Abstract
Cognitive impairment plays a role in the development and maintenance of chronic pain. Patients with painful disorders are reported to show attentional biases toward pain-related information. However, these findings are controversial, and rarely has any study examined whether chronic pain patients have attentional biases to pain-related conditioned stimuli (CS). In this study, twenty-one patients diagnosed with trigeminal neuralgia (TN) were recruited from the neurosurgical department of a large urban general hospital. Sixteen family members and twenty-one pain-free volunteers were included as two separate control groups. Pain ratings, pain-related anxiety, general anxiety, and depression were measured in all subjects using questionnaires. Two dot probe tests were performed, one that used pictures of painful versus neutral faces as cues, and another that presented three types of CS as cues that predicted certain, uncertain, or no pain. Our results demonstrate that the TN patients showed attentional biases towards painful faces and the CSs that signaled uncertain pain. Moreover, the ratings of negative emotion about their pain conditions correlated significantly with the presence of attentional biases. The patients’ close family members, however, displayed biases towards uncertain-pain CS. This study demonstrates that patients with chronic pain have increased attention towards pain-related information, and the fearful thinking about pain was positively correlated with this phenomenon.
Keywords: attentional bias, chronic pain, dot probe task, pain signal
1. Introduction
The experience of chronic pain is commonly accompanied by cognitive impairment (Moriarty, McGuire, & Finn, 2011), which may be due to the sharing of limited resources in the brain by pain and cognition (Moriarty, et al., 2011; Wiech, Ploner, & Tracey, 2008). Pain can easily capture one's attention, helping the individual to act quickly to prevent further tissue damage. In the presence of chronic pain, however, the adaptive response may evolve into a hypervigilance to pain that can disrupt the normal cognitive processes. Patients with chronic pain have been demonstrated to show attentional biases towards pain-related materials, such as words and pictures (Khatibi, et al., 2009; Liossi, White, & Schoth, 2011; Roelofs, et al., 2005; Schoth & Liossi, 2010; Sharpe, Dear, & Schrieber, 2009). These findings are in accordance with the schema enmeshment model of pain (Pincus & Morley, 2001) and the fear avoidance model of pain (Cook, Brawer, & Vowles, 2006; Leeuw, et al., 2007), both highlighting the cognitive bias towards pain information in subjects suffering from pain.
However, the literature data are not always consistent. Some research has failed to demonstrate the attentional bias to pain-related stimuli in subjects experiencing pain (Pincus & Morley, 2001; Schoth, Nunes, & Liossi, 2012). The inconsistency may be due to the nature of the stimulus material used in these experiments (Khatibi, et al., 2009). Most research has used sensory and negative affective pain words as stimuli, which are usually not specific for patients with different pain conditions (Asmundson, Carleton, & Ekong, 2005; Asmundson, Wright, & Hadjistavropoulos, 2005; Haggman, et al., 2010; Liossi, et al., 2011). In this case, there may be only limited words that can describe the patients’ conditions and thus draw their attention. Since the limitations of verbal stimuli have been realized, more vivid stimuli, e.g., facial expressions of pain, have been introduced to study the attentional bias to pain. It has been reported that the chronic pain patients show selective attention for pictures depicting painful faces (Khatibi, et al., 2009). Some research even has found that the caregivers of pain patients have biases towards painful faces(Mohammadi et al., 2012).
In addition to direct stimulators, indirect stimuli may also draw the attention of subjects who suffer from pain. For example, one such stimulus might be an otherwise innocuous conditioned stimulus (CS) that is paired with somatosensory pain. Pain patients are particularly sensitive to signals of impending pain, which induce them to attempt to prevent the pain episode (Atlas & Wager, 2012; Notebaert, et al., 2011). Van Demmeand and colleagues have conducted a series of studies on the modulation of attentional biases by pain anticipation using a spatial cueing paradigm (Van Damme, Crombez, & Eccleston, 2004b; Van Damme, et al., 2006; Van Damme, et al., 2006; Van Damme, et al., 2004). They have investigated the attentional biases towards pain signals during acquisition, extinction, and reinstatement of pain conditioning in healthy individuals. They found that during acquisition phase, attention was biased to threat signals and was more strongly modulated by the anticipation of pain than by the anticipation of vibrotactile stimulation (Van Damme, Crombez, & Eccleston, 2004a). It was also found that participants were still hypervigilant to pain signals after extinction of pain conditioning (Van Damme, et al., 2006). Furthermore, during the reinstatement phase, attentional bias to threat signals reemerged (Van Damme, et al., 2006). Most recently, a meta-analysis has systematically reviewed the studies that examined the attentional biases towards pain-related information, and demonstrated significant attentional bias towards signals of impending pain in healthy volunteers (Crombez, et al., 2013).
Nevertheless, some issues of experimental design arise in the previous studies. First, attentional bias task was performed simultaneously with pain during the acquisition phase. Although the CS+ (i.e., those followed by pain) were presented merely in a portion of trials (one third to half) and the trials containing pain were not analyzed. A recent study revealed that the presence of painful stimulation can not only interfere with the ongoing task, but also have prolonged interference effect on the subsequent task (D. M. Van Ryckeghem, Crombez, Eccleston, Liefooghe, & Van Damme, 2012). Second, since there were a number of trials in which the cue was not followed by pain, predictions of pain from stimuli were made with uncertainty by the participants. It has been reported that uncertain expectation of pain has differential effect on cognitive task in comparison to the accurately predicted pain (Arntz & Hopmans, 1998; Yoshida, Seymour, Koltzenburg, & Dolan, 2013). Additionally, most studies employed only healthy individuals. And as far as we know, noly one study has been conducted to investigate whether chronic pain patients show an attentional bias towards pain-related CS (Van Ryckeghem, et al., 2013). Surprisely, this research didn't find attentional bias towards pain-related information, but attentional bias may predicted the disability and pain severity in future. According to Apkarian et al., chronic pain is a state of continuous learning, in which pain experience is associated with incidental events, and coupled with reduced opportunity for extinction of those associations (Apkarian, 2008; Apkarian, Baliki, & Geha, 2009; Apkarian, Hashmi, & Baliki, 2011). The predictive value of attentional bias towards pain-related information probably implied that hypervigilance to pain signals plays a crucial role in the development and maintenance of the chronic pain conditions.
To address issues we mentioned above, the present study applied a dot-probe paradigm and a unique design to test two hypotheses. First, compared to pain-free healthy control, chronic pain patients as well as their family members would demonstrate attentional bias towards both painful facial expressions and pain-related CSs. Second, uncertain pain-related CSs may draw more attentional resources in participants than certain pain-related CSs. Additionally the relationship between emotion measures and attentional biases towards pain was analyzed, with the goal of elucidating potential predictive factors for pain hypervigilance in chronic pain sufferers.
2. Materials and Methods
2.1. Participants
Fifty-eight individuals participated in this study: 21 patients with TN, 16 pain-free family members of these patients, and 21 pain-free healthy controls. TN patients were recruited from the neurosurgical department of the Chinese PLA General Hospital of Jinan Military Command which received patients from all over the country. The duration of pain of TN patients was 8.76±1.26. And there are 13 patients where right side faces were affected, the rest were left side faces affected. Family members were spouses or children of the patients who had lived with the patients for at least 1 year. Healthy controls were recruited from among workers of the hospital or via advertisements placed around the institute. The controls were strangers to the patients and their family members. The exclusion criteria for family group and healthy controls were the presence of any current or previous medical, psychosocial, or emotional conditions.
Inclusion criteria for the TN patients were: (a) idiopathic TN, as defined by the International Headache Society (IHS) criteria (Headache Classification Subcommittee, 2004), and (b) duration of pain ≥ 1 year. Patients were excluded if they: (a) had other types of acute or chronic pain, (b) had a history of any other medical, neurological, or psychiatric disorder, or (c) were under the influence of drugs or alcohol. The patients participating in the experiment had not yet undergone neurosurgical interventions (i.e., microvascular decompression) and were not experiencing pain at the time of the study. All participants were confirmed to have no serious visual impairments and were able to complete both the questionnaires and the computerized task.
Subjects gave their written informed consent after they had received a detailed explanation of the study protocol. They acknowledged that they would experience a painful shock during the course of the experiment. All subjects were informed that they could terminate the experiment at any time without prejudice. The experimental protocol was approved by the Institutional Review Board of the first author's academic institution).
2.2. Procedure
Participants were tested individually in a quiet room. Questionnaires were used to collect information on demographics, medical history, and emotional status. All participants were asked to fill out the short versions of the Pain Anxiety Symptoms Scale (PASS-20), Self-Rating Depression Scale (SDS), and Self-Rating Anxiety Scale (SAS). Patients also completed the short form of the McGill Pain Questionnaire (SF-MPQ), to provide a quantitative evaluation of their pain in its sensory, affective, and cognitive aspects. If the participant could not read, then the experimenter read the scales to them, and the participant reported their answers orally.
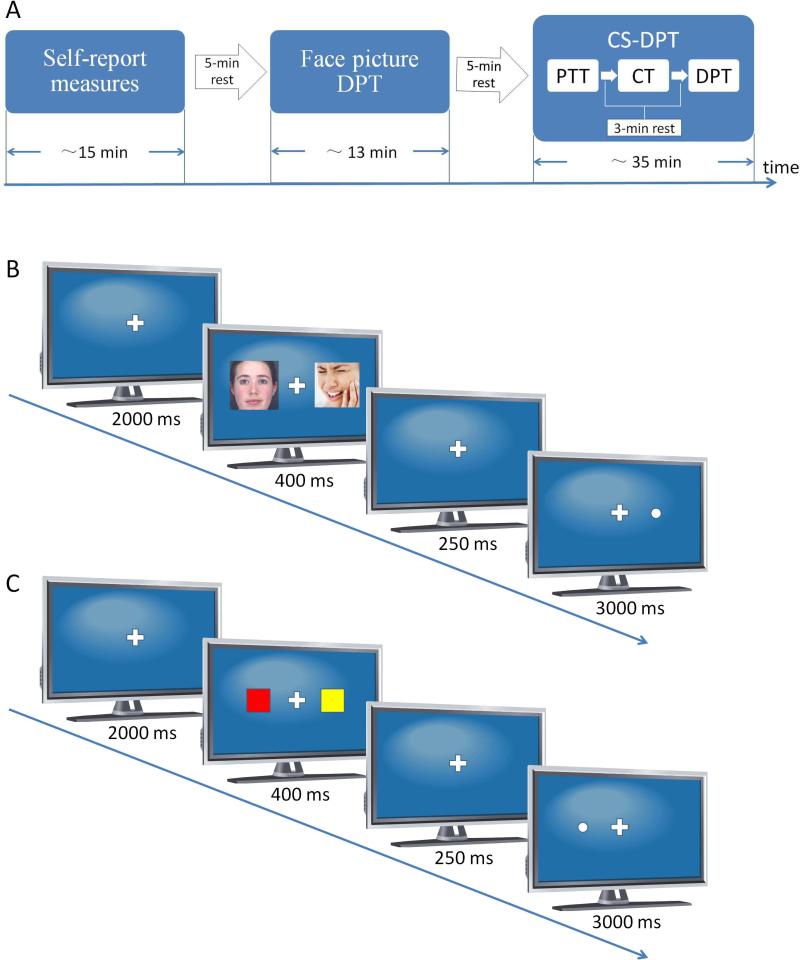
After completing the questionnaires, participants were required to perform two dot-probe tasks (DPTs) in two consecutive sessions. In the first session, an emotional pictures DPT was conducted to measure the attentional bias towards painful faces. The second session consisted of three parts: pain-threshold testing, pain-related conditioning, and followed by a conditioned stimulus DPT (CS-DPT), in which the attention bias towards pain-related color cues was examined. There was a 3-minute rest period between different parts. The two sessions were 5 minutes apart. The experimental protocol is illustrated in Fig. 1A. Because patient recruitment was very difficult, the faces DPT and CS-DPT were run in the same participant.
FIGURE 1.
Experimental design of the current study, based upon the dot-probe paradigm. (A)General procedure. (B) Face-pictures dot-probe task (DPT). (C) Conditioned stimuli (CS) DPT. PTT: pain-threshold test; CT:conditioning training.
2.3. Materials
2.3.1. Questionnaires
The SF-MPQ consists of 15 pain descriptors on a scale from 0 (none) to 3 (severe) designed to assess the sensory and affective aspects of pain, as well as two single-item measures of current pain severity (Melzack, 1987). The factorial validity of the sensory and affective components of the SF-MPQ has been empirically supported, with internal consistency estimates for the sensory and affective dimensions reported as .78 and .76, respectively (Wright, Asmundson, & McCreary, 2001).
The short version of the PASS-20 provides a measure of pain-related fear, avoidance, and both physical and cognitive anxiety responses (McCracken & Dhingra, 2002). Participants rate anxiety responses such as “I think that if my pain gets too severe, it will never decrease” on a scale from 0 (never) to 5 (always), indicating how often they experience each of the actions or thoughts described. The PASS-20 has demonstrated good internal consistency (mean α = .81) and has strong correlations (mean r = .95) with the original subscales (McCracken & Dhingra, 2002).
The SAS is a 20-item scale representing 15 somatic and 5 affective symptoms of anxiety (Zung, 1971). Respondents are required to rate how each item applied to them during the last week (1 = none or little of the time, 4 = most or all of the time). Scores range from 20 to 80, with higher scores representing higher levels of anxiety. This scale has adequate reliability, with Zung reporting a Cronbach's alpha of .85 (Zung, 1971).
The SDS is one of the most widely used depression scales in both research and clinical settings (Zung, Richards, & Short, 1965). Respondents are required to rate the frequency of 20 depressive symptoms, using a 4-point scale ranging from 1 (none or little of the time) to 4 (most or all of the time). This scale has good reported internal consistency, with Cronbach's alpha coefficients ranging from .73 to .92 (Zung, Richards, & Short, 1965).
2.3.2. Face-pictures DPT
Eighty pictures, half with painful and the other half with neutral, facial expressions, were downloaded from the Internet. During the pre-experimental phase, 30 undergraduate and graduate students were recruited to rate how painful the faces in the pictures appeared to them. These preliminary subjects rated how painful the faces were using a numerical rating scale between 0 (“no pain”) and 10 (“extremely painful”). For the cues in the DPT experiments of the current study, 20 pictures were chosen within the upper 75% of the scores that were rated higher than 7 on the numerical rating scale (i.e., painful faces) and 20 pictures within the lower 25% of the scores that were rated lower than 3 (i.e., neutral faces).
The paradigm of the face-pictures DPT is shown in Fig. 1B. Each trial in the task began with a 2000-ms presentation of a fixation cross in the middle of the computer screen. This was followed by a 400-ms presentation of a pair of face pictures (one painful and one neutral, or both neural) located on the left and right sides of the cross, each being 3 cm in width and 6.8 cm in height. The distance from the center of either picture to the fixation point was 4.0 cm. Subsequently, both pictures disappeared, and the central fixation point was presented alone for 250 ms. A dot with a diameter of 1.4 cm then appeared in the place of one of the pictures. Participants were asked to press the left/right button of the mouse as quickly as possible to indicate the location of the dot. The dot disappeared if the reaction time was longer than 2500 ms. Each participant underwent a total of 288 trials divided across two blocks, with an interblock interval of 2 min.
Three types of trials were used in the current study: congruent, incongruent, and neutral. Each of them included 96 trials. In congruent trials, the dots appeared on the same side as the pictures with the painful facial expressions. In incongruent trials, the dots were presented on the side opposite to that of the painful faces. The neutral trials, unlike the congruent and incongruent trials, which both incorporated painful faces, were comprised solely of neutral faces. Trials of each type were randomly mixed within the blocks.
2.3.3. Pain-related conditioned stimulus DPT
2.3.3.1 Pain-threshold test
Electrical stimuli were delivered to test subjects with a constant-current stimulator (DS7A, Digitimer Ltd., Glenwyn Garden City, UK). A pair of Ag/AgCl electrodes (1-cm diameter) was symmetrically positioned on the palm and the back (both near the thumb) of the nondominant hand. The stimulator sent a series of 25 rectangular pulses (2-ms duration and 6-ms interval) in each shock, for a total duration of 200 ms. Parameters of the electrical stimuli were chosen based on previous studies (Notebaert, et al., 2011).
The electrical pain threshold of each subject was determined with a method incorporating a limits-type paradigm (Snijders, et al., 2010). The applied stimulus intensity began at 0 mA and increased gradually until the subject reported pain. The stimulation intensity was then set above the pain threshold and gradually decreased until the subject reported no pain. The endpoints of both series were averaged to obtain the individual's threshold for pain. The stimulus intensity was changed in steps of 2 mA, with the interstimulus intervals ranging between 6 and 10 s.
2.3.3.2 Conditioning training
Participants were trained to associate visual cues (conditioned stimuli, CSs) with the electrical stimuli (unconditioned stimuli, USs). In each trial, a noxious or innocuous electrical stimulus was delivered .5 to 1.5 s after the presentation of a solid square with a specific color on a computer screen for 1-s duration. To ensure an efficient aversive stimulation, the intensity of the noxious stimulus was set to be 30% above the individual's pain threshold. The innocuous electrical stimulus was set to be 30% below the individual's pain threshold. Three differential conditioning trials (each n = 20) were used: (1) a red square was a CS that predicted pain with a 100% probability (certain pain); (2) a green square was a CS that predicted pain with a 50% probability (uncertain pain); (3) a blue square was a CS that predicted no pain. All 60 trials were randomly mixed within one block.
2.3.3.3 CS-DPT
The CS-DPT procedure was the same as that of the face-pictures DPT, except that the faces were replaced with differently colored squares (Fig. 1C). Five colors were used. All colored squares were 3.2 cm in width and 3.6 cm in height on the computer screen. Squares were presented two at a time, in two different color pairs: CS-neutral and neutral-neutral. The CSs consisted of the certain-pain CS (red square), the uncertain-pain CS (green square), and the nonpain CS (blue square) employed in the prior conditioning session. The neutral stimuli were represented by yellow and purple squares. Congruent trials were those in which the dots appeared on the same side as the CS; incongruent trials were those in which the dots appeared on the opposite side of the CS. As before, participants were asked to press the left/right button of the mouse as quickly as possible to indicate the location of the dot. The CS-DPT was comprised of three blocks of 144 trials each (432 trials). Every block contained equal numbers of congruent, incongruent and neutral trials.
2.4. Data Analysis
The reaction time (RT) and the number of trials with incorrect responses were recorded. A RT of less than 200 ms or of more than three standard errors above the participant's mean was excluded as an outlier. An index of attentional bias was calculated by using the following formula: Index = Mean RT of incongruent trials – Mean RT of congruent trials.
Statistical comparisons were performed and graphs constructed with GraphPad's Prism 5.0 software and SPSS 13.0. Differences between groups in general data (age and education), questionnaire scores, and pain thresholds were evaluated by one-way analysis of variance (ANOVA). The gender composition of the groups was compared with a chi-square statistic. To determine differences between groups in the average RT of the DPTs, a mixed-model ANOVA was conducted with “group” as the between-group factor and “trial type” as the within-group factor, followed by Newman-Keuls post hoc test. Pearson's correlation coefficients were calculated between the indices of attentional bias and questionnaire measures. Data are presented as means ± SEM. The significance level was set at P < .05.
3. Results
3.1. Subject Characteristics
Table 1 presents the demographic characteristics for all three groups (TN patients, patients’ family members, and healthy controls). A one-way ANOVA was performed to determine differences between groups in age or educational level. The main effects of group were significant (age: F (2, 55) = 8.54, P < .001, education: F (2, 55) = 10.08, P < .001). The post hoc test revealed that there was no significant difference between the TN patients and healthy controls, suggesting that the control group was individually matched to the patients in age and education. The family members were, on average, younger and had higher levels of education than the other two groups. Six (37.5%) of the family members were children of the patients, whereas the rest (63.5%) were spouses. No differences were found in the gender distribution between the three groups.
Table 1.
Demographics and questionnaire scores of participants
| Patients (n = 21) | Family (n = 16) | Controls (n = 21) | F/χ2 | P value | |
|---|---|---|---|---|---|
| Age | 57.1 ± 1.7 | 46.2 ± 2.8** | 55.3 ± 1.4 | 8.54 | <.001 |
| Gender | 9 male | 8 male | 9 male | .24 | .887 |
| Education level (years) | 6.2 ± .7 | 1.3 ± .4* | 7.5 ± .7 | 10.08 | <.001 |
| Pain threshold (mA) | 11.9 ± 1.1*** | 8.1 ± .4 | 8.0 ± .4 | 9.41 | <.001 |
| SF-MPQ | |||||
| Sensory score (0-33) | 14.9 ± .9 | -- | -- | -- | -- |
| Affective score (0-12) | 8.7 ± .6 | -- | -- | -- | -- |
| PPI (0-5) | 1.5 ± .3 | -- | -- | -- | -- |
| VAS (0-100) | 84.2 ± 3.4 | -- | -- | -- | -- |
| PASS-20 | |||||
| Cognitive (0-25) | 13.7 ± 1.2*** | 4.2 ± .7 | 2.9 ± .5 | 46.80 | <.0001 |
| Escape/avoidance (0-25) | 14.2 ± 1.5*** | 7.2 ± .6** | 2.5 ± .4 | 34.77 | <.0001 |
| Fear (0-25) | 11.4 ± 1.5*** | 10.2 ± 1.2*** | 1.9 ± .3 | 21.52 | <.0001 |
| Physiological anxiety (0-25) | 10.6 ± 1.1*** | 3.5 ± .6 | 2.3 ± .3 | 38.53 | <.0001 |
| Total (0-100) | 499 ± 3.4*** | 25.1 ± 1.4*** | 9.6 ± 1.0 | 82.43 | <.0001 |
| SDS | .40 ± .03*** | .39 ± .02*** | .21 ± .02 | 18.29 | <.0001 |
| SAS | 40.2 ± 2.9 | 37.0 ± 3.4 | 31.7 ± 2.3 | 2.56 | .086 |
P < .05
P < .01
P < .001, all compared to the healthy controls.
SF-MPQ, short-form McGill pain questionnaire; PPI, present pain intensity; VAS, visual analogue scale; PASS-20, short version of the Pain Anxiety Symptoms Scale; SDS, Self-rating Depression Scale; SAS, Self-rating Anxiety Scale.
Individual pain thresholds, measured with electrical shocks, were compared and a significant main effect of group was seen in the ANOVA (F (2, 55) = 9.41, P < .001). The results of the post hoc test showed that the pain threshold of patients are higher than that of healthy controls (11.9 ± 1.1 vs. 8.1 ± .4, t = 3.87, P < .001) and family members (11.9 ± 1.1 vs. 8.0 ± 0.4, t = 3.53, P < .01). No significant differences in pain threshold were found between the family and control groups.
3.2. Questionnaire Data
The results and statistics of the self-report measures are presented in Table 1. One-way ANOVA revealed significant main effects of group on the PASS-20 total and subscale scores. Post hoc tests using Bonferroni's multiple comparisons showed that the differences between any two of the three groups were significant in the total scores (patients vs. family: 49.9 ± 3.4 vs. 25.1 ± 1.4, t = 6.82, P < .001; patients vs. control: 49.9 ± 3.4 vs. 9.6 ± 1.0, t = 11.93, P < .001; family vs. control: 25.1 ± 1.4 vs. 9.6 ± 1.0, t = 4.28, P < .001). The main effect of group was also significant for the SDS score (F (2, 55) = 18.29, P < .0001). The SDS scores of both patients and family members were higher than those of the control group (patients vs. control: .40 ± .03 vs. .21 ± .02, t = 5.54, P < .001; family vs. control: .39 ± .02 vs. .21 ± .02, t = 4.73, P < .001). No differences were found in the SAS scores between the three groups.
3.3. Analyses of Attentional Bias
The family group did not match the other two groups in terms of age or educational level. To exclude these confounding effects, additional analyses were performed by using age and education level as covariates in the analysis of RT in the DPTs. Controlling for these covariates in the analysis did not qualitatively affect the results. Therefore, we report only the results based on analyses conducted without controlling for covariates.
3.3.1. Face-pictures DPT
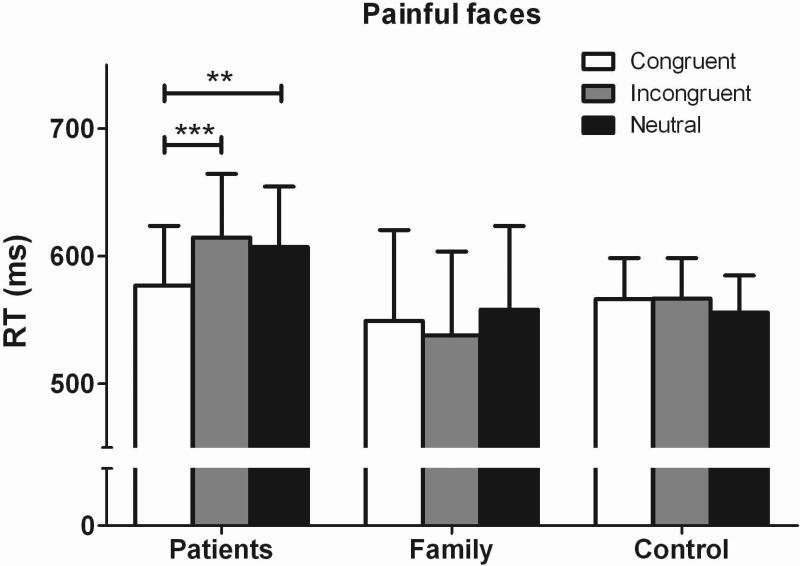
A 3 × 3 ANOVA of group (patients, family, control) × trial type (congruent, incongruent, neutral) was conducted with RT as the dependent variable, group as the between-subject factor, and trial type as the within-subject factor. Figure 2 presents the comparisons of RT in the face-pictures DPT between the three groups. A significant group × trial type interaction was found (F (4, 55) = 5.00, P < .001, η2 = .15), although there was no main effect for group (F (2, 55) = .30, P = .74, η2 = .01) or for trial type (F (2, 55) = 1.81, P = .17, η2 = .03). Post hoc comparisons revealed that TN patients responded more quickly in the congruent trials than in the neutral (t = 3.29, P < .01) or incongruent (t = 4.10, P < .001) trials, indicating that the patients displayed attentional biases towards painful facial expressions. No significant difference was observed between RTs in the neutral versus incongruent trials in the TN patients. In contrast to the attentional bias displayed by the pain patients, the average RT of family members and healthy controls did not differ between congruent, incongruent, and neutral trials.
FIGURE 2.
The mean RT of all the conditions in face picture dot-probe tests. Patients responded faster to congruent trials than neutral and incongruent trials. There are no difference between congruent trials and neutral and incongruent trials in the family or control group. **p < 0.01 and ***p < 0.001, compared with congruent trials.
3.3.2. CS-DPT
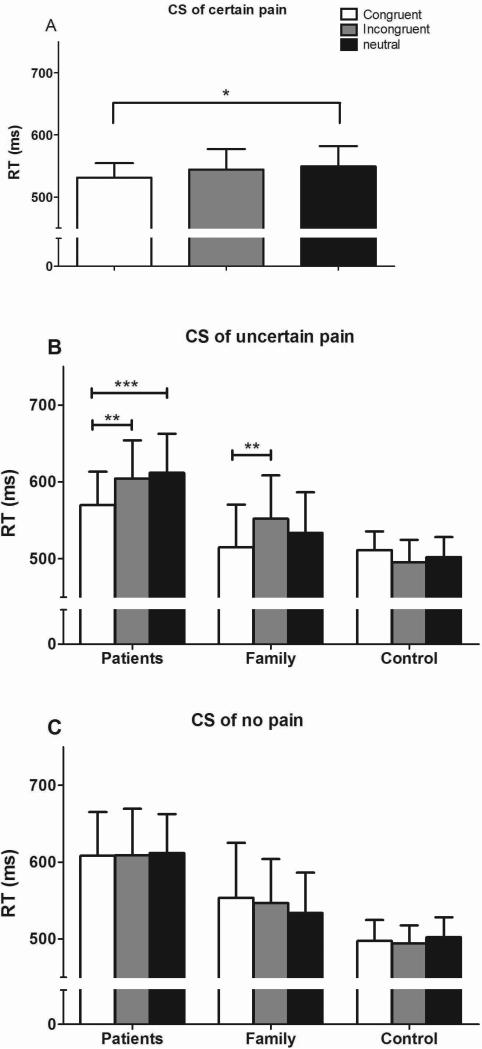
To confirm the attentional vigilance exhibited by pain patients in the face-pictures DPT, a modified dot-probe paradigm was employed, in which differently colored squares that predicted either pain or no pain were used as CSs. The results are illustrated in Figure 3. A 3 × 3 ANOVA (group × trial type) was conducted, similar to that used in the face-pictures DPT. For the certain-pain CS, the analysis revealed a main effect for trial type (F (2, 55) = 3.84, P = .02, η2 = .07) but not for group (F (2, 55) = 1.49, P = .23, η2 = .05), and no interaction effect (F (4, 55) = 1.85, P = .12, η2 = .06, Fig. 3A). Multiple comparisons (adjusted by bonferroni) revealed significant differences in the average RT between congruent and neutral trials (F = 4.11, P < .05). There were no differences between incongruent and congruent or neutral trials. Although the main effect of group wasn't significant, the effect size for trial type in patients group (η2 = .20) was greater than the effect size of trial type in family group (η2 = .03) and in control group (η2 = .02).
Figure 3.
Reaction times of subjects in the conditioned stimuli dot-probe task (CS-DPT). The results revealed only an effect of trial type, indicating that response time of congruent trials were shorter than response time of neutral trials and there are no main effect of group or interaction effect in the certain-pain CS-DPTs (A).Patients responded faster to congruent trials than to neutral or incongruent trials in the uncertainpainCS- DPTs (B). The family group responded more quickly to congruent trials than to incongruent trials in the uncertain-pain CS-DPT (B). There were no significant differences between the different trials in the nonpain CS-DPT (C). *P < 0.05 **P < 0.01, and ***P < 0.001.
Similarly, in the analyses of the uncertain-pain CS, there was a significant main effect for trial type (F (2, 55) = 6.29, P < .01, η2 = .10) but not for group (F (2, 55) = 1.30, P = .28, η2 = .18; Fig. 3B), and a significant interaction for group and trial (F (4, 55) = 5.89, P < .001, η2 = .06). When comparing the average RT between different trial types within each group, significant attentional biases were found in the patient group (congruent vs. incongruent: t = 3.58, P < .01; congruent vs. neutral: t = 4.34, P < .001) as well as in the family group (congruent vs. incongruent: t = 3.33, P < .01). For the nonpain CS, there was no main effect for group (F (2, 55) = 1.50, P = .23, η2 = .02) or trial type (F (2, 55) = .13, P = .88, η2 = .004), and no interaction effect (F (4, 55) = .55, P = .70, η2 = .03; Fig. 3C). These data indicate that subjects did not show attentional biases toward the cues that were unrelated to pain.
3.4. Correlation Analyses
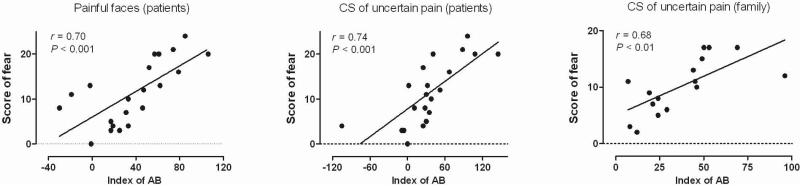
To determine which factor(s) may affect the likelihood of a patient exhibiting attentional biases toward pain stimuli, a series of correlations were calculated between the questionnaire measures and the bias indices. Table 2 presents the results of the correlation analyses. In the patient group, significant positive correlations were found between the presence of attentional biases and the responses to the fear subscale of PASS-20 for both the face-pictures DPT (r = .70, P < .001) and the uncertain-pain CS-DPT (r = .74, P < .001). That is, the higher the scores on the PASS-20 indicating fearfulness, the greater the degree of attentional bias. However, no correlation was observed between the PASS-20 responses and the presence of attentional bias in the certain-pain CS-DPT in these patients. Interestingly, we found significant positive correlations in the family group between the indices of attentional bias and the scores of the fear subscale of the PASS-20 (r = .68, P < .01) as well as between the bias and the total PASS-20 score (r = .58, P < .05). No correlations were observed between the bias indices and other questionnaire measures in the different groups (including SF- MPQ, SDS, and SAS). Examples of correlations between the bias index and the fear subscale score are shown in Figure 4.
Table 2.
Correlations between questionnaire measures and attentional bias to pain-related stimuli
| Patients (n = 21) |
Family members (n = 16) |
Healthy controls (n = 21) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Face pictures | Conditioned stimuli |
Face pictures | Conditioned stimuli |
Face pictures | Conditioned stimuli |
|||||||
| CP | UP | NP | CP | UP | NP | CP | UP | NP | ||||
| Pain duration | −.19 | .04 | .02 | .02 | -- | -- | -- | -- | -- | -- | -- | -- |
| SF-MPQ | ||||||||||||
| Sensory (0-33) | −.23 | .05 | .31 | −.05 | -- | -- | -- | -- | -- | -- | -- | -- |
| Affective (0-12) | .08 | −.16 | .38 | .25 | -- | -- | -- | -- | -- | -- | -- | -- |
| PPI (0-5) | .01 | .02 | .02 | −.18 | -- | -- | -- | -- | -- | -- | -- | -- |
| VAS (0-100) | .10 | −.06 | .42 | .24 | -- | -- | -- | -- | -- | -- | -- | -- |
| PASS-20 | ||||||||||||
| Cognitive (0-25) | .04 | .03 | .16 | .12 | .39 | .15 | .02 | −.03 | −.33 | .08 | −.23 | .16 |
| Escape (0-25) | .09 | −.18 | .24 | −.10 | .36 | −.14 | −.23 | .26 | −.07 | .17 | .00 | −.06 |
| Fear (0-25) | .70*** | −.31 | .74*** | .02 | −.23 | −.05 | .68** | .21 | .08 | .30 | .08 | .21 |
| Anxiety (0-25) | −.14 | −.04 | −.01 | −.15 | .15 | .01 | .18 | .06 | −.10 | −.01 | −.36 | .21 |
| Total (0-100) | .10 | .01 | .10 | −.07 | .22 | −.03 | .58* | .31 | −.20 | .20 | −.20 | .18 |
| SDS | .28 | −.43 | −.34 | −.31 | −.09 | −.26 | .21 | .35 | .05 | .04 | .14 | .05 |
| SAS | −.001 | −.15 | −.34 | −.04 | −.30 | −.10 | .23 | −.35 | −.22 | .03 | −.02 | .07 |
The figures in the table show the Pearson correlation coefficients between questionnaire scores and indices of attentional bias.
P < .05
P < .01
P < .001.
SF-MPQ, short-form McGill pain questionnaire; PASS-20, short version of the Pain Anxiety Symptoms Scale; CP, certain pain; UP, uncertain pain; NP, no pain.
FIGURE 4.
Examples of correlations betwee the fear subscale score of the PASS-20 and the index of attentional bias. Significant positive correlations are shown. AB: attentional bias.
4. Discussion
The present study used a DPT paradigm to investigate whether chronic pain patients and their close relatives exhibit attentional biases towards pain-related information. We conducted correlational analyses to isolate potential factors that related to the presence of hypervigilance to pain. Our research yielded three major findings. First, we confirmed and extended previous studies by demonstrating that chronic pain patients displayed attentional biases not only towards painful facial expressions, but also towards visual cues that signaled unpredictable pain. Second, the family members of the patients selectively attended to visual cues that signaled pain. Third, the attentional biases towards pain-related stimuli that we observed in patients and their caregivers were positively correlated with measures of fearful thinking about pain in both groups. Of particular importance, our study demonstrated that patients with chronic pain showed an attentional bias towards cues that signal pain. This study is unique because, to our knowledge, no study has examined whether chronic pain patients have attentional biases to pain-related CSs. Several recent studies have involved data on attentional biases to pain signals using pain-free participants (Schrooten, et al., 2012; Van Damme, et al., 2004a; Van Damme, et al., 2006; Van Ryckeghem, et al., 2012), but studies that include chronic pain patients in the sample are rare.
Unlike the TN patients in this study, their family members did not show attentional biases toward painful expressions. This finding is inconsistent with previous results from Mohammadi et al., (2012) who found that the main caregivers of chronic pain patients demonstrated a bias towards painful faces. The differences between the studies may be due to the fact that we analyzed the biases using painful versus neutral faces, whereas Mohammadi et al. (2012) used painful versus happy faces. Unexpectedly, we found that the family group displayed attentional biases towards a visual CS previously paired with a painful US. This finding suggests that the family members of pain patients are themselves hypervigilant to the pain-related CSs.
Previous studies have provided evidence that exposure to the pain of others has important effects upon the psychological and physical well being of family caregivers (Goubert, et al., 2005). Family caregivers may even tend to overestimate the pain experienced by their loved one, and this overestimation can exacerbate their own personal distress (Redinbaugh, et al., 2002). Importantly, we found that the family members only showed attentional biases towards uncertain pain CSs associated with fear subscale of PASS-20, but not that of certain pain. One possible explanation is that the two conditions (uncertain expectation of pain vs. certain expectation of pain) produce different levels of hypervigilance. Previous findings have shown that lowering the stimulus predictability leads to higher levels of anxiety, fear, and associated physiological arousal (Carlsson, et al., 2006; Oka, et al., 2010).
There was a correlation between the fear of pain and attentional biases towards pain-related stimuli (including the painful faces and the cues predicting pain). Fearful thinking about pain has been shown to play a role in modulating one's attentional bias towards pain-related information (Asmundson & Hadjistavropoulos, 2007; Keogh, et al., 2001; Yang, et al., 2012). Patients with chronic pain consistently report worries about the causes and consequences of pain due to the stubborn persistence of the discomfort (Crombez, et al., 2013; De Vlieger, Crombez, & Eccleston, 2006; Eccleston & Crombez, 2007). In our study, most of the items in the fear subscale of PASS-20 were aimed towards measuring whether the patients were anxious about the potential bad outcomes of their pain. For example, items included “When pain comes on strong, I think that I might become paralyzed or more disabled” and “I think that if my pain gets too severe, it will never decrease” (McCracken & Dhingra, 2002). We found that the patients’ negative expectation about their pain correlated significantly with the attentional bias they exhibited towards pain-associated stimuli. In other words, if the patient thinks that his/her pain will worsen, he/she will pay more attention to pain-related stimuli. The findings from the present study revealed that the attentional biases were independent of pain intensity or duration in the TN patients. And it seems even more surprising that the TN patients were less sensitive to shock pain than the family or control groups. However, we should note that the electric stimulus was delivered to the patients’ hands and not the areas affected by TN. In similar fashion, earlier studies also found no correlation between pain threshold or intensity and attentional bias (Schoth, Nunes, & Liossi, 2012; Yang, Jackson, Gao, & Chen, 2012). More importantly, these results may indicate that attentional biases to pain-related stimulus are focused on the negative emotions (such as anxiety or fear) or thoughts (catastrophic thinking) induced by pain, and are not directly related to physical qualities of pain.
Attentional training paradigms have been designed that intend to distract patient's attention away from pain-related stimuli (McGowan, et al., 2009; Sharpe, et al., 2012). The results of those studies demonstrated that patients’ pain condition getting better right after the intervention and at 6-month follow-up. Our results suggest that the use of a pain-related CS may be an efficient tool for this kind of attentional training, given that it has high personal relevance and, thus, high ecological validity (Dear, et al., 2011). According to Apkarian et al., chronic pain represents a state of continuous learning, in which pain experiences are associated with incidental events (Apkarian, et al., 2009; Apkarian, et al., 2011). Training pain patients to shift their attention away from pain-related cues may break the association between pain and random events and thereby disrupt, to some extent, the sustained state of chronic pain.
Several limitations should be acknowledged in this study. First, the sample size of the family group was slightly smaller than that of the other two groups, because some of the patients were not accompanied by their close family members. Second, we made the conclusion that the negative expectation of pain was a predictor of hypervigilance or attentional bias to pain-related stimuli based solely on the fear subscale scores of the PASS-20. A special questionnaire for measuring pain expectation is not currently available, so it is not clear whether the patient's anxiety concerning bad pain outcomes is a stable state or only an occasional occurrence. Third, the conditioning paradigm did not counter balance the color of the CS across pain certainty conditions. In the future study, all kinds of color square should have the equal chance to become the CS of certain/uncertain pain or nonpain between subjects. So the influence of CS stimulus salience to results would be counteracted.
Acknowledgements
This work was funded by an NNSF grant (31271092) and a Chinese Academy of Sciences Knowledge Innovation Project grant (KSCX2-EW-Q-18) to J.Y.W., and by NNSF grants (30970959, 61033011, and 31171067), Chinese Academy of Sciences Knowledge Innovation Project grants (YZ200944, KSCX2-YW-R-254, and KSCX2-EW-J-8), and a grant from the NIH Fogarty International Center (R03 TW008038) to F.L. This research was also supported by the Key Laboratory of Mental Health at the Institute of Psychology, Chinese Academy of Sciences, China.
Footnotes
The authors declare no competing financial interests.
References
- Apkarian AV. Pain perception in relation to emotional learning. Current Opinion in Neurobiology. 2008;18(4):464–468. doi: 10.1016/j.conb.2008.09.012. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Progress in Neurobiology. 2009;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(3 Suppl):S49–64. doi: 10.1016/j.pain.2010.11.010. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntz A, Hopmans M. Underpredicted pain disrupts more than correctly predicted pain, but does not hurt more. Behaviour Research and Therapy. 1998;36(12):1121–1129. doi: 10.1016/s0005-7967(98)00085-0. doi: 10.1016/S0005-7967(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Carleton RN, Ekong J. Dot-probe evaluation of selective attentional processing of pain cues in patients with chronic headaches. Pain. 2005;114(1-2):250–256. doi: 10.1016/j.pain.2004.12.025. doi: 10.1016/j.pain.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Hadjistavropoulos HD. Is High Fear of Pain Associated With Attentional Biases for Pain-Related or General Threat? A Categorical Reanalysis. The Journal of Pain. 2007;8(1):11–18. doi: 10.1016/j.jpain.2006.05.008. doi: 10.1016/j.jpain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Wright KD, Hadjistavropoulos HD. Hypervigilance and Attentional Fixedness in Chronic Musculoskeletal Pain: Consistency of Findings Across Modified Stroop and Dot-probe Tasks. The Journal of Pain. 2005;6(8):497–506. doi: 10.1016/j.jpain.2005.02.012. doi: 10.1016/j.jpain.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neuroscience Letters. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32(4):1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Cook AJ, Brawer PA, Vowles KE. The fear-avoidance model of chronic pain: Validation and age analysis using structural equation modeling. Pain. 2006;121(3):195–206. doi: 10.1016/j.pain.2005.11.018. doi: 10.1016/j.pain.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Crombez G, Van Ryckeghem DM, Eccleston C, Van Damme S. Attentional bias to pain-related information: A meta-analysis. Pain. 2013;154(4):497–510. doi: 10.1016/j.pain.2012.11.013. doi: 10.1016/j.pain.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Crombez G, Viane I, Eccleston C, Devulder J, Goubert L. Attention to pain and fear of pain in patients with chronic pain. Journal of Behavioral Medicine. 2013;36(4):371–378. doi: 10.1007/s10865-012-9433-1. doi: 10.1007/s10865-012-9433-1. [DOI] [PubMed] [Google Scholar]
- De Vlieger P, Crombez G, Eccleston C. Worrying about chronic pain. An examination of worry and problem solving in adults who identify as chronic pain sufferers. Pain. 2006;120(1-2):138–144. doi: 10.1016/j.pain.2005.10.022. doi: 10.1016/j.pain.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Dear BF, Sharpe L, Nicholas MK, Refshauge K. Pain-related attentional biases: the importance of the personal relevance and ecological validity of stimuli. The Journal of Pain. 2011;12(6):625–632. doi: 10.1016/j.jpain.2010.11.010. doi: 10.1016/j.jpain.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Worry and chronic pain: A misdirected problem solving model. Pain. 2007;132(3):233–236. doi: 10.1016/j.pain.2007.09.014. doi: 10.1016/j.pain.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Goubert L, Craig KD, Vervoort T, Morley S, Sullivan MJ, de C, Williams AC, Crombez G. Facing others in pain: the effects of empathy. Pain. 2005;118(3):285–288. doi: 10.1016/j.pain.2005.10.025. doi: 10.1016/j.pain.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Haggman SP, Sharpe LA, Nicholas MK, Refshauge KM. Attentional biases toward sensory pain words in acute and chronic pain patients. The Journal of Pain. 2010;11(11):1136–1145. doi: 10.1016/j.jpain.2010.02.017. doi: 10.1016/j.jpain.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Keogh E, Ellery D, Hunt C, Hannent I. Selective attentional bias for pain-related stimuli amongst pain fearful individuals. Pain. 2001;91(1-2):91–100. doi: 10.1016/s0304-3959(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Khatibi A, Dehghani M, Sharpe L, Asmundson GJG, Pouretemad H. Selective attention towards painful faces among chronic pain patients: Evidence from a modified version of the dot-probe. Pain. 2009;142(1-2):42–47. doi: 10.1016/j.pain.2008.11.020. doi:10.1016/j.pain.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Leeuw M, Goossens M, Linton S, Crombez G, Boersma K, Vlaeyen J. The Fear-Avoidance Model of Musculoskeletal Pain: Current State of Scientific Evidence. Journal of Behavioral Medicine. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Schoth DE. Time-course of attentional bias for threat-related cues in patients with chronic daily headache-tension type: evidence for the role of anger. European Journal of Pain. 2011;15(1):92–98. doi: 10.1016/j.ejpain.2010.05.008. doi: 10.1016/j.ejpain.2008.11.007. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Research & Management. 2002;7(1):45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- McGowan N, Sharpe L, Refshauge K, Nicholas MK. The effect of attentional re-training and threat expectancy in response to acute pain. Pain. 2009;142(1-2):101–107. doi: 10.1016/j.pain.2008.12.009. doi:10.1016/j.pain.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Mohammadi, Somayeh, Dehghani, Mohsen, Sharpe, Louise, Heidari, Mahmoud, Sedaghat, Maryam, Khatibi, Ali Do main caregivers selectively attend to pain-related stimuli in the same way that patients do? Pain. 2012;153(1):62–67. doi: 10.1016/j.pain.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Progress in Neurobiology. 2011;93(3):385–404. doi: 10.1016/j.pneurobio.2011.01.002. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Notebaert L, Crombez G, Vogt J, De Houwer J, Van Damme S, Theeuwes J. Attempts to control pain prioritize attention towards signals of pain: An experimental study. Pain. 2011;152(5):1068–1073. doi: 10.1016/j.pain.2011.01.020. doi: 10.1016/j.pain.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Oka S, Chapman CR, Kim B, Shimizu O, Noma N, Takeichi O, et al. Predictability of painful stimulation modulates subjective and physiological responses. Journal of Pain. 2010;11(3):239–246. doi: 10.1016/j.jpain.2009.07.009. doi: 10.1016/j.jpain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychological Bulletin. 2001;127(5):599–617. doi: 10.1037/0033-2909.127.5.599. [DOI] [PubMed] [Google Scholar]
- Redinbaugh EM, Baum A, DeMoss C, Fello M, Arnold R. Factors associated with the accuracy of family caregiver estimates of patient pain. Journal of Pain Symptom Management. 2002;23(1):31–38. doi: 10.1016/s0885-3924(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Peters ML, Fassaert T, Vlaeyen JW. The role of fear of movement and injury in selective attentional processing in patients with chronic low back pain: a dot-probe evaluation. Journal of Pain. 2005;6(5):294–300. doi: 10.1016/j.jpain.2004.12.011. doi: 10.1016/j.jpain.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schoth DE, Liossi C. Attentional bias toward pictorial representations of pain in individuals with chronic headache. The Clinical Journal of Pain. 2010;26(3):244–250. doi: 10.1097/AJP.0b013e3181bed0f9. doi: 10.1097/AJP.0b013e3181bed0f9. [DOI] [PubMed] [Google Scholar]
- Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clinical Psychology Review. 2012;32(1):13–25. doi: 10.1016/j.cpr.2011.09.004. doi: 10.1016/j.cpr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Schrooten MGS, Van Damme S, Crombez G, Peters ML, Vogt J, Vlaeyen JWS. Nonpain goal pursuit inhibits attentional bias to pain. Pain. 2012;153(6):1180–1186. doi: 10.1016/j.pain.2012.01.025. doi: 10.1016/j.pain.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Dear BF, Schrieber L. Attentional Biases in Chronic Pain Associated With Rheumatoid Arthritis: Hypervigilance or Difficulties Disengaging? The Journal of Pain. 2009;10(3):329–335. doi: 10.1016/j.jpain.2008.10.005. doi: 10.1016/j.jpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Ianiello M, Dear BF, Nicholson K, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain. 2012;153(3):722–731. doi: 10.1016/j.pain.2011.12.014. doi: 10.1016/j.pain.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Snijders TJ, Ramsey NF, Koerselman F, van Gijn J. Attentional modulation fails to attenuate the subjective pain experience in chronic, unexplained pain. European Journal of Pain. 2010;14(3):282.e281–282.e210. doi: 10.1016/j.ejpain.2009.05.019. doi: 10.1016/j.ejpain.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C. The anticipation of pain modulates spatial attention: evidence for pain-specificity in high-pain catastrophizers. Pain. 2004;111(3):392–399. doi: 10.1016/j.pain.2004.07.022. doi: 10.1016/j.pain.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C, Koster EHW. Hypervigilance to learned pain signals: A componential analysis. Journal of Pain. 2006;7(5):346–357. doi: 10.1016/j.jpain.2005.12.006. doi: 10.1016/j.jpain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Hermans D, Koster EHW, Eccleston C. The role of extinction and reinstatement in attentional bias to threat: A conditioning approach. Behaviour Research and Therapy. 2006;44(11):1555–1563. doi: 10.1016/j.brat.2005.11.008. doi: 10.1016/j.brat.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Lorenz J, Eccleston C, Koster EHW, De Clercq A, Crombez G. Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophysiologie Clinique-Clinical Neurophysiology. 2004;34(1):33–39. doi: 10.1016/j.neucli.2003.11.001. doi: 10.1016/S0987-7053(03)00102-3. [DOI] [PubMed] [Google Scholar]
- Van Ryckeghem DM, Crombez G, Eccleston C, Liefooghe B, Van Damme S. The interruptive effect of pain in a multitask environment: an experimental investigation. Journal of Pain. 2012;13(2):131–138. doi: 10.1016/j.jpain.2011.09.003. doi: 10.1016/j.jpain.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Van Ryckeghem DM, Crombez G, Goubert L, De Houwer J, Onraedt T, Van Damme S. The predictive value of attentional bias towards pain-related information in chronic pain patients: A diary study. Pain. 2013;154(3):468–475. doi: 10.1016/j.pain.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Van Ryckeghem DM, Crombez G, Van Hulle L, Van Damme S. Attentional bias towards pain-related information diminishes the efficacy of distraction. Pain. 2012;153(12):2345–2351. doi: 10.1016/j.pain.2012.07.032. doi: 10.1016/j.pain.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Wright KD, Asmundson GJG, McCreary DR. Factorial validity of the short-form McGill pain questionnaire (SF-MPQ). European Journal of Pain. 2001;5(3):279–284. doi: 10.1053/eujp.2001.0243. doi: 10.1053/eujp.2001.0243. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jackson T, Gao X, Chen H. Identifying selective visual attention biases related to fear of pain by tracking eye movements within a dot-probe paradigm. Pain. 2012;153(8):1742–1748. doi: 10.1016/j.pain.2012.05.011. doi: 10.1016/j.pain.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ. Uncertainty Increases Pain: Evidence for a Novel Mechanism of Pain Modulation Involving the Periaqueductal Gray. The Journal of Neuroscience. 2013;33(13):5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. doi: 10.1523/jneurosci.4984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WK, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic: Further validation of the sds. Archives of General Psychiatry. 1965;13(6):508–515. doi: 10.1001/archpsyc.1965.01730060026004. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]
- Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13(6):508–515. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]