Abstract
Recently we reported (Mehra et al., 2013), that lung granulomas from Mycobacterium bovis Bacille Calmette–Guérin (BCG)-vaccinated cynomolgus macaques exhibit upon challenge with M. tuberculosis a more balanced expression of α- and β-chemokines, relative to comparable samples from sham-vaccinated animals by comparative transcriptomics. Here, we studied the recruitment of immune cells to blood and lungs in M. tuberculosis-infected macaques as a function of prior BCG-vaccination. Vaccination initially enhanced the levels of both macrophages and lymphocytes in blood. In contrast, significantly more CD4+ lymphocytes were later recruited to the lungs of sham-vaccinated animals compared with earlier times/BCG vaccinated animals. BCG-vaccination had a short-lived impact on the anti-M. tuberculosis response. M. tuberculosis continued to replicate in the lung even in the wake of increased CD4+ T cell recruitment to primate lungs, indicating that immune subversive mechanisms are key to its survival in vivo.
Keywords: M. tuberculosis, BCG, Animal model, Granuloma, Macrophage, Lymphocyte
Introduction
Tuberculosis is responsible for the death of over 1.5 million people every year [1]. This situation has worsened due to the emergence of drug-resistance [2], HIV co-infection and the insufficient protection of the Bacillus Calmette–Guérin (BCG) vaccine, which is unable to protect adults against pulmonary TB [3,4]. The efficacy of BCG in protecting against adult pulmonary TB is unsatisfactory [5]. New and efficacious vaccines against TB are therefore urgently needed [6,7]. This requires both a better understanding of the correlates of protection in appropriate experimental models [8,9], and also a clearer understanding of the shortcomings of BCG such that they can be avoided with future vaccine design.
Nonhuman Primates (NHPs), such as rhesus [10–13] or cynomolgus [11,14] macaques are excellent models of Mycobacterium tuberculosis (Mtb) infection [15]. Upon experimental infection via either the intrabronchial or the aerosol route, these animals recapitulate the entire breadth of the human TB infection syndrome, including acute TB [10] characterized by massive caseous immunopathology [13] and asymptomatic, latent infection (LTBI). Furthermore, in macaques, LTBI can be reactivated by SIV co-infection [12], TNFα inhibition [12,16] or CD4-depletion [17]. More importantly, macaques demonstrate a spectrum of lung pathological outcomes upon experimental infection with Mtb, similar to naturally infected human beings [10–13,15,18–20]. Akin to humans, vaccination with BCG can protect NHPs against Mtb challenge, but the protection is incomplete [11,21,22]. These observations reinforce our contention that macaques best represent the human TB infection syndrome, allowing a study of pathology as well as protective responses not possible in other model systems [23].
It is known that prior BCG vaccination leads to a higher expression of β-chemokines in lung lesions following Mtb challenge [11]. Vaccinated animals expressed a better balance of α- and β-chemokines in lung granulomas at week 5, relative to unvaccinated NHPs, which only expressed high levels of α- but not β-chemokines. Unsurprisingly, more macrophages were present in the lesions of vaccinated relative to sham-vaccinated macaques at earlier time points, as many β-chemokines promote specific monocyte/macrophage chemotaxis.
We reasoned that the presence of significantly more macrophages in the lesions of BCG-vaccinated primates causes a more robust antigen-presentation against the pathogen resulting in the generation of a more effective adaptive immune response. Here we compared both systemic and local cellular responses to Mtb infection in the two groups of BCG and sham vaccinated-NHPs by phenotyping for effector cells in blood, broncho-alveolar lavage (BAL) and in granulomatous as well as non-granulomatous regions of the lung at necropsy.
Materials and methods
Animals, vaccination, challenge, sample collection and phenotypic analyses of immune cells
Blood and BAL samples were obtained from BCG- and sham-vaccinated cynomolgus macaques (2/group), which were challenged with highly virulent Mtb Erdman 17 weeks post-vaccination [11]. Lung (granuloma as well as adjoining normal) samples were obtained from necropsy of two animals each in both groups at week 5 and 10 post-infection, a cellular fraction obtained by suspending the tissue samples in complete Roswell Park Memorial Institute (RPMI) medium containing collagenase and proteinase-K (1 h, 37 °C) and immunophenotyped as described [18]. The experimental design and the schedule/intervals at which the various blood, BAL and lung samples were collected for immunophenotyping are shown in Fig. 1. For flow cytometry, cells were stained with appropriately diluted concentrations of specific monoclonal antibodies are shown in Table 1.
Fig. 1.
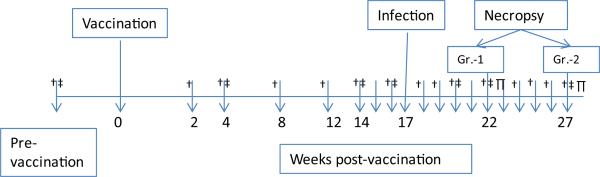
Vaccination, challenge and sampling schedule. Cynomolgus macaques were divided into two groups (BCG- and sham-vaccinated control) of four per group as follows: Animals Group 1: IF16 & IF21 (BCG, TB + 5 weeks); IF23 & IF18 (saline, TB + 5 weeks). Animals Group 2: IF20 & IF17 (BCG, TB + 10 weeks); IF19 & IF22 (saline, TB + 10 weeks). Groups 1 and 2 were sacrificed after 5 and 10 weeks post-challenge with Mycobacterium tuberculosis Erdman (250–500 CFU, bronchoscopically), respectively. Whole blood (†), BAL (‡) and lung (g=P) samples were collected at indicated time points.
Table 1.
List of antibodies used for FACS analysis.
| Antibody | Fluorochrome | Cat# | Company | Volume used per reaction (μl) |
|---|---|---|---|---|
| CD8 | PE-TR | MHCD0817 | Caltag | 3 |
| CXCR4 | PE-Cy5 | 555975 | BD Biosciences | 20 |
| CD4 | PerCP-Cy5.5 | 552838 | BD Biosciences | 10 |
| CD14 | AL700 | 557923 | BD Biosciences | 10 |
| CD69 | APC Cy7 | 557756 | BD Biosciences | 5 |
| CD3 | PB | 558124 | BD Biosciences | 7 |
| CASP3 | AL647 | 558124 | BD Biosciences | 20 |
| CD68 | FITC | F7135 | Dako | 20 |
| CD163 | PE | 556018 | BD Biosciences | 20 |
Data evaluation and statistical analysis
Data was analyzed using FlowJo version 9.5.2 and statistical comparisons utilized ONE-way ANOVA with Bonferroni multiple hypothesis correction within GraphPad Prism, a P value of <0.05 was considered significant.
Results
Recruitment of macrophages in peripheral blood and lungs in response to Mtb infection in BCG-vaccinated and sham-vaccinated NHPs
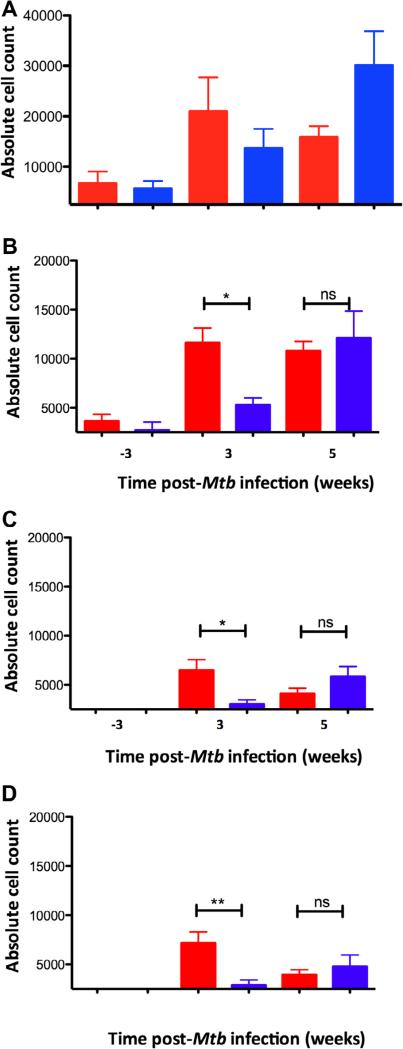
Significantly more macrophages (CD14+ CD68+) were recruited to peripheral blood of BCG vaccinated compared to sham-vaccinated NHPs at an early stage (week 3) post-Mtb infection (Fig. 2A). This is consistent with our earlier report [11] that more macrophages are present within early granulomatous lesions derived from BCG-vaccinated rather than sham-vaccinated NHPs. The increase in blood macrophages is possibly, in part due to increased trafficking of these cells to the lungs of vaccinated animals. That observation, using confocal microscopy, is itself consistent with our transcriptomics data, where TB lesions derived from BCG-vaccinated, Mtb-challenged animals expressed greater amounts of CCL2 and CCL3 (molecules primarily responsible for macrophage recruitment) at week 5 [11] but not at the later time-points. The timing of the immunophenotyping observations was also consistent with the chemokine expression data. Similarly, our immunophenotyping data also indicates that while early on (at week 3), more macrophages were recruited to the lungs of animals that received vaccination, by the later time point (week 5), the higher recruitment of macrophages to the lung of vaccinated animals dissipated and in fact more macrophages were recruited to the lungs of sham vaccinated animals. It is important to note that Mtb replication levels, while undetectable for the first few weeks, were significantly higher in the sham-vaccinated animals relative to the BCG-vaccinated animals at the latter time-point (week 5). Thus, the higher macrophage recruitment levels in the sham-vaccinated animals at week 5 could be attributed to differences in antigen load. Monocytes generated in bone marrow enter the peripheral blood and in response to the appropriate chemotactic signal home to lungs where they differentiate into activated macrophages that can recognize and begin innate phagocytosis of Mtb. Thus, one of the mechanisms by which BCG vaccination may confer partial protection against Mtb infection may be via increased accumulation of innately activated macrophages.
Fig. 2.
Accumulation of macrophages and lymphocytes in whole blood following Mtb infection as a function of prior BCG vaccination. Absolute cell counts in whole blood are shown (on Y-axis) for BCG-vaccinated (red) and sham-vaccinated (blue) NHPs. The numbers on the X-axis indicate weeks prior to or post-Mtb infection. (A) CD68+ macrophages in whole blood. (B) CD3+ cells, (C) CD3+CD4+ cells, (D) CD3+CD8+ cells in whole blood. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Recruitment of lymphocytes in peripheral blood and lungs in response to Mtb infection in BCG-vaccinated and sham-vaccinated NHPs
Granuloma formation requires the interplay between both innate and adaptive immunity. Hence, we analyzed the recruitment of T cells following Mtb-infection as a function of vaccination. NHPs in the BCG-vaccinated group exhibited a significant increase in the peripheral blood levels of total CD3+, CD3+CD4+ and CD3+CD8+ cells at 3 weeks post-infection (Fig. 2B–D). By week 5, the total number of CD3+, CD4+ and CD8+ T cells present in the peripheral blood of sham-vaccinated NHPs were higher than in the vaccinated NHPs and the differences were no longer statistically significant (Fig. 2B–D), although there remained more T cells in the vaccinated group. The absolute lymphocyte counts further increased at later time-points in the unvaccinated (week 7 and 9) (data not shown). We also analyzed if CD4+ cells from the vaccinated group also exhibited a higher level of activation, as determined by the co-expression of CD69 (week 8) as well as CD28 and CD95 (week 3 and 8) (data not shown). Distinct populations of T cells were typed as belonging to naïve (CD28+CD95– ), central memory (CD28+− CD95+) or effector memory (CD28 CD95+) phenotypes. However, we were unable to detect any significant differences in these sub-populations between the vaccinated and the sham-vaccinated group. Our results showing a higher absolute recruitment of CD4+ T cells in the absence of any increase in their activation levels indicates that BCG vaccination allows for an earlier recruitment of potentially antigen-specific T cells upon Mtb infection. This may potentially represent another possible mechanism by which BCG partially protects against TB.
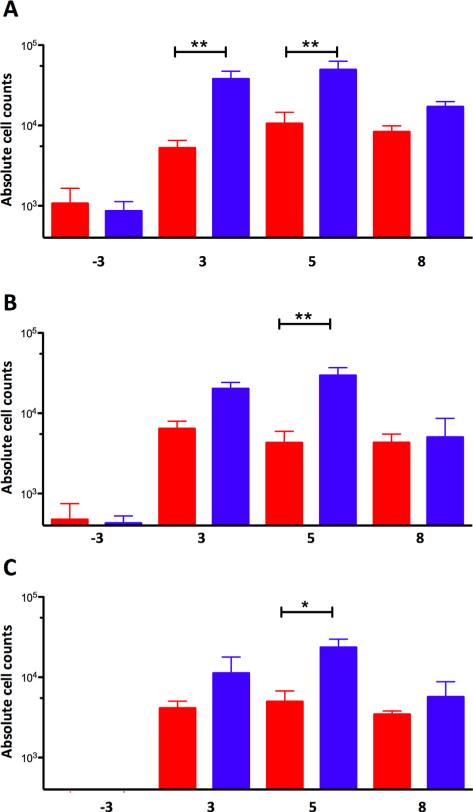
While significantly more lymphocytes were present in whole blood immediately following infection, a different result was observed in lungs. In BAL, significantly higher numbers of CD3+, CD4+ and CD8+ T cells were obtained from sham-vaccinated rather than BCG-vaccinated NHPs at all times observed (weeks 3, 5 and 8) post-Mtb infection (Fig. 3). Hence, the recruitment of lymphocytes to the lung occurred largely in an antigen-dependent manner, as a higher Mtb load was prevalent in the lungs of sham- rather than BCG-vaccinated macaques.
Fig. 3.
Accumulation of macrophages and lymphocytes in lung samples, following Mtb infection as a function of prior BCG vaccination. Absolute cell counts in BAL samples are shown (on the Y-axis) for BCG-vaccinated (red) and sham-vaccinated (blue) NHPs. X-axis in all cases represents time post-Mtb challenge. Thus, a time-point denoted as 3 corresponds to a post-vaccination time-point 3-weeks prior to Mtb challenge. (A) CD3+ cells. (B) CD3+CD4+ cells. (C) CD3+CD8+ cells. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
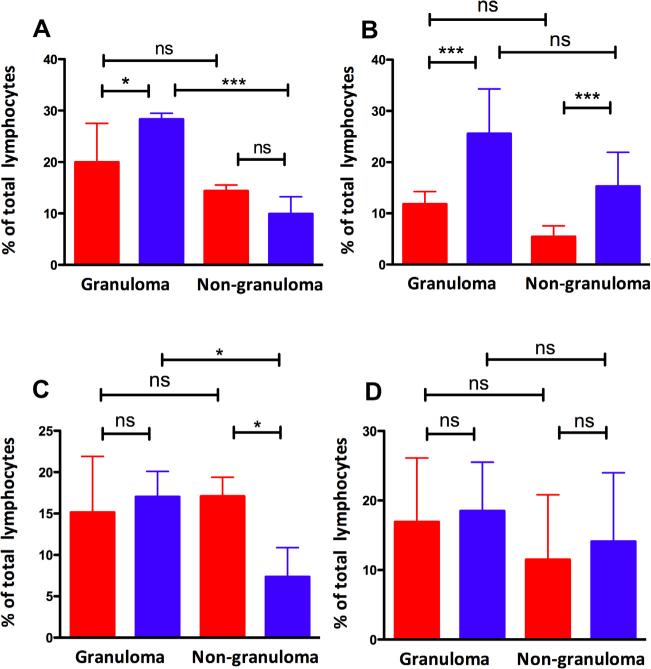
Next, we analyzed the total number of lymphocytes and their activation status in the lung lesions derived from the two groups of animals, relative to adjoining non-lesion areas. At week 5, a statistically significant higher number of CD4+ T cells were present in the lung lesions derived from sham-vaccinated, relative to vaccinated NHPs (Fig. 4A). Importantly, this difference, at least at week 5, was restricted to the granulomatous lesions, because the CD4+ T cell counts were not different in normal tissue isolated from lung regions adjoining granulomatous lesions (Fig. 4A). We conclude that at this time, the T cells being recruited in an antigen-specific manner were specifically routed to the granuloma. These differences increased in both magnitude and significance in lesions, but not in normal tissue at week 10 (Fig. 4B). We also analyzed the total CD8+ T cell levels in these samples and observed no differences between the BCG vaccinated and sham-vaccinated animals and between granuloma and adjoining tissue at either 5 or 10 weeks (Fig. 4C, D). Thus, the excess recruitment of T cells, in response to the higher replication of Mtb in the sham-vaccinated group, only manifested itself in the case of CD4 T cells.
Fig. 4.
CD4+ T cells expressed as a % of total lymphocytes are shown for lung (granuloma as well as non-granuloma) samples on the Y-axis for BCG-vaccinated (red) and sham-vaccinated (blue) NHPs at both 5 (A) and 10-week (B) post-Mtb infection. CD8+ T cells expressed as a % of total lymphocytes are shown for lung (granuloma as well as nongranuloma) on the Y-axis for BCG-vaccinated (red) and sham-vaccinated (blue) NHPs at both 5 (C) and 10-week (D) post-Mtb infection. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion
Long-term control of TB requires the development of efficacious vaccines [24]. This in turn requires an understanding of the immune responses that have the potential to protect against increasingly virulent Mtb infections. The outcome of infection with Mtb – acute TB, latent infection or reactivation, is decided at the level of the granuloma [25,26].
Primate granulomas exhibit highly organized architecture, commonly with central necrosis surrounded by a peripheral rim of fibrosis [11,18]. This is critical for containing Mtb replication, and conversely, permitting the latent survival of the pathogen [27,28]. Recently, it has been proposed that Mtb may actively promote granulomatous inflammation as part of its life cycle, since granulomas allow it to persist and disseminate [29–31]. However, Mtb must manage this Th1 response through novel mechanisms [32,33]. Hence, we characterized the cellular response in NHP granuloma's, as an addendum to characterization at the molecular level [11,13,18] and compared it to that observed in blood and BAL.
Vaccinated animals initially recruited significantly higher numbers of macrophages, as well as CD3+ and CD3+CD4+ lymphocytes in peripheral blood upon Mtb challenge, relative to sham-vaccinated animals. However, these differences rapidly normalized and by 5 weeks post-Mtb infection, higher lymphocyte recruitment occurred in sham-vaccinated animals, in an antigen load-dependent manner. Moreover, immunophenotyping of both lung tissue and BAL showed higher CD4+ T cell counts in sham-vaccinated relative to BCG-vaccinated macaques during early time-points (Fig. 2). The differences manifested themselves primarily in the granulomatous lesions obtained from sham-vaccinated relative to BCG-vaccinated macaques at week 5 and further increased at week 10. The differences were not observed for the surrounding non-granulomatous normal tissue and were specific to lesion tissue only. Significant differences were also not observed for CD8+ T cells at either 5 or 10 weeks. Thus, recruitment and accumulation of likely antigen-specific, Th1-type, activated CD4+ T cells in granulomatous lesions occurred as a function of antigen load. However, the accumulation of these CD4+ T cells in the granulomas of sham-vaccinated NHPs failed to check both the replication of Mtb and the onset of acute TB at this site [11].
Since CD4+ T cells are absolutely required for the protective immune response to Mtb infection [34], our results suggest that their recruitment to the lung by itself is insufficient in controlling the infection. This underscores the ability of Mtb in modulating host immunity and persisting in the wake of protective immune responses.
One mechanism by which Mtb may survive in lungs is by inducing IDO, which encodes indoleamine-2,3,-dioxygenase. Its expression is highly induced in lungs following Mtb infection in an antigen-specific manner and occurs exclusively within macrophages in the inner part of the granuloma closer to the central necrotic region [11]. Since IDO exerts powerful T cell suppressive effects, this expression pattern could prevent activated T cells from accessing the central regions of the granuloma, potentially preventing the exacerbation of immunopathology. A similar mechanism has been uncovered in lung granulomas formed by Listeria monocytogenes infection [35].
While preventing immunopathology, IDO may also inadvertently provide Mtb with a way to survive in the face of activated CD4 cells, by forming a physical barrier. This likely allows distance to be maintained between macrophages infected with Mtb, and CD4+ cells capable of eliminating the pathogen. Antigens secreted by the necrosis of macrophages infected with Mtb potentially causes increased lymphocyte influx to the lesion, but the expression of IDO around the ring-wall prevents them from directly engaging Mtb. Hence, an increased antigen-specific CD4+ T cell response in the sham-vaccinated NHPs is unable to check Mtb replication in NHP lung granulomas. Removal of IDO by inhibitors or siRNA, or the removal of T cells from this microenvironment, may in fact overcome this inhibitory effect. Experiments are underway to address this question. In this regard, it is important to point to the work by Rubin and coworkers [36], who recently found that Mtb can in fact synthesize tryptophan, thus countering the immune-function of IDO in the granuloma.
Acknowledgements
This work was supported by National Institutes of Health Grants (HL106790, AI089323, AI091457, RR026006, RR020159, OD011104, AI058609); and by support from the Louisiana Vaccine Center, the Tulane Research Enhancement Fund, the Tulane Center for Infectious Diseases, the Tulane National Primate Research Center Office of the Director, and the Tulane University Office of the Vice-President for Research.
Abbreviations
- NHP
Nonhuman Primate
- TB
tuberculosis
- Mtb
Mycobacterium tuberculosis
- BCG
Bacille Calmette–Guerin
- BAL
broncho-alveolar lavage
Footnotes
The Tulane National Primate Research Center facilities are accredited by the American Association for Accreditation of Laboratory Animal Care and licensed by the US Department of Agriculture. All animals were routinely cared for according to the guidelines prescribed by the NIH Guide to Laboratory Animal Care. NHP studies were conducted following the recommendations of the institutional animal care and use committee. Humane endpoints were pre-defined in this protocol.
The procedures reported in this manuscript were approved by the relevant oversight committees (IACUC and IBC).
Author contributions
Funding – D.K.; research design – D.K., S.M.; research – N.K.D.; data analysis – S.M., J.B.M.; writing – D.K. with input from N.K.D., J.B.M. and S.M.
Conflict of interest
The authors declare no conflict of interest in connection with this manuscript.
References
- 1.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 2.Phillips L. Infectious disease: TB's revenge. Nature. 2013;493:14–16. doi: 10.1038/493014a. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 4.Orme IM. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 5.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 6.Lalvani A, Sridhar S, von Reyn CF. Tuberculosis vaccines: time to reset the paradigm? Thorax. 2013;68:1092–1094. doi: 10.1136/thoraxjnl-2013-203456. [DOI] [PubMed] [Google Scholar]
- 7.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray DN. A nonhuman primate model for preclinical testing of new tuberculosis vaccines. Clin. Infect. Dis. 2000;30(Suppl. 3):S210–S212. doi: 10.1086/313885. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, et al. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin. Vaccine Immunol.: CVI. 2010;17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta NK, Mehra S, Didier PJ, Roy CJ, Doyle LA, Alvarez X, et al. Genetic requirements for the survival of tubercle bacilli in primates. J. Infect. Dis. 2010;201:1743–1752. doi: 10.1086/652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J. Infect. Dis. 2013;207:1115–1127. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, et al. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J. Med. Primatol. 2011;40:233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra S, Golden NA, Stuckey K, Didier PJ, Doyle LA, Russell-Lodrigue KE, et al. The Mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. J. Infect. Dis. 2012;205:1203–1213. doi: 10.1093/infdis/jis102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roodgar M, Lackner A, Kaushal D, Sankaran S, Dandekar S, Trask JS, et al. Expression levels of 10 candidate genes in lung tissue of vaccinated and TB-infected cynomolgus macaques. J. Med. Primatol. 2013;42:161–164. doi: 10.1111/jmp.12040. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. J. Med. Primatol. 2012;41:191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res. Hum. Retroviruses. 2012;28:1693–1702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, et al. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS ONE. 2010;5:e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet. Pathol. 2012;49:423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- 20.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu. Rev. Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 21.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, et al. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS ONE. 2009;4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushal D, Bohm RP, Jr., Lackner AA. How well do you know your monkeys? J Med. Primatol. 2013;42:48–49. doi: 10.1111/jmp.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann SH. Tuberculosis vaccines: time to think about the next generation. Semin. Immunol. 2013;25:172–181. doi: 10.1016/j.smim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Rubin EJ. The granuloma in tuberculosis – friend or foe? N Engl. J. Med. 2009;360:2471–2473. doi: 10.1056/NEJMcibr0902539. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 27.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell. Microbiol. 2010;12:301–309. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 28.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013;191:773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell DG. The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr. Opin. Microbiol. 2013;16:78–84. doi: 10.1016/j.mib.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silver RF, Walrath J, Lee H, Jacobson BA, Horton H, Bowman MR, et al. Human alveolar macrophage gene responses to Mycobacterium tuberculosis strains H37Ra and H37Rv. Am. J. Respir. Cell Mol. Biol. 2009;40:491–504. doi: 10.1165/rcmb.2008-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baird MA, Hart DN, Abernethy N, Watson JD. Dendritic cell presentation of PPD and 19 kDa protein of Mycobacterium tuberculosis and emergent T helper cell phenotype. Immunol. Cell Biol. 1995;73:537–543. doi: 10.1038/icb.1995.84. [DOI] [PubMed] [Google Scholar]
- 34.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 35.Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Invest. 2006;116:3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell. 2013;155:1296–1308. doi: 10.1016/j.cell.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]