of the patients with morphea were positive for anti-Ro and 5 had anti–RNA-pol 3.
Figure 2. Serum Samples That Had Antinuclear Antibodies (ANAs) Present at a Cutoff Titer of 1:80 Were Serially Diluted up to a Titer of 1:1280.
The frequency (number of patients) of a positive ANA at each dilution and pattern of immunofluorescence on Hep-2 cells are presented.
aData are given as number of patients
Autoantibodies and Markers of Disease Severity
The presence of specific autoantibodies was associated with measures of morphea severity including functional limitation, extensive BSA, LoSSI, and LoSDI (Table 4). Among patients with linear morphea, the presence of ssDNA abs or AHAs was associated with functional limitation (P = .005 and P = .006, respectively). In patients with linear morphea, the presence of either ANAs or ssDNA abs was also associated with extensive BSA involvement (P = .005 and P = .01, respectively). This association was not present in other subtypes.
Table 4.
Autoantibody Associations With Clinical Markers of Disease Activity and Severity in Morphea and its Subtypesa
| Morphea Subtype/Autoantibody |
P Value
|
||||||
|---|---|---|---|---|---|---|---|
| Limited Function | BSAb | mRSS | Localized Scleroderma Skin
|
PGA
|
|||
| Severity Index | Damage Index | Activity | Damage | ||||
| Linear | |||||||
|
| |||||||
| ANA | NS | .005 | <.001 | NS | NS | NS | NS |
|
| |||||||
| Anti-ssDNA | .005 | .01 | NS | NS | NS | NS | NS |
|
| |||||||
| Antihistone | .006 | NS | NS | NS | NS | NS | NS |
|
| |||||||
| Total group of patients with morphea | |||||||
|
| |||||||
| ANA | NS | NS | <.001 | NS | NS | NS | NS |
|
| |||||||
| Anti-ssDNA | .03 | NS | NS | NS | NS | NS | NS |
|
| |||||||
| Antihistone | .007 | NS | NS | NS | NS | NS | NS |
Abbreviations: ANA, antinuclear antibody; Anti-ssDNA, anti–single-stranded DNA; BSA, body surface area; mRSS, modified Rodnan Skin Score; NS, nonsignificant; PGA, Physician Global Assessment.
Only subtypes with statistically significant associations are shown. Generalized and plaque subtypes do not have any significant associations.
Extensive BSA is defined as having 3 or more body sites involved. For limited functionality and extensive BSA, 2-tailed χ2 tests with Yates correction were used, except when one of the expected values in the 2 × 2 table is less than 5, in which case the 2-tailed Fisher exact test was used. For mRSS, Localized Scleroderma Skin Severity Index, Localized Scleroderma Skin Damage Index, PGA of Activity, and PGA of Damage, Wilcoxon rank sum tests were used.
Autoantibodies and Markers of Disease Activity
The presence of autoantibodies (ANAs, AHAs, ssDNA abs) was compared with measures of disease activity (LoSSI and PGA-A) to determine if an association exists (Table 4). There were no significant associations between the tested antibodies with morphea activity.
Autoantibodies and Concomitant Autoimmune Conditions
There were 46 of 187 patients who had concomitant autoimmune disorders confirmed by direct examination or review of medical records. Analysis for an association between the presence of ANAs, ssDNA abs, and AHAs and concomitant autoimmune conditions did not reveal any significant associations. The most common concomitant autoimmune conditions included psoriasis (7 patients), rheumatoid arthritis (7 patients), and Hashimoto thyroiditis (4 patients).
Discussion
The aim of the present study was to determine the prevalence and clinical significance of autoantibodies in morphea via a prospective case-control study of the MAC cohort. Similar to prior reports, ANAs were present with greater frequency in patients with morphea than in controls. However, ANAs were not associated with a particular morphea subtype. In addition, AHAs were present with greater frequency in morphea than in controls and were associated specifically with the linear subtype. All 3 of these autoantibodies were associated with increased disease severity in linear morphea, but not activity, underscoring their limited clinical utility.
The overall ANA prevalence of 34% in the present study was within the range of previous reports (18%–68%),5–7,14–21 although it is lower than that seen in the 2 largest prior studies.5,6 In 1987, Falanga et al6 reported presence of ANAs in 11 of 22 patients (50%) of all ages with either “generalized morphea” or “morphea,” with the latter described as having circumscribed lesions, which likely represented plaque morphea as defined by Zulian et al.1 In 2008, Arkachaisri et al5 investigated 72 patients with adult- and childhood-onset linear morphea and found ANAs present in 68%. The high prevalence of ANAs reported in these 2 studies may be due to referral bias (pediatric rheumatology practice may be enhanced with patients positive for ANAs) and selection bias (not all patients were tested a priori). Furthermore, direct comparison is difficult because the methodology and titer cutoff for determination of a positive result were also different. Nonetheless, our results confirm that ANAs are present with increased frequency at significant titers in a diverse morphea cohort vs a healthy control population, providing further evidence for the autoimmune underpinnings of morphea.
In contrast to prior reports, including our own,2 in which ANAs were more frequently present in generalized and linear subtypes, ANAs were not associated with a particular morphea subtype in the present study. This is likely due to differences in study design. Prior studies were retrospective or only tested for ANAs in a limited number of morphea subtypes. It is likely that patients with more widespread and severe morphea (eg, generalized morphea) were more likely to have had ANA testing than were those with less severe disease, which may account for the previously reported association of ANA with generalized morphea, as reported in our retrospective review.2 Furthermore, in other studies, ANAs were only determined in 1 or 2 morphea subtypes, making it difficult to accurately determine associations across the entire spectrum of morphea.5,6 In contrast, as part of enrollment of the MAC cohort, serum samples are prospectively drawn from all patients with morphea and assayed in a single laboratory. This implies that while ANAs are present with higher frequency in morphea, they are not specific to a particular subtype.
The predominance of speckled ANA pattern at a high titer was unexpected. Furthermore, most of these patients did not have commonly identified extractable nuclear antigens or an association with concomitant autoimmune conditions. These results raise the question of whether there could be an as-yet unrecognized nuclear antigen to which these serum samples are reacting. Further studies are warranted.
To date, the prevalence and clinical utility of ssDNA ab in morphea has been controversial. We found that the overall prevalence of ssDNA abs in morphea was 8%, which is similar to controls. Almost all patients with ssDNA abs had linear morphea, an association that was not statistically significant (P = .06). Although our results are similar to prior studies in that there is a trend toward an association between ssDNA ab and linear subtype,5,14,15 the prevalence is far lower (29%–39% in prior reports).5,6 We confirmed that AHA may be more frequently present in patients with linear morphea than in controls (18%) (although this association did not retain significance with correction for multiple comparisons), but we failed to find an association between the presence of these AHAs and generalized morphea in contrast to prior reports by Sato et al.7 The prevalence of antihistone antibodies in our study was also lower than in prior studies (39%–42% in prior reports).5,7 These differences may represent referral bias among rheumatologist-assembled cohorts as well as differences in study populations and assays. However, our results indicate that among patients with morphea in general, these autoantibodies are infrequently present, even among the linear subtype cases.
The assayed autoantibodies were of limited clinical significance, with the exception of patients with linear morphea. In patients with linear morphea, presence of ssDNA abs was associated with both extensive BSA and functional limitation, and a positive AHA was associated with extensive BSA, findings that are similar to those of Arkachaisri and colleagues.5 Among patients with linear morphea, we also found an association between the presence of ANAs and extensive BSA, findings that differ from those of Arkachaisri et al,5 who did not find this association in their population. Among the clinical scoring systems (mRSS and the components of the LoSCAT), the only association was between positive ANA and higher mRSS specifically in patients with linear morphea. We suspect that this finding relates predominantly to the BSA involvement aspect of the mRSS and the relatively few patients who had LoSCAT scores available. We did not find an association between autoantibodies and clinical markers of disease activity (namely, LoSSI or PGA-A). This finding is consistent with the results of Arkachaisri et al,5 who did not find an association between autoantibodies and “clinical disease activity,” defined as the development of lesional erythema or a new or enlarging lesion within the prior 6 months.5 Thus, AHAs and possibly ssDNA abs may be useful markers of increased disease severity in patients with linear morphea (although they are rarely present).
Our study, while the largest of its kind, is still limited by small sample size, especially with regard to certain morphea subtypes and the relative infrequent presence of the tested auto-antibodies. The same is true of the association between AHAs, ss-DNA abs, and morphea activity because very few patients with morphea had these autoantibodies. Also, some associations fail to retain statistical significance with adjustment for multiple comparisons, specifically the association of AHA and linear subtype, and should be interpreted with caution.
Our results have several implications for practice. First, testing for ANAs, AHAs, and ssDNA abs is most useful for patients with linear morphea as a potential marker of disease severity. There is little indication for repeated testing of these autoantibodies as a marker of disease activity or remission, although longitudinal studies are needed for confirmation. Unfortunately, despite these associations, these antibodies are relatively infrequently present, even among patients with linear morphea. This underscores the need for studies to identify biomarkers relevant to morphea.
Acknowledgments
Funding/Support: Research for this manuscript was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health (NIAMS/NIH) grant K23AR056303-4. This work was conducted with support from University of Texas–Science Teacher Access to Resources at Southwestern (UT-STARS), National Institutes of Health/National Center for Research Resources/National Center for Advancing Translational Sciences grant UL1RR024982, and NIAMS/NIH grant 1R01 AR055258.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STARS, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, or the National Institutes of Health.
Additional Information: Dr Ahn, Department of Clinical Sciences, University of Texas Southwestern Medical Center, is the biostatistician.
Author Contributions: Dr Jacobe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Warner Dharamsi, Ahn, Arnett, Mayes, Jacobe.
Acquisition of data: Warner Dharamsi, Victor, Aguwa, Arnett, Jacobe.
Analysis and interpretation of data: Warner Dharamsi, Ahn, Arnett, Mayes, Jacobe.
Drafting of the manuscript: Warner Dharamsi, Victor, Ahn, Mayes, Jacobe.
Critical revision of the manuscript for important intellectual content: Warner Dharamsi, Aguwa, Arnett, Mayes, Jacobe.
Statistical analysis: Ahn, Jacobe.
Obtained funding: Warner Dharamsi, Jacobe.
Administrative, technical, and material support: Victor, Aguwa, Arnett, Mayes, Jacobe. Study supervision: Warner Dharamsi, Jacobe.
Additional Contributions: Rose Ann Cannon and Loderick Matthews, BS, provided administrative and laboratory support.
References
- 1.Zulian F, Athreya BH, Laxer R, et al. Juvenile Scleroderma Working Group of the Pediatric Rheumatology European Society (PRES) Juvenile localized scleroderma: clinical and epidemiological features in 750 children: an international study. Rheumatology (Oxford) 2006;45(5):614–620. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 2.Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145(5):545–550. doi: 10.1001/archdermatol.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martini G, Murray KJ, Howell KJ, et al. Juvenile-onset localized scleroderma activity detection by infrared thermography. Rheumatology (Oxford) 2002;41(10):1178–1182. doi: 10.1093/rheumatology/41.10.1178. [DOI] [PubMed] [Google Scholar]
- 4.Li SC, Liebling MS, Haines KA. Ultrasonography is a sensitive tool for monitoring localized scleroderma. Rheumatology (Oxford) 2007;46(8):1316–1319. doi: 10.1093/rheumatology/kem120. [DOI] [PubMed] [Google Scholar]
- 5.Arkachaisri T, Fertig N, Pino S, Medsger TA., Jr Serum autoantibodies and their clinical associations in patients with childhood- and adult-onset linear scleroderma: a single-center study. J Rheumatol. 2008;35(12):2439–2444. doi: 10.3899/jrheum.080098. [DOI] [PubMed] [Google Scholar]
- 6.Falanga V, Medsger TA, Jr, Reichlin M. Antinuclear and anti-single-stranded DNA antibodies in morphea and generalized morphea. Arch Dermatol. 1987;123(3):350–353. [PubMed] [Google Scholar]
- 7.Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Clinical characteristics associated with antihistone antibodies in patients with localized scleroderma. J Am Acad Dermatol. 1994;31(4):567–571. doi: 10.1016/s0190-9622(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim A, Marinkovich N, Jacobe H. Clinical features of generalized morphea patients with the pansclerotic subtype: a prospective study from the Morphea in Adults and Children (MAC) cohort. Presented as a poster at: Annual Meeting of the Rheumatologic Dermatology Society (RDS); November 10, 2012; Washington, DC. [Google Scholar]
- 9.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–613. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 10.Johnson W, Jacobe H. Morphea in Adults and Children cohort II: patients with morphea experience delay in diagnosis and large variation in treatment. J Am Acad Dermatol. 2012;67(5):881–889. doi: 10.1016/j.jaad.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Furst DE, Clements PJ, Steen VD, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25(1):84–88. [PubMed] [Google Scholar]
- 12.Arkachaisri T, Vilaiyuk S, Li S, et al. Localized Scleroderma Clinical and Ultrasound Study Group. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36(12):2819–2829. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora-Singh RK, Assassi S, del Junco DJ, et al. Autoimmune diseases and autoantibodies in the first degree relatives of patients with systemic sclerosis. J Autoimmun. 2010;35(1):52–57. doi: 10.1016/j.jaut.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falanga V, Medsger TA, Jr, Reichlin M. High titers of antibodies to single-stranded DNA in linear scleroderma. Arch Dermatol. 1985;121(3):345–347. doi: 10.1001/archderm.121.3.345. [DOI] [PubMed] [Google Scholar]
- 15.Falanga V, Medsger TA, Jr, Reichlin M, Rodnan GP. Linear scleroderma: clinical spectrum, prognosis, and laboratory abnormalities. Ann Intern Med. 1986;104(6):849–857. doi: 10.7326/0003-4819-104-6-849. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa I, Hasegawa M, Takehara K, Sato S. Anti-DNA topoisomerase IIα autoantibodies in localized scleroderma. Arthritis Rheum. 2004;50(1):227–232. doi: 10.1002/art.11432. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi K, Takehara K, Ishibashi Y. Antinuclear antibodies in localized scleroderma: unique staining in chromosome spreads. J Am Acad Dermatol. 1989;21(6):1301–1303. doi: 10.1016/s0190-9622(89)80310-x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg AM, Uziel Y, Krafchik BR, et al. Antinuclear antibodies in children with localized scleroderma. J Rheumatol. 1995;22(12):2337–2343. [PubMed] [Google Scholar]
- 19.Ruffatti A, Peserico A, Rondinone R, et al. Prevalence and characteristics of anti-single-stranded DNA antibodies in localized scleroderma: comparison with systemic lupus erythematosus. Arch Dermatol. 1991;127(8):1180–1183. [PubMed] [Google Scholar]
- 20.Sato S, Ihn H, Soma Y, et al. Antihistone antibodies in patients with localized scleroderma. Arthritis Rheum. 1993;36(8):1137–1141. doi: 10.1002/art.1780360815. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Kodera M, Hasegawa M, Fujimoto M, Takehara K. Antinucleosome antibody is a major autoantibody in localized scleroderma. Br J Dermatol. 2004;151(6):1182–1188. doi: 10.1111/j.1365-2133.2004.06256.x. [DOI] [PubMed] [Google Scholar]