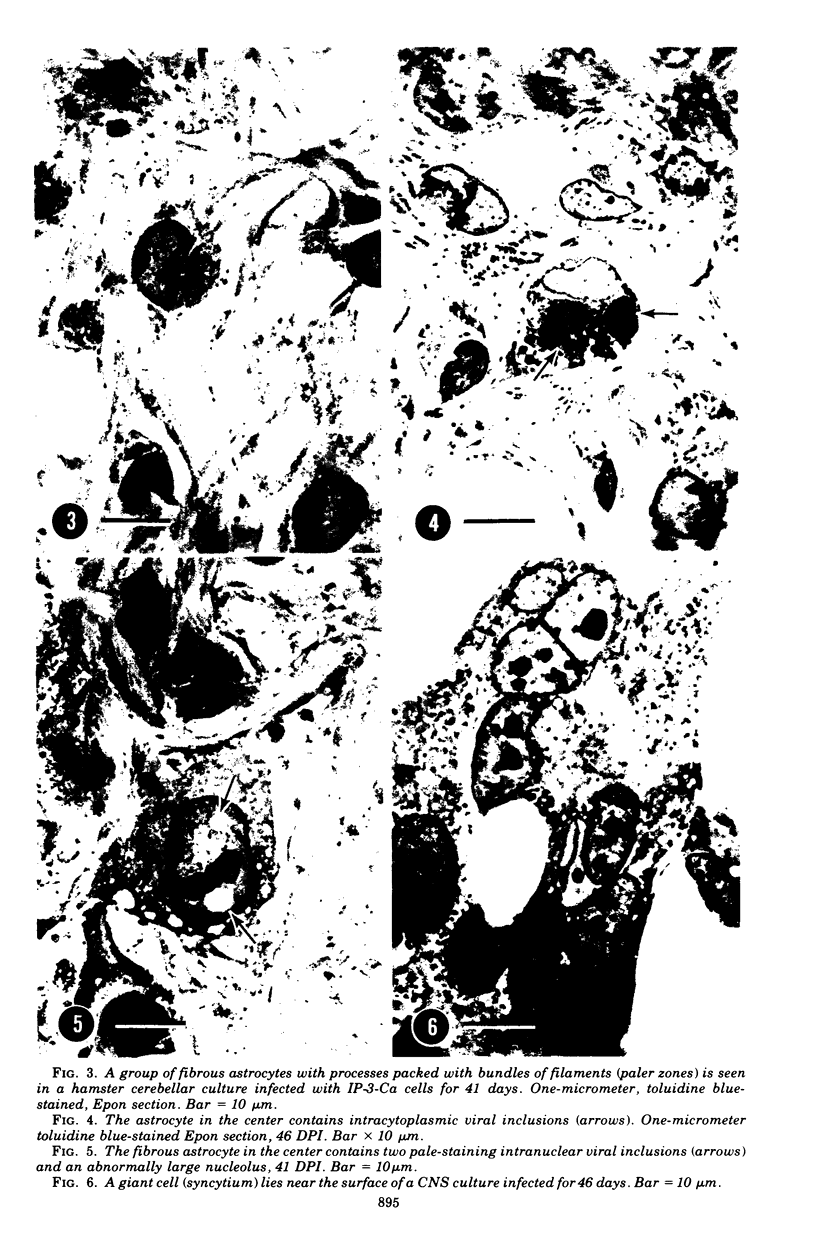
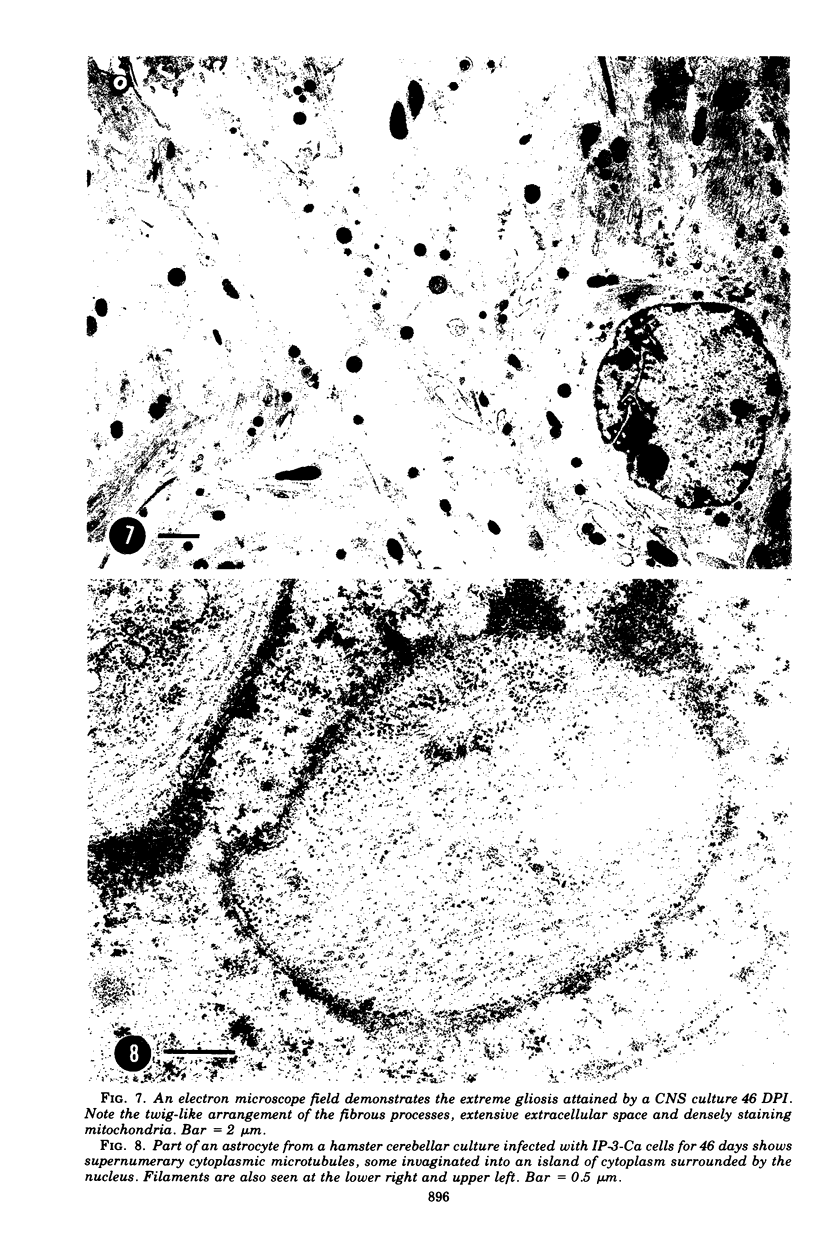
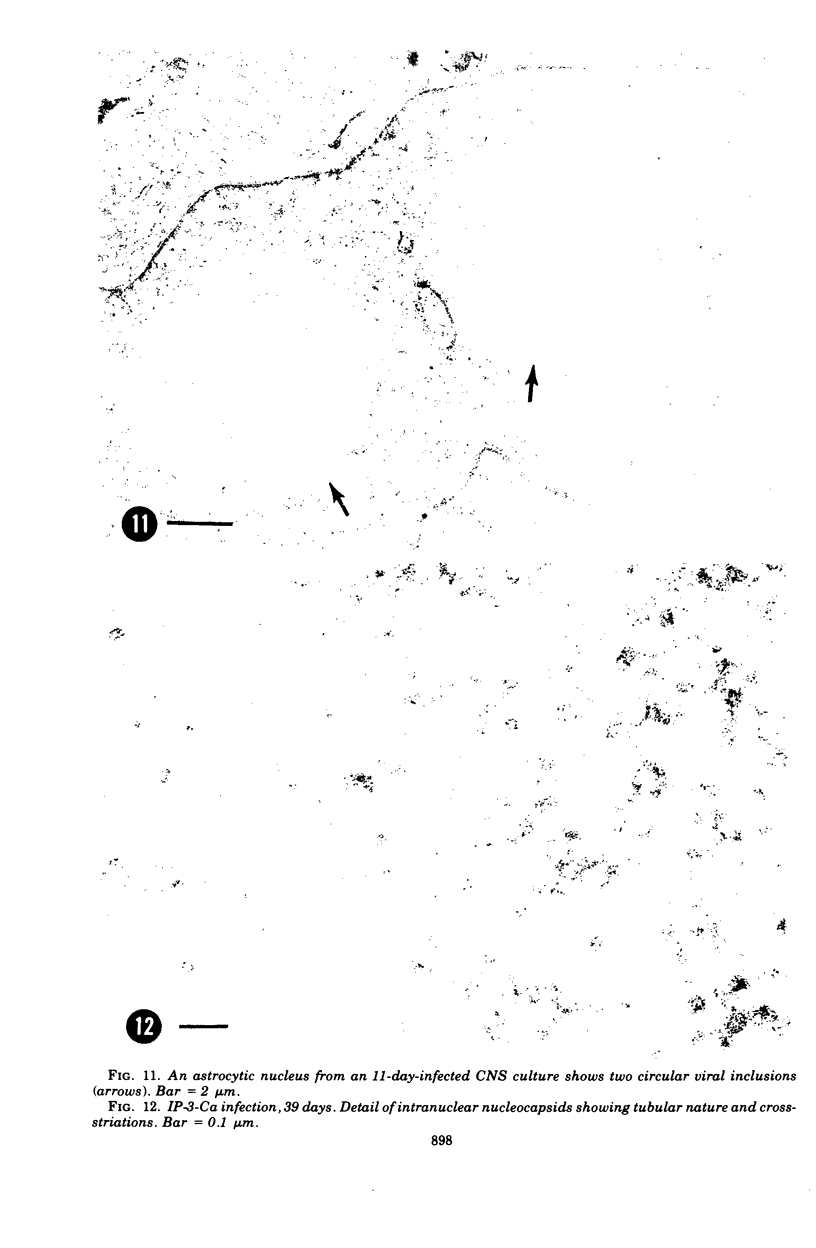
Abstract
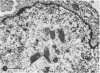
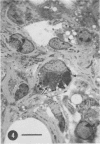
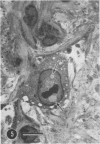
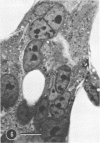
Organotypic cultures of hamster cerebellum were exposed to the IP-3-Ca cell line , which contains a cell-associated subacute sclerosing panencephalitis agent. Central nervous system (CNS) cultures were examined by light and electron microscopy as well as standard virological techniques from 3 to 46 days postinfection. The results indicate that although viral nucleocapsid material was transferred to elements of the CNS, cell-free virus could not be detected by virological techniques and by electron microscopy, and budding viral particles were not observed. Attempts to recover cell-free virus from hamster CNS tissue exposed to IP-3-Ca cells were generally negative. However, 2% of the cultures yielded low levels of infectious virus. IP-3-Ca cells were able to transfer the cell-associated viral material to all cell types found in the CNS cultures and were capable of inducing polykaryocytes in the CNS cultures. The role of cell-associated virus-like agents in subacute sclerosing panencephalitis and other chronic CNS infections is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN M. B., MURRAY M. R. Serial observations on patterns of growth, myelin formation, maintenance and degeneration in cultures of new-born rat and kitten cerebellum. J Biophys Biochem Cytol. 1958 Sep 25;4(5):499–504. doi: 10.1083/jcb.4.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringer J. R., Griffith J. F. Experimental measles virus encephalitis. A light, phase, fluorescence, and electron microscopic study. Lab Invest. 1970 Sep;23(3):335–346. [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Doi Y., Sanpe T., Nakajima M., Okawa S., Koto T. Properties of a cytopathic agent isolated from a patient with subacute sclerosing panencephalitis in Japan. Jpn J Med Sci Biol. 1972 Oct;25(5):321–333. doi: 10.7883/yoken1952.25.321. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H., Hamilton R., Sever J. L. Comparison between productive and latent subacute sclerosing panencephalitis viral infection in vitro. An electron microscopic and immunoperoxidase study. Lab Invest. 1974 Mar;30(3):241–250. [PubMed] [Google Scholar]
- Feldman L. A., Raine C. S., Sheppard R. D., Bornstein M. B. Virus-host cell relationships in measles-infected cultures of central nervous tissue. J Neuropathol Exp Neurol. 1972 Oct;31(4):624–638. doi: 10.1097/00005072-197210000-00006. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- Notkins A. L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974 Mar 30;11(1-3):478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Jr, Klintworth G. K., Graham D. G., Griffith J. F. Uncommon morphologic features in subacute sclerosing panencephalitis (SSPE). Report of two cases with virus recovery from one autopsy brain specimen. Am J Pathol. 1970 Nov;61(2):275–292. [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Barbosa L. H., Bornstein M. B. Subacute sclerosing panencephalitis virus. Observations on a neuroadapted and non-neuroadapted strain in organotypic central nervous system cultures. Lab Invest. 1974 Jul;31(1):42–53. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructural study of long-term measles infection in cultures of hamster dorsal-root ganglion. J Virol. 1971 Sep;8(3):318–329. doi: 10.1128/jvi.8.3.318-329.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructure of measles virus in cultures of hamster cerebellum. J Virol. 1969 Aug;4(2):169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]