Abstract
Emotional instability is a hallmark feature of borderline personality disorder (BPD), yet its biological underpinnings are poorly understood. We employed functional MRI to compare patterns of regional brain activation in BPD patients and healthy volunteers as they process positive and negative social emotional stimuli. fMRI images were acquired while 19 BPD patients and 17 healthy controls (HC) viewed emotion-inducing pictures from the IAPS set. Activation data were analyzed with SPM5 ANCOVA models to derive the effects of diagnosis and stimulus type. BPD patients demonstrated greater differences in activation than controls, when viewing negative pictures compared to rest, in the amygdala, fusiform gyrus, primary visual areas, superior temporal gyrus (STG), and premotor areas, while healthy controls showed greater differences than BPD’s in the insula, middle temporal gyrus and dorsolateral prefrontal cortex (BA46). When viewing positive pictures compared to rest, BPD patients showed greater differences in the STG, premotor cortex, and ventrolateral prefrontal cortex. These findings suggest that BPD patients show greater amygdala activity and heightened activity of visual processing regions than HC’s, when processing negative social emotional pictures compared to rest. They activate neural networks in emotion processing that are phylogenetically older and more reflexive than healthy controls.
Keywords: Affective Instability, Emotion, fMRI, Social-Emotional Cues, Borderline Personality Disorder
1. INTRODUCTION
Emotional instability is one of the most striking features of borderline personality disorder (BPD) and is central to many of the behavioral and interpersonal symptoms of the disorder (Linehan 1993; Stone 1988), including some of the most disabling, even life-threatening, symptoms of BPD, such as suicidality, outbursts of intense anger, stormy relationships, and identity disturbances (Koenigsberg et al 2001). This emotional instability may be related to a heightened attention or sensitivity to social-emotional cues in interpersonal senarios (Lynch et al 2006; Meyer et al 2004; Taylor and Fragopanagos 2005; Wagner and Linehan 1999), a tendency to self-referential emotional processing (Schnell et al 2007), or to dysregulated emotional processing mechanisms (Phillips et al 2003b). Understanding the nature of the disturbances in emotion processing in BPD may provide important insights into the mechanisms of affective instability, the underlying pathology of the disorder, understanding the relationship between BPD and the axis I mood disorders, and help identify endophenotypes that could focus genetic studies of BPD, and target biological or psychological treatments to more specifically address affective instability in BPD.
Neuroimaging studies have begun to identify networks that are engaged in emotion processing in healthy individuals and in those with disturbed affect. A number of studies have employed images from the International Affective Pictures System (IAPS; Lang et al 2001) as emotional stimuli. The IAPS is a set of positive, negative and neutral valence pictures for which normative data for picture valence and arousal level is available. In healthy individuals, viewing of emotional pictures is associated with activation in the visual cortex (Britton et al 2006; Takahashi et al 2004), ventromedial prefrontal cortex and medial orbitofrontal cortex (Britton et al 2006; Grimm et al 2006; Northoff et al 2000; Takahashi et al 2004), anterior cingulate (Grimm et al 2006; Takahashi et al 2004), the dorsolateral prefrontal cortex (Grimm et al 2006; Northoff et al 2000), amygdala- hippocampal region (Britton et al 2006; Takahashi et al 2004) and basal ganglia (Takahashi et al 2004). Differences in activation patterns in these regions have been identified in schizophrenic subjects with and without affective flattening (Takahashi et al 2004), phobics (Goossens et al 2007), and individuals high in neuroticism (Britton et al 2007).
Little is known about the neurobiological underpinnings of the emotional instability in BPD, but the BPD syndrome itself has been associated with regional hypometabolism and deficits in serotonergic activity (De La Fuente et al 1997; Juengling et al 2003; Leyton et al 2001; New et al 2002; Siever et al 1999; Soloff et al 2000). Structural MRI studies have found smaller amygdala, hippocampal (Driessen et al 2000); (Schmahl et al 2003); (Tebartz van Elst et al 2003), anterior cingulate (Hazlett et al 2005; Tebartz van Elst et al 2003) and orbitofrontal cortex (Tebartz van Elst et al 2003) volumes in BPD patients compared to controls. Two functional neuroimaging studies of borderline patients performing an emotion-related task have been reported. In the first, BOLD fMRI images were obtained of six BPD patients and controls as they viewed negative compared to neutral pictures (inanimate objects). Compared to healthy controls, the BPD patients showed an increased activation of the amygdala bilaterally and of the medial and inferolateral prefrontal cortex when viewing the negative versus the neutral images (Herpertz et al 2001). The second study examined the processing of facial expressions of emotion (Donegan et al 2003). The BPD patients showed increased left amygdala activation to fearful, sad, happy and neutral faces.
The emotional instability in BPD is associated with emotional reactivity to social events (Stiglmayr et al 2005), yet the neuroimaging studies of emotion processing in BPD have thus far been confined to studies of face perception (Donegan et al 2003) and to scenes intermixing social and non-social stimuli (e.g images of attacking animals, offensive insects and reptiles, and disfigured bodies), making it impossible to characterize the processing of social cues in particular. This is a serious limitation since social and non-social emotional stimuli are processed differently in the brain (Britton et al 2006). The present study represents an important advance because of its focus on social emotional processing in particular.
A network comprising the amygdala, fusiform gyrus, superior temporal sulcus (STS), primary visual regions, and the prefrontal cortex has been implicated in visual social emotional cognition (Adolphs and Spezio 2006; Allison et al 2000; Bokde et al 2006). This model posits that visual social stimuli are processed by the fusiform face area in interaction with the STS, which attributes motivation and social intension. Emotional salience is then assigned by the amygdala, together with other prefrontal areas such as the insula. The amygdala, via feedback loops to the STS and more primary visual areas, may activate attentional amplification (Allison et al 2000) to relevant features of the stimuli. Building upon this formulation, Satpute and Lieberman (Satpute and Lieberman 2006) have proposed a dual-process model of social cognition in which there is a division between “reflexive” and “reflective” neural systems. The former, including the amygdala, STS, orbitofrontal (OFC) cortex, dorsal anterior cingulate (dACC) and basal ganglia, provides an automatic, fast operating emotional response, while the latter, incorporating the lateral and medial prefrontal areas, the medial temporal lobe and the rostral anterior cingulate (rACC), provides a more nuanced, experienced-based, but slower-responding emotional appraisal. We hypothesize that the increased emotional reactivity characteristic of BPD patients may be a consequence of their inability to adequately engage the reflective system and thus to rely heavily upon the more primitive reflexive system. This model would imply that when processing social emotional stimuli, BPD patients compared to healthy subjects would show greater activation of the amygdala, fusiform gyrus, primary visual areas, STS, dACC and OFC, while healthy subjects would demonstrate greater activation of lateral and medial prefrontal areas and medial temporal regions compared to BPD subjects. To test these hypotheses we obtained BOLD fMRI images of BPD patients and healthy volunteers as they viewed social emotional pictures.
2. METHODS
2.1 Subjects
Subjects were 19 BPD patients and 17 healthy volunteers (HC) recruited from the outpatient clinics at the Mount Sinai Medical Center in New York City, and the Bronx Veterans Affairs Medical Center, and by advertisements in local newspapers. They were male and female between 18 and 50 years of age. BPD subjects met DSM-IV criteria for BPD and had prominent affective instability as evidenced by the presence of three of four BPD criteria associated with affective instability (Koenigsberg et al 2001), i.e. (1) affective instability due to a marked reactivity of mood, (2) chronic feelings of emptiness, (3) a pattern of unstable and intense interpersonal relationships, and (4) identity disturbance. BPD subjects could not meet DSM-IV criteria for present or past bipolar I disorder, schizophrenia, schizoaffective disorder, substance dependence, or organic mental syndromes, and could not have histories of significant head trauma, CNS neurological disease, or significant medical illness, or a substance abuse disorder within the previous 6 months. All subjects were free of psychotropic medication for at least two weeks (6 weeks in the case of fluoxetine) prior to the scan.
The healthy volunteers could not meet criteria for any current or past Axis I or Axis II disorder and could not have a family history of an Axis I disorder. Subjects with contraindications to MRI, pregnant women and patients with current active suicidal ideation were excluded.
All subjects received a physical examination, EKG, complete blood count, electrolyte, liver and renal function tests, thyroid function tests, urine analysis and a urine toxicology screen. The Structured Clinical Interview for DSM-IV (SCID-I/P) was utilized to evaluate Axis I diagnoses. The Schedule for Interviewing DSM-IV Personality Disorders-IV (SIDP-IV) was utilized to evaluate criteria for DSM-IV personality disorders on the basis of Ph.D. or Master’s level psychologists interviewing the patient and an informant close to the patient when available. In previous studies (Koenigsberg et al 2002) we have documented an interrater reliability of kappa = 0.81 for diagnosing BPD. Subjects signed an informed consent after the study was explained to them.
As a measure of affective instability, subjects completed the Affective Lability Scale (ALS) (Harvey et al 1989), a 54-item self report scale which has been shown to correlate with clinician-rated affective instability in patients with BPD (Koenigsberg et al 2002). Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield 1971).
The two groups did not differ in age (BPD: 34.9 ± 11.1 vs. HC: 31.2 ± 10.6; t34 = .997, NS), or gender (BPD: 7 females vs. HC: 8 females; χ2 = 0.39, NS). Both groups were primarily right-handed (BPD: 14 right-handed, 4 left-handed, 1 mixed; HC: 15 right-handed, 1 left-handed, 1 mixed; χ2 = 1.834, df = 5, NS). Seven BPD subjects had a past history of major depression; none met criteria for a current major depressive episode. One BPD subject met criteria for current bipolar II disorder. Six subjects had a history of PTSD, of whom 4 currently met PTSD criteria. Comorbid personality disorders in the BPD sample included 11 subjects with paranoid personality disorders, 7 with avoidant, 4 with antisocial, 6 with schizotypal, 4 with obsessive compulsive, 1 with histrionic and 6 with narcissistic personality disorders. Hamilton depression score ratings indicated that the BPD’s were more depressed than the HC’s, but their level of depression was mild (BPD: 9.38 ± 4.86; HC: 1.33 ± 0.89; t23=5.65, p<.001). Consistent with a higher level of affective instability, the BPD subjects attained a significantly higher total ALS scale score than the normal controls (BPD: 1.60 ± 0.41 [range: 0.78 – 2.50] vs. HC: 0.31 ± 0.25 [range: 0.00 – 0.81]; t34 = 11.20, p < .0001).
2.2 Experimental Paradigm
Each subject viewed 25 negative and 25 positive pictures while BOLD fMRI images were acquired. Pictures were selected from the International Affective Pictures System (IAPS) (Lang PJ 2001), a collection of photographic images that have been shown to induce positive, negative or neutral affective states. We selected pictures with social-emotional content1 by including IAPS pictures that showed two or more persons in interaction or one person emotionally relating to the viewer, and excluding those IAPS pictures whose negative valence derived from non-interpersonal situations such as scenes of fearsome animals, reptiles or insects, or bodily deformity. The included images were rated as high in either positive or negative valence and high in arousal, based upon the normative data provided for the IAPS (Lang PJ 2001).
The pictures were presented in blocks of 5 images of a given valence, with each individual image present for 6 seconds. Five blocks of positive and negative images were presented in alternation beginning with a positive block. A blue screen bearing the word “relax”, the rest condition, appeared for 30 seconds before the first image block and between each positive and negative image block.
Subjects rested supine in the scanner and viewed the images via a set of fiber optic goggles (SV2000; Avotec, Inc.) positioned in the head coil above their eyes. They were instructed to keep their eyes open, to watch all images and to allow themselves to “feel fully whatever emotion the slides produced.” The subjects were also told that should they find any slide too disturbing, they could ask that the image be turned off. No subjects chose this option.
2.3 Manipulation Check
Immediately following the scanning session, the subjects were asked to view, on a 15 inch laptop display, the IAPS pictures that they had seen during the scan and to rate their subjective reaction to each image using the Self-Assessment Manikin (SAM, (Bradley and Lang 1994), a pictorial assessment instrument that measures valence (pleasure vs. unpleasure) and arousal (stimulated vs. calm), each on a 9-point scale (for valence: 1= most positive, 9= most negative; for arousal: 1 = most calm, 9 = most aroused). After they completed the SAM rating, the subjects were then debriefed about their experience.
2.4 Image Acquisition and Analysis
MRI scanning was performed on a Siemens 1.5T Symphony with enhanced (Quantum) gradients using the standard quadrature head coil. Following a localizer, anatomical images (T1W) were acquired with a spin-echo sequence (TR/TE/FA 524/14/90, 20 axial 5 mm slices with 1.5mm gap, FOV 220 mm, matrix 256×256). BOLD images were acquired at the same slices with single-shot EPI (TR/TE/FA 3000/60/90, matrix 64×64 with fat saturation); 220 BOLD images were acquired, but the first 10 were discarded to ensure magnetization steady-state and the last ten were also discarded. Each block (10 BOLD images, 30 sec) consisted of either a rest period or negative or positive image viewing (5 images of 6 sec duration).
Image analysis was carried out using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). Motion correction was applied by realigning all images to the first image using 6 parameter rigid body transformation and reslicing with 4th degree B-spline interpolation. The mean BOLD image was then spatially normalized with SPM5’s EPI template using both affine and non-linear normalization. Estimated normalization parameters are then applied to realigned BOLD images followed by 6mm Gaussian kernel smoothing. Non-linear frequency cutoff, iterations, and regularization were set to 25mm, 16, and 1, respectively. Following motion correction and temporal and spatial filtering, each subject was individually checked for image quality and analyzed for the contrasts: positive vs. rest, negative vs. rest, negative vs. positive, and positive vs. negative.
The first-level fixed-effects analysis included motion realignment parameters as regressors, and identified regions of significantly different BOLD activation in subjects viewing negative pictures vs. rest, positive pictures vs. rest, positive vs. negative pictures and negative vs. positive pictures. To test for differences between groups, we carried out a random-effects ANOVA design in each activation response (BPD>HC and HC>BPD) for the negative picture-rest (N-R), positive picture-rest (P-R), negative-positive picture (N-P), and positive-negative picture (P-N) contrasts. We repeated the same analyses entering handedness as a covariate (separate regressors for right and left handedness where mixed handedness was defined as right and left handedness). The findings with and without the handedness covariate were essentially the same. For economy of space we present below the results of the analysis with handedness covaried. These statistical tests employed a random-effects model, defining clusters with a voxel-wise significance level of p=.005 uncorrected and a minimum cluster size of k = 20 voxels. Final anatomical labeling of findings in MNI space was based on the MSU Matlab toolbox (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html).
Additional procedures were carried out for analysis of amygdala and fusiform activation, due to their importance for our a priori hypotheses and their isolation by previous authors as relevant structures. We assessed their activation by three separate methods. First, we relaxed the overall SPM second-level (random effects) analysis to a voxel significance threshold of 0.10, within a cluster size of k=10, to search for clusters anatomically belonging to the amygdala and fusiform gyrus. Such clusters were found in the left amygdala (at coordinates −16, −6, −14, with 18 contiguous voxels) and right fusiform (coordinates 42, −12, −32, 22 voxels). We then conducted the SPM small volume correction as suggested by previous authors, with a 12mm diameter sphere for the amygdala (Strange & Dolan, 2004) and 20mm diameter sphere for the fusiform gyrus (Winston et al., 2002). Second, we extracted the signal magnitude, defined by contrast image intensity of each subject, at the original cluster voxels, averaged them, and subjected this mean VOI signal to additional tests. Finally, we defined anatomical VOIs on both right and left amygdala and fusiform gyrus, using the Wake-Forest University digital atlas (Maldjian et al., 2003), averaged the signal magnitude in those anatomical VOIs, and subjected them to statistical analyses. These numbers extracted from contrast images define the difference in the slope fitted with HRF “beta(Negative)-beta(Rest)”. Thus Positive and negative values refer to “beta(Positive)>beta(Rest)” and “beta(Negtaive)<beta(Rest)”, respectively.
3. RESULTS
3.1 Self-Report
The manipulation check confirmed that the positive and negative pictures elicited emotional reactions of the expected valence. For the BPD subjects, the mean SAM valence rating for the negative pictures was 7.18 ± 0.19 and 3.63 ± 0.17 for the positive pictures. The healthy controls rated the negative pictures at 7.49 ± 0.19 and the positive pictures 3.50 ± 0.17. A repeated measures ANOVA with SAM valence rating as the dependent variable, IAPS picture valence category (positive vs. negative) as the within subjects variable and diagnosis as the between subjects variable revealed a main effect for picture valence category (F[1,33] = 259.0, p<.0001), but no diagnosis X valence category interaction (F[1,33] = 0.85, NS), nor a main effect of diagnosis. Post hoc t-tests showed that within each group the positive and negative picture ratings were significantly different, confirming that subjects within each group differentiated the positive from negative IAPS pictures.
The negative pictures were more arousing than the positive pictures for both groups. The BPD subjects rated their subjective level of arousal for the negative pictures at 6.00 ± 0.29 and for the positive pictures at 4.71 ± 0.27. The healthy controls rated their arousal to the negative pictures at 6.40 ± 0.30 and to the positive pictures at 4.64 ± 0.28. Repeated measures analysis of variance demonstrated a main effect in arousal for picture valence category (F[1,33] = 27.91, p = .000008), without a main effect of diagnosis or an interaction. Post hoc tests showed the differences were significant within each group as well. In the debriefing, all subjects reported that their reactions to the pictures in the immediate post-scan rating session were similar to their reactions while viewing the pictures during the scan.
3.2 BOLD Activation
To compare patterns of brain activation between BPD patients and HC’s as they viewed emotional stimuli, we obtained statistical maps of the difference in BOLD response when viewing negative pictures compared to rest (N-R) and when viewing positive pictures compared to rest (P-R) for BPD subjects and healthy controls. For each of these contrasts we examined the between group differences (i.e. BPD > HC and HC > BPD) (Tables 1 & 2; Figs. 1 & 2). Examining the difference in BOLD activation when viewing negative compared to positive emotional scenes (N-P) permitted subtracting out the effects of looking at faces and social scenes. The between group differences for the N-P contrast are presented in Table 3.
Table 1.
Group Differences in Regional Activation for Negative-Rest Contrasts
| Region | k | MNI Coordinates | T | p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BPD>HC | ||||||
| R Cuneus/Middle Occipital G. (BA18) | 82 | 16 | −92 | 10 | 4.36 | 0.000 |
| R Sup Occipital G/ Cuneus (BA19) | 22 | 32 | −86 | 28 | 3.33 | 0.002 |
| R Middle Occipital G. (BA19) | 26 | 48 | −74 | −12 | 3.36 | 0.001 |
| R Middle Occipital/Temporal G. | 57 | 30 | −76 | 18 | 3.34 | 0.001 |
| L Sup. Temporal G. | 48 | −46 | −42 | 16 | 3.45 | 0.001 |
| R Lingual G. (BA18/19) | 28 | 18 | −68 | 0 | 3.33 | 0.001 |
| R Precuneus/ Post Cingulate | 24 | 6 | −66 | 18 | 3.51 | 0.001 |
| R Parahippocampal G. (BA19/30) | 37 | 16 | −48 | 0 | 3.24 | 0.001 |
| R Sup Temp G/Intraparietal Lob (BA13) | 87 | 46 | −44 | 14 | 4.14 | 0.000 |
| R Middle Frontal G. (BA6) | 26 | 36 | −2 | 48 | 3.33 | 0.001 |
| HC>BPD | ||||||
| L Middle Temporal G. | 60 | −44 | −28 | −10 | 3.85 | 0.000 |
| R Insula (BA13) | 44 | 48 | −10 | 22 | 3.54 | 0.001 |
| R Middle Frontal G. (BA46) | 25 | 40 | 44 | 10 | 3.32 | 0.001 |
Clusters of activation for >20 contiguous voxels with local maxima of t>2.74, p<.005 uncorrected. k = cluster size in voxels, x,y,z are MNI coordinates of local voxel with maximum t, t= t-score of local maximum, p = p-value of that local maximum voxel.
Table 2.
Group Differences in Regional Activation for Positive-Rest Contrasts
| Region | k | MNI Coordinates | T | p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BPD>HC | ||||||
| R Cerebellar Declive | 27 | 16 | −76 | −28 | 3.29 | 0.001 |
| R Middle Occipital G (BA19) | 36 | 48 | −70 | −14 | 3.76 | 0.000 |
| L Middle/Superior Temporal G. (BA39) | 42 | −54 | −64 | 22 | 3.62 | 0.000 |
| R/L Superior Frontal G (BA6) | 45 | 4 | 6 | 64 | 4.19 | 0.000 |
| L Inferior Frontal G (BA45/47) | 24 | −54 | 22 | 6 | 3.56 | 0.001 |
| L Thalamus (Vent Lat Nuc) | 29 | −16 | −12 | 4 | 3.83 | 0.000 |
| Post Cingulate (BA23) | 40 | 0 | −62 | 14 | 3.61 | 0.001 |
| L Inf Frontal G (BA47) | 46 | −40 | 24 | 2 | 3.34 | 0.001 |
| R Lingual G. (BA19/30) | 21 | 18 | −50 | 0 | 3.22 | 0.001 |
| R Superior Temporal G. | 21 | 48 | −40 | 16 | 3.09 | 0.002 |
| HC>BPD | ||||||
| R Caudate | 32 | 18 | 28 | 4 | 3.36 | 0.001 |
| L Caudate/Lat Ventricle | 101 | −20 | 34 | 6 | 4.04 | 0.000 |
Clusters of activation for >20 contiguous voxels with local maxima of t>2.74, p<.005 uncorrected. k = cluster size n voxels, x,y,x are MNI coordinates of local voxel with maximum t, t= t-score of local maximum, p = p-value of that local maximum voxel.
Figure 1.
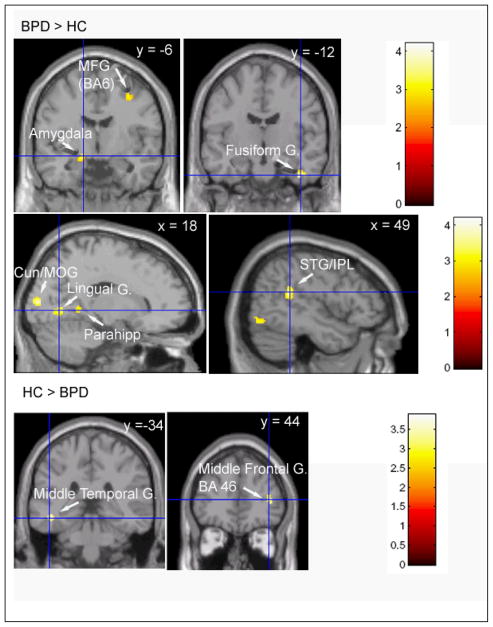
Regions in which BPD’s show a greater Negative Picture vs Rest Activation than Healthy Volunteers (BPD>HC) and Healthy volunteers show a greater Negative Picture vs. Rest Activation than BPD’s (HC>BPD). MFG – Middle Frontal Gyrus, Cun – Cuneus, STG- Superior Temporal Gyrus, IPL – Intraparietal Lobule.
Figure 2.
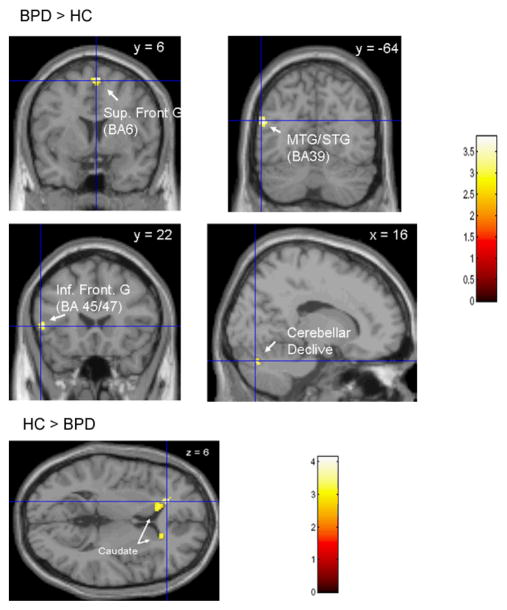
Regions in which BPD’s show a greater Positive Picture vs Rest Activation than Healthy Volunteers (BPD>HC) and Healthy volunteers show a greater Positive Picture vs. Rest Activation than BPD’s (HC>BPD). MTG – Middle Temporal Gyrus, STG- Superior Temporal Gyrus.
Table 3.
Group Differences in Regional Activation for Negative-Positive Contrasts
| Region | k | MNI Coordinates | T | p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BPD>HC | ||||||
| R Middle Occipital G./Cuneus | 117 | 16 | −94 | 12 | 4.07 | 0.000 |
| L Middle Occipital G. | 88 | −34 | −88 | 14 | 4.20 | 0.000 |
| L Middle Occipital G. | 26 | −26 | −84 | −2 | 3.61 | 0.001 |
| R Middle Occipital G. | 20 | 26 | −90 | −2 | 3.36 | 0.001 |
| R Cuneus | 22 | 6 | −88 | 22 | 3.40 | 0.001 |
| L Fusiform G. | 109 | −38 | −54 | −18 | 4.15 | 0.000 |
| R Temporal Lobe (White Matter) | 26 | 30 | −60 | 24 | 4.06 | 0.000 |
| R Cerebellum (Culmen) | 74 | 30 | −48 | −22 | 3.99 | 0.000 |
| R Paracentral Lobule/Precuneus (BA5/7) | 65 | 18 | −42 | 42 | 3.70 | 0.000 |
| R Inferior/Middle Frontal G. (BA9/46) | 21 | 44 | 20 | 22 | 3.24 | 0.001 |
| HC>BPD | ||||||
| L Superior/Middle Temporal G. | 39 | −44 | −26 | −8 | 3.98 | 0.000 |
| R Frontal Lobe White Matter | 32 | 30 | −20 | 40 | 4.07 | 0.000 |
| R Insula | 64 | 42 | −12 | 16 | 4.30 | 0.000 |
Clusters of activation for >20 contiguous voxels with local maxima of t>2.74, p<.005 uncorrected. k = cluster size n voxels, x,y,x are MNI coordinates of local voxel with maximum t, t= t-score of local maximum, p = p-value of that local maximum voxel.
The BPD’s demonstrate a greater difference in BOLD activation to negative pictures vs. rest than HC’s in the primary visual areas (BA18, BA19), premotor cortex (BA6), superior temporal gyrus, precuneus/posterior cingulate, and parahippocampal gyrus.
Left amygdala activation was noted by the following methods. The small volume SPM analysis yielded a significant voxel-level outcome after appropriate correction for multiple comparisons (T=3.12, p < .05 by FWE corrected). Larger search diameters did not improve the results, suggesting that this was indeed a highly focal activation. This was confirmed by the anatomical VOI analyses, which failed to reach significance for either the right or left amygdala. The extracted cluster voxels yielded a higher significant diagnostic effect when gender was controlled: in an ANOVA of diagnosis by gender, while neither the gender main effect nor interaction reached significance, the diagnostic main effect was similar (BPD > HC, p<.006, F1,32=8.73). Similarly, in an ANCOVA with age covariance, the diagnostic difference reached p<.005 (F1,32=9.51).
Right fusiform gyrus activation was also detected by the small volume correction (T=3.80, p=0.015 FWE correction). Similar to the amygdala, the larger anatomical VOIs failed to reach significance, but the functional cluster demonstrated significantly higher activation for BPD patients than controls when gender was controlled (F1,32=13.01, p=.001), as well as when age was controlled by ANCOVA (F1,32=13.68, p=.0008).
In a model that examined diagnosis, age, gender, and their interactions with diagnosis, only diagnosis was significant. The signal magnitude was significantly higher in the BPD patients for left amygdala (t34=3.20, p=.003) and right fusiform (t34=3.82, p=.0005).
When viewing positive pictures, compared to rest, the BPD’s showed a greater difference in BOLD activation than the HC’s in the premotor cortex, superior temporal gyrus, inferior frontal gyrus, posterior cingulate and cerebellar declive. The HC’s showed a greater difference than the BPD’s in the insula, middle temporal gyrus and middle frontal gyrus (BA46) when viewing negative pictures compared to rest. When viewing positive pictures compared to rest, HC’s showed a greater difference in BOLD activation than BPD’s in the caudate bilaterally.
The pattern of activation for the N-P contrast was similar to that for the N-R contrast, with BPD’s showing greater activation than HC’s in the primary visual areas, precuneus, fusiform gyrus and left lateral globus pallidus extending to the amygdala and the HC’s showing greater activation than the BPD’s in the right insula.
4. DISCUSSION
The manipulation check confirmed that the IAPS pictures selected as negative in valence were in fact experienced by the subjects as negative and as significantly more negative than the pictures selected as positive. There were no group differences in the ratings of valence or arousal of the pictures. Nevertheless, the BPD subjects and the normal controls show different patterns of neural activation during the processing of the emotional pictures. These observations are similar to those of Herpertz et al (Herpertz et al 2001), who also found no group differences in the subjective valence and arousal rating to IAPS pictures, but did find different patterns of regional brain activation. This raises the possibility that, although borderline patients demonstrate a greater reactivity of mood and a higher sensitivity to emotional stimuli than healthy controls, their subjective experience of emotional intensity does not differ from that of the healthy volunteers. This is consistent with the finding reported previously by us (Koenigsberg et al 2002) that whereas BPD patients report greater levels of affective lability than a comparison group of personality disorder patients, they do not report a higher level of affective intensity.
When viewing negative pictures compared to rest, BPD patients showed a greater difference in BOLD activation in the amygdala and fusiform gyrus than HC’s. The amygdala plays a central role in assessing emotional salience, in face processing and in the generation of an affective state and is particularly reactive to aversive stimuli (Dickstein and Leibenluft 2006; Phillips et al 2003a). The fusiform cortex is implicated in face processing as well. The finding of a greater N-R difference in BOLD activation in BPD’s compared to HC’s in the left amygdala and fusiform gyrus is consistent with the findings of Herpertz et al (Herpertz et al 2001) and Donegan et al (Donegan et al 2003). Activation of the left amygdala in particular has been associated with the viewing of sad or fearful faces (Lane et al 1997b). The BPD’s also showed a greater N-R BOLD difference than HC’s in primary visual processing areas (the middle occipital gyrus, the cuneus, and the lingual gyrus). This is consistent with an upregulation of the visual processing stream in response to feedback from the amygdala, which has projections to the visual cortex (Morris J 2004; Taylor and Fragopanagos 2005; Vuilleumier et al 2004). Thus one possible interpretation of our finding is that in the presence of negative visual emotional stimuli, BPD patients enhance the activation of primary visual processing regions. Duncan and Barrett (Duncan and Barrett 2007) have suggested that such heightened visual activation may allow individuals to be aware of affective stimuli that may be invisible to others. Such a neural mechanism could account for the finding that borderline patients have a heightened visual sensitivity to identifying facial expressions of emotion (Lynch et al 2006; Wagner and Linehan 1999). This intriguing idea raises the possibility that borderline patients’ exquisite interpersonal sensitivity may be related to a hyperawareness of facial expression or other social cues.
Further along in the visual processing stream, BPD patients show greater N-R and P-R differences in BOLD activation than HC’s in the superior temporal gyrus (STG). The STG is implicated in the processing of human actions and in the assessment of intensions (Allison et al 2000) and is considered to be part of a fast-response, phylogenetically older, and more reflexive social processing system (Satpute and Lieberman 2006). When processing negative pictures compared to rest, the healthy volunteers show greater difference in activation than the BPD’s in BA46, a dorsolateral prefrontal region involved in reflective cortical processing and executive control. Although these observations require replication, they raise the possibility that, when processing social-emotional cues, BPD patients rely more upon reflexive, automatically responding networks, whereas healthy volunteers call upon networks with access to higher level conscious cortical processing. The BPD patients also showed greater N-R and P-R differences in activation in the precuneus and posterior cingulate than HC’s. The precuneus and posterior cingulate have been implicated in self-referential processing and first person perspective (see (Vogt 2005)and review by (Cavanna and Trimble 2006)), features consistent with the tendency for BPD patients to become emotionally overinvolved in interpersonal situations.
The healthy subjects demonstrated a greater difference in BOLD activation in the insula than the BPD’s when viewing negative pictures compared to rest and negative pictures compared to positive pictures. The insula is involved in the processing of facial emotion (Adolphs and Spezio 2006) and in the subjective awareness of one’s emotional state (Craig 2004). Recently, increased insula activation has been reported in trauma exposed individuals who did not go on to develop PTSD, in contrast to similarly exposed subjects who developed PTSD (A. New, personal communication, May 2007). Thus insula activation may be associated with adaptive processing of emotional stimuli. The BPD patients show greater N-R and P-R differences in BOLD activation in premotor (BA6) regions compared to healthy volunteers. The activation of these regions may be a marker of a readiness to act, which is consistent with the tendency of borderline patients to ‘act out” when confronted with strong emotional states. BPD patients show smaller activation differences in the caudate than healthy controls when viewing positive pictures compared to rest. Caudate activation is associated with pleasurable experience (Aron et al 2005). BPD patients demonstrate greater P-R activation differences in the cerebellar declive than HC’s. The cerebellum receives afferents from the parahippocampal and anterior cingulate cortices and the hypothalamus, and has efferents to the prefrontal and cingulate cortices and the hypothalamus (Parvizi et al 2001) and has been shown to participate in emotional processing (Lane et al 1997a; Paradiso et al 1999).
fMRI examines task-dependent activation differences within subjects rather than absolute BOLD values, hence the findings we report of increased activation differences in one group compared to the other may reflect either an increased activation in the task condition (viewing negative or positive pictures) or decreased activation in the rest state or some combination of the two.
Strengths of the current study include the sample size, which is to our knowledge the largest sample of BPD subjects in an fMRI study of emotion processing. In addition, all subjects were unmedicated. Another strength of the present study is we have examined responses to social-emotional cues exclusively, excluding non-interpersonally related negative valence stimuli.
The present study has a number of limitations. The first results from our choice to contrast BOLD responses when viewing negative or positive pictures to the resting state rather than to the viewing of neutral pictures. This did not allow us to directly subtract out the effects of simply looking at complex scenes or faces. Despite this potential limitation, we chose the present design because we could not assure that borderline patients would perceive neutral pictures as such. A number of investigators have reported that BPD patients experience neutral faces as negative (Donegan et al 2003; Wagner and Linehan 1999), often attributing negative motives to neutral expressions. However, we did examine the BOLD negative minus positive (N-P) and positive minus negative (P-N) contrasts to obtain analyses which subtract out the effects of simply processing complex scenes and faces. Overall, we found the between group comparison for the N-P contrast similar to our findings in the negative-rest contrast, with BPD’s showing greater N-P differences in activation than HC’s in the amygdala, fusiform, and primary visual regions, and HC’s showing a greater N-P difference in the insula than the BPD’s. Thus, even when the effect of simply looking at scenes and faces is subtracted out, we find differences in BOLD activation between BPD’s and HC’s in their differential response to negative vs positive social emotional scenes. A second potential limitation in the present study arises from the fact that negative valence IAPS pictures are more arousing than positive IAPS pictures. Thus the differences between BPD and HC subjects with respect to negative pictures could be attributed either to a differential response to valence or to arousal. An arousal effect however is unlikely explain the group differences in processing the positive images, as they were not rated as highly arousing. Finally, since the BPD patients were, as is typical of clinical samples, more depressed than the HC’s, we can not exclude the possibility that the differences we find are related to depression rather than to the personality diagnosis. However, based on the mean Hamilton depression scores, the level of depression among the BPD patients in this sample was quite mild, close to the score typically used to identify remitted depressed patients (Thase and Ninan 2002).
The present study suggests that borderline personality disorder patients respond to negative and positive social emotional scenes with a more hyperarroused visual processing system than healthy volunteers and with a more activated premotor cortex. In addition, when the stimuli are negative, BPD subjects appear to show greater activity in the amygdala, fusiform, precuneus and parahippocampal regions than HC’s, who mobilize dorsolateral and insular regions instead. These findings are consistent with the model that borderline patients use a more reflexive, hypervigilant and action-prone system to process social emotional stimuli, whereas healthy volunteers call upon a more reflective and less reactive network. These observations may help explain the greater emotional reactivity of borderline personality disorder patients.
Acknowledgments
This work was supported by Grant Number MO1-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the Mental Illness Research Education and Clinical Center, VISN 3 Veterans Health Administration, and an educational grant from Siemens Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIH, or the VA. We thank Dr. Sergei Pakhomov (Institute of the Human Brain, St. Petersburg, Russia) for the MSU software.
Footnotes
The negative stimuli presented were IAPS pictures Nos. 2205, 6560, 9810, 6311, 6510, 2900, 2053, 6315, 2800, 3350, 6540, 6313, 3015, 9433, 9040, 9800, 3230, 6020, 6530, 6570, 3181, 3301, 6312, 6821, 6370, and the positive stimuli Nos. 2360, 2091, 2020, 2340, 2500, 2550, 4700, 2530, 2391, 2341, 2050, 8200, 8033, 2540, 8470, 8120, 7325, 5831, 4614, 5470, 2080, 2650, 1340, 2370, 2501.
Presented in part at the Annual Meeting of the Society for Biological Psychiatry, San Diego, CA May 2007.
FINANCIAL DISCLOSURES
The authors have no potential conflicts of interest.
References
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Britton JC, Ho SH, Taylor SF, Liberzon I. Neuroticism associated with neural activation patterns to positive stimuli. Psychiatry Res. 2007;156:263–267. doi: 10.1016/j.pscychresns.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- De La Fuente JM, Goldman S, Stanus E, Vizuete C, Morlan I, Bobes J, et al. Brain glucose metabolism in borderline personality disorder. J Psychiatr Res. 1997;31:531–541. doi: 10.1016/s0022-3956(97)00001-0. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Dev Psychopathol. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. The role of the amygdala in visual awareness. Trends Cogn Sci. 2007;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Schruers K, Peeters R, Griez E, Sunaert S. Visual presentation of phobic stimuli: amygdala activation via an extrageniculostriate pathway? Psychiatry Res. 2007;155:113–120. doi: 10.1016/j.pscychresns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. Neuroimage. 2006;30:325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, et al. Reduced Anterior and Posterior Cingulate Gray Matter in Borderline Personality Disorder. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Schmahl C, Hesslinger B, Ebert D, Bremner JD, Gostomzyk J, et al. Positron emission tomography in female patients with borderline personality disorder. J Psychiatr Res. 2003;37:109–115. doi: 10.1016/s0022-3956(02)00084-5. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, New AS, Goodman M, Silverman J, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Personal Disord. 2001;15:358–370. doi: 10.1521/pedi.15.4.358.19181. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, Schmeidler J, New AS, Goodman M, et al. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159:784–788. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997a;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997b;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lang PJBM, Cuthbert BN. International Affective Pictures System (IAPS):Technical Manual and Affective Ratings: NIMH Center for the Study of Emotion and Attention. 2001. [Google Scholar]
- Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, et al. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158:775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- Linehan M. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York: Guilford Press; 1993. [Google Scholar]
- Lynch TR, Rosenthal MZ, Kosson DS, Cheavens JS, Lejuez CW, Blair RJ. Heightened sensitivity to facial expressions of emotion in borderline personality disorder. Emotion. 2006;6:647–655. doi: 10.1037/1528-3542.6.4.647. [DOI] [PubMed] [Google Scholar]
- Meyer B, Pilkonis PA, Beevers CG. What’s in a (neutral) face? Personality disorders, attachment styles, and the appraisal of ambiguous social cues. J Personal Disord. 2004;18:320–336. doi: 10.1521/pedi.2004.18.4.320. [DOI] [PubMed] [Google Scholar]
- Morris JDR. Functional Neuroanatomy of Human Emotion. In: Frackowiak SJFK, Frith CD, et al., editors. Human Brain Function. 2. London: Elsevier; 2004. pp. 383–384. [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, et al. Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Lieberman MD. Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Res. 2006;1079:86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Schnell K, Dietrich T, Schnitker R, Daumann J, Herpertz SC. Processing of autobiographical memory retrieval cues in borderline personality disorder. J Affect Disord. 2007;97:253–259. doi: 10.1016/j.jad.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, et al. d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: a computer-based controlled field study. Acta Psychiatr Scand. 2005;111:372–379. doi: 10.1111/j.1600-0447.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- Stone M. Toward a Psychobiological Theory of Personality Disorder. Dissociation. 1988;1:2–15. [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Taylor JG, Fragopanagos NF. The interaction of attention and emotion. Neural Netw. 2005;18:353–369. doi: 10.1016/j.neunet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Thase ME, Ninan PT. New goals in the treatment of depression: moving toward recovery. Psychopharmacol Bull. 2002;36(Suppl 2):24–35. [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wagner AW, Linehan MM. Facial expression recognition ability among women with borderline personality disorder: implications for emotion regulation? J Personal Disord. 1999;13:329–344. doi: 10.1521/pedi.1999.13.4.329. [DOI] [PubMed] [Google Scholar]