Abstract
The inability to detect all individuals with active tuberculosis has led to a growing interest in new approaches to improve case detection. Policy makers and program staff face important challenges measuring effectiveness of newly introduced interventions and reviewing feasibility of scaling-up successful approaches. While robust research will continue to be needed to document impact and influence policy, it may not always be feasible for all interventions and programmatic evidence is also critical to understand what can be expected in routine settings. The effects of interventions on early and improved tuberculosis detection can be documented through well-designed program evaluations. We present a pragmatic framework for evaluating and measuring the effect of improved case detection strategies using systematically collected intervention data in combination with routine tuberculosis notification data applying historical and contemporary controls. Standardized process evaluation and systematic documentation of program implementation design, cost and context will contribute to explaining observed levels of success and may help to identify conditions needed for success. Findings can then guide decisions on scale-up and replication in different target populations and settings.
Keywords: Active case finding, Case detection, Screening, Tuberculosis
Introduction
Disease registration systems are maintained in many countries for both communicable and non-communicable diseases. The tuberculosis (TB) registration and reporting system is amongst the first established and best developed, with approximately 200 countries and territories reporting data annually1 using standardized forms developed through successive WHO guidance.2,3 The purposes of the system include both operational management of TB control efforts and surveillance of local, national and international trends. Quarterly routine reports from the system are either published or can be made available, comprising counts of diagnostic activities, cases enrolled on treatment and outcome of treatment disaggregated by a number of different measures. It is this system that has permitted the tracking of progress in TB control, nationally and globally. Although millions of people are treated for TB and millions of lives have been saved since 1990, each year an estimated 3 million incident cases of TB are ‘missed’, either never diagnosed or diagnosed and not reported to national TB programs (NTPs).1
Reaching all individuals ill with TB is becoming an increasingly important goal for national and global policy makers.4–6 The inability to detect all in need of care, has renewed interest in using active case finding (ACF) approaches as opposed to only waiting for people with undiagnosed TB to seek care, the so-called passive case finding (PCF). Recent multi-country initiatives7,8 supported by the publication of guidelines9–13 have focused efforts to improve TB case detection. The ultimate goal of improved TB case finding approaches should be reducing incidence and mortality through early detection and cutting transmission,14 but evidence and guidance on different strategies to improve case detection is yet to fully mature.10 The gold standard for evaluating the impact of an intervention is through randomized control trials (RCT) using measurable indicators in a well-defined population. To date, there have been two such trials measuring the impact of ACF on prevalence or incidence, which did not show a lasting impact on TB prevalence,15,16 and one cluster randomized trial without controls showing that ACF could significantly reduce TB prevalence.17 However, extrapolating their findings to other settings, is hampered by considerable variations in TB epidemiology, infrastructure and site-specific realities. RCTs are expensive, resource-intensive, require relatively stable conditions in the intervention and control zones, and require strict adherence to the study protocol for the entirety of the trial. Furthermore, the effect of improved case detection on transmission and incidence is likely to be seen only after several years of delay;18 therefore, a trial must take place over a period long enough to measure such impact. Finally, programmatic evidence is important for feedback to shape and revise policy guidance.19 NTPs and their partners aiming to reach, identify and successfully treat all people with TB as outlined in the recent post-2015 TB strategy20 require efforts to measure the effectiveness of different approaches. Consequently, a need remains to identify pragmatic solutions for measuring program performance and for evaluating outcomes and feasibility of different intervention strategies.
In this paper we discuss the approaches to selecting a TB case finding intervention and describe a pragmatic framework for monitoring and evaluation based primarily on routinely collected surveillance data, optimizing the strengths and limitations of different research and evaluation methodologies. Finally, we suggest a number of important implications that must be considered before introducing or scaling-up case detection interventions as part of TB control programs.
Selecting approaches to improve case detection
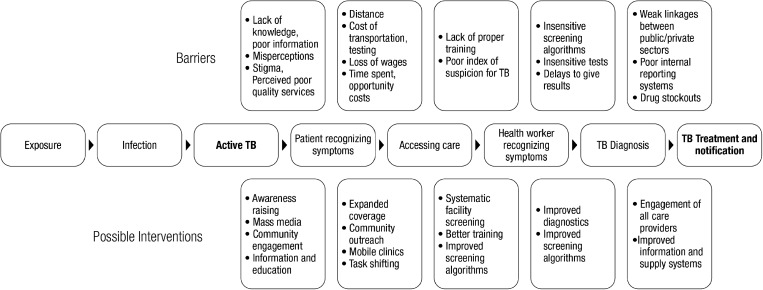
The decision of which case detection strategies to introduce should consider the local context and be based on a careful assessment of the epidemiological situation, potential benefits and risks, costs and available resources.14 Identifying key populations with high numbers of undiagnosed TB and understanding health-seeking behavior and the barriers to TB detection are necessary first steps when developing an intervention and an approach to measure its effectiveness. The pathways for identification of people with suspected TB, their diagnosis and treatment provide several entry points for potential improvement (Figure 1). The gaps between boxes are areas where people with TB may be ‘missed’. Five entry points for improving detection and notification are: improving awareness/knowledge, increasing access to care, better identification of people for testing, more sensitive and rapid diagnostic tests and stronger linkages for notification to NTPs.4 The first four areas are entry points to increase case finding and treatment.The last approach focuses on improving notification rather than necessarily increasing detection. It can be used when a sizable proportion of ‘missed’ cases are due to under notification; for example, by linking the private sector to NTPs and improving recording and reporting within NTPs. Studies on improved case detection have focused on different entry points such as improving access,15–17,21–26 accuracy of diagnostics27–31 and notification,32–34 and less frequently on measuring the effects of raising awareness35 and identification of people for testing.36
Figure 1.
Pathway to care with barriers and possible interventions.
The diagnostic algorithm for screening and testing must be considered when undertaking these ACF interventions. Highly sensitive screening tests are preferred in combination with highly specific confirmatory tests. While culture testing (both highly sensitive and specific) is not feasible in many settings, caution must be taken when actively screening people with a low pre-test probability of TB, such as house-to-house approaches. Using smear microscopy alone may lead to false positive results due to lower specificity. A recent ACF intervention in Uganda found half of smear-positive cases to be culture-negative.37 The use of chest x-ray as a screening rather than diagnostic tool can be highly sensitive for identifying people needing further testing.38
All interventions to improve TB case detection will attempt to identify and diagnose prevalent TB and cut the cycle of transmission. Based on the barriers addressed in the pathway to care, the intervention type, population targeted and testing procedures used, a monitoring and evaluation framework can be tailored.
A pragmatic framework for monitoring and evaluating TB case finding interventions
When introducing interventions aimed at improving case detection, program managers will want to know if cases are detected earlier, but also that they effectively target cases that would otherwise have been missed. This framework employs both intervention data (cases found by the project) and TB notification data from a larger population, and uses both historical and contemporary controls to evaluate the impact of a given intervention to improve TB case detection. The primary outcome is measured as additional TB cases above what would be expected without the intervention, and can include a number of other measures.
Measuring intervention specific effects
Interventions to improve case detection should collect data for calculating a number of project-specific indicators based on the type of interventions proposed. Many ACF studies are designed to measure the yield of screening efforts and use this to predict the prevalence of active and undiagnosed TB in a population. When people are asked about the presence of TB symptoms or approached about inclusion in diagnostic procedures, they are screened. A diagnostic test is then applied to a subset of those screened who have met criteria for further testing to identify TB. Direct yield of an intervention can be defined as the number of people identified with TB through efforts of the intervention. Contact investigation39,40 and screening of prisoners,41 people with HIV42 or other easily identified risk groups lend themselves to measure direct yield. Interventions should attempt to collect a basic set of core data including: number of people approached, screened, screening positive, tested, diagnosed, initiated on treatment and successfully treated. Reviewing the numbers of people passing through each step of the screening process is essential to evaluate drop out or inefficiencies throughout the system, and to identify areas to be improved in order to optimize case detection. Intervention-specific indicators are detailed in Box 1. The number of people needed to screen (NNS) to identify a case of TB can provide a reasonable estimate of the effort required in an ACF intervention, however not all screening approaches are the same. Some screening efforts can be done at little or no cost, while others may require significant time, effort and financial resources.43 Thus, interventions that only report yield or NNS without measures such as time, cost and effort are of limited value when trying to plan scale-up, while using resources efficiently.
Box 1.
Key data and indicators for evaluating TB case detection interventions
| Intervention description and assumptions | |
|
|
| Intervention-level case detection data and indicators | |
| |
| |
| Population-level case detection data and indicators | |
Target population
| |
| External factors affecting notifications in the evaluation and/or control populations | |
| |
Bac+: bacteriologically positive; BMUs: basic management units, EPTB: extrapulmonary TB; SS+: sputum smear positive; SS-: sputum smear negative.
Measuring detection/intention to treat, rather than actual treatment initiation and treatment completion as the endpoint for analysis will provide an overestimation of an intervention's impact. Even with PCF, pre-treatment loss to follow-up can be an important concern.44–46 A recent study in South Africa found large differences in the cost per person for TB diagnosed, treated or cured through ACF.47 Other studies have found small differences48 but without measuring both diagnosis and treatment initiation, it will be harder to draw useful conclusions about the intervention.
Measuring change in notification
To be successful, interventions need to achieve direct yield, however intervention-specific indicators are insufficient to measure the larger impact of the activities on TB notifications. Studies have shown that a sizable proportion of TB cases identified during ACF (measured by direct yield and NNS) would presumably have found their way to treatment without the intervention.7 The concept of ‘an additional TB case’ can be defined as a case that would not have been notified in the absence of the intervention. By tracking notifications in a larger intervention area and ideally a control population both historically and prospectively, the contribution of the intervention to increased case notification can be approximated.
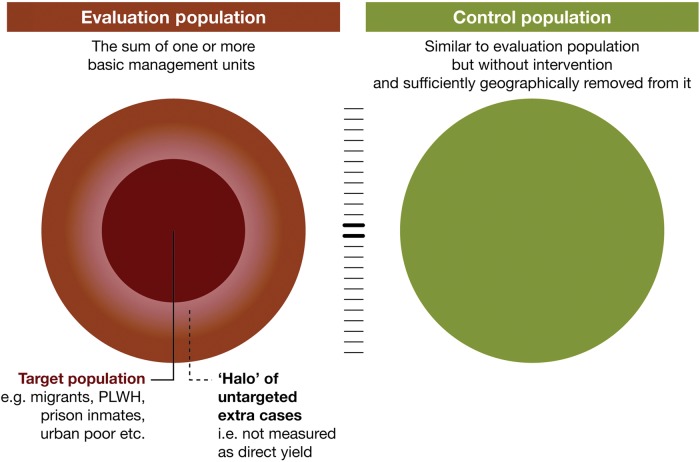
Target, evaluation and control populations
The delineation of target, evaluation and control populations to measure impact of an intervention is an important step in setting up the evaluation framework underlining the difference between yield and additional cases. When choosing the ACF intervention the target population must be identified and described. Some populations, such as people with HIV or all diabetics are relatively easy to define. In other cases the target population will be more dispersed e.g., people living in slum areas with poor access to care. To show impact on TB notifications at a population level, the group targeted with the intervention needs to contain a substantial number of people with TB who are unlikely to present with symptoms to services (PCF) within a given future time period without the intervention.
The next step is to determine the population in which notification and treatment outcome data will be monitored and evaluated (evaluation population). To capture possible spill-over effects in neighboring non-intervention areas, and to account for potential redistribution of cases (e.g., shifting patients from one health facility to another without increasing the total number of patients receiving care) notification data will need to be monitored in an evaluation area geographically larger and more populous than the intervention area alone. The selection of this evaluation population is a delicate exercise. The evaluation population needs to be large enough to capture all potential effects of an intervention, but it should not be so large that any potential effect is diluted if the intervention targets only a small part of this population. In most cases it is possible and desirable to make use of the existing NTP notification system and select one or more basic management units where patients in the target population would normally present for care and be notified. While this approach works well in most interventions, small or dispersed target populations may require an adapted definition of evaluation population. Furthermore, it is important to verify where cases (e.g., migrant labourers and prisoners) diagnosed in a different catchment area than their registered domicile would normally be notified to ensure consistency in the intervention's notification approach.
The use of control populations is important to identify contemporary trends and other factors influencing case notification. Comparing year-over-year increases without taking into account both secular trends in notification and using control populations can give a false impression of the impact of an intervention,8 or conversely, a lack thereof. Currently, there is a steady decline in national TB case notification in a number of high-burden countries such as Russia, Ethiopia and Kenya, while others such as Mozambique and Indonesia have increasing trends.1 A few well-designed studies using TB case notification as an outcome measure have used control populations to help evaluate the effects of an intervention at a population level.15,16,25,34 An intervention in Myanmar measuring the impact of involving private providers on TB case notification provides a clear example of a good use of a control population, as large increases in the intervention area were somewhat tempered by smaller gains in the control areas.33
Control populations should be comparable, but isolated from the evaluation population. They must share the same TB control system and ideally be similar in size and notification trend. A similar initial case notification rate supports plausibility of any measured difference between the two populations during the intervention. External factors influencing case notification in the evaluation and/or control population should be considered. Randomizing both control and intervention areas adds strength to the results.21–22 If randomization is not feasible, external factors should be systematically monitored and documented, and their effect on case notification in both evaluation and control populations estimated. Examples of such factors are the introduction or closure of other interventions affecting case notification, or political and natural events that impact health seeking or shape the effectiveness of screening efforts (Box 1).
TB case notifications in both evaluation and control populations should be collected both historically and prospectively. Historical quarterly TB notifications, prior to the intervention period, need to be compiled in the absence of a local prevalence survey. At least 1 year of historical (baseline) data should be obtained to account for seasonal variation in notifications. Obtaining data for additional years would be ideal to better control for secular trends. In many situations a simple linear projection can be used to predict the standard passive system performance, while a logarithmic projection could be considered in other situations depending on the best fit. An illustration of target, evaluation and control populations for this framework are presented in Figure 2. In exceptional cases, where no TB control activities have been available and a new diagnostic center is established, there could be a baseline of zero.
Figure 2.
Conceptual model of monitoring and evaluating efforts to improve TB case notification. This figure is available in black and white in print and in color at International Health online.
Interpreting case notification results
Evaluating intervention results must be based on where the entry point lies in the patient pathway for improving case detection (Figure 1). For some interventions an improvement will be shown through better notification systems, others will rely on better sensitivity of diagnostic tests, while most of the interventions will focus on the direct yield of previously undiagnosed TB cases, the target and evaluation populations.
Interventions that can show direct yield of the ACF strategy with concurrent improvements in TB notifications, controlling for trend and control populations can demonstrate additional cases above the PCF system. However, a short period of increased TB case notification may be primarily due to identifying cases earlier and notifications will return to pre-intervention levels as soon as the back-log of prevalent cases is cleared. An intervention that is also successful in identifying cases that would otherwise not have reached any services is likely to show increased notifications over a longer period until an impact can be expected on transmission and incidence and notifications begin to decline. A study in Brazil documented increased TB notifications during the intervention but the effect was not sustained during a post-intervention monitoring period.The findings could mean that people were diagnosed earlier, rather than in increased numbers overall.23
An increase in bacteriological-confirmed cases in absence of an increase in all forms of TB may suggest a shift from unconfirmed to confirmed cases, rather than an increased number of people with TB treated. A recent RCT comparing Xpert MTB/RIF to smear microscopy found no difference in overall numbers of people treated,27 highlighting the importance of reporting both bacteriologically-confirmed, as well as all forms of TB. A rapid, sensitive test could actually decrease overall notification in settings where many people are being put on treatment without bacteriological evidence, since such a test could convince a clinician to rule out TB. Analyzing the trends in bacteriologically-confirmed cases, all forms of TB (especially pulmonary TB), and the proportion of confirmed cases among all forms will provide a better understanding of the impact of different interventions. Increases in TB case detection are unlikely without substantial increases in laboratory testing and tracking the number of tests performed historically and prospectively can provide a good indicator of efforts for finding more people with TB.
The use of surveillance data (TB notification and treatment outcomes) has limitations; data completeness, consistency and reliability can vary over time and may be influenced by changes in surveillance practice (such as changing from paper-based to electronic registration, district boundaries, or changes in case definitions). Data outliers are not always easy to explain. These issues should be considered when analyzing the results of interventions. Therefore, systematic monitoring of external factors influencing notification in the evaluation and control population is crucial.
Other intervention benefits
A number of individual level benefits can be measured during ACF including early detection and improved treatment outcomes.48ACF interventions attempt to find people with TB earlier than they would have been detected (if at all) without the intervention.24,49 Measuring changes in delay in diagnosis can be done by administering questionnaires about their health-seeking behavior to people identified with TB.50 Documenting previous attempts to seek care for TB-related symptoms can be used to measure delay or missed opportunities.51 There are a number of biases inherent in these types of measurements including recall and selection bias. The results of a systematic review showed that ACF may find cases earlier, and that smear grading is one of the better ways to measure this difficult indicator.48 In addition to early diagnosis, other indicators of intervention success can include how the intervention affects the acceptability of screening52 and the proportion of people identified with TB who immediately start treatment.44
Treatment outcome data for both individuals found though ACF as well as the larger evaluation population should be monitored. There is no clear evidence that ACF alone will improve treatment outcomes; but it does not worsen them.48 A number of studies have shown that among actively found cases, better treatment outcomes can be obtained by providing treatment support to the patient.21,25,53 Improved treatment outcomes through ACF may be mitigated by the possibility that people who delay or fail to seek care through PCF due to migration patterns, release from prison, substance abuse etc., may be harder to keep on treatment. Other mitigating factors include health systems unable to cope with the increased case load including drug shortages.
Implications for program decisions
The feasibility of scaling-up an intervention that has been proven to be effective and the likelihood of sustaining the same effect under regular program conditions are particular concerns for NTPs. For instance, ACF can easily lead to large increases in numbers of people to be tested, but laboratory capacity, supply lines and quality assurance systems may fail to keep up. A careful analysis of capacity and cost requirements is crucial. An assessment of the views of providers who need to be convinced of the usefulness of the proposed intervention and acceptance of the intervention by beneficiaries can predict future fidelity to a new approach when scaled up under routine program conditions.54 The latter would include considerations of cost and time implications. To enable proper evaluation of interventions complete, quality notification data by BMU is critical. Routine data on people identified as symptomatic, people tested, diagnosed with TB and initiated on treatment would ideally be available.55
ACF will require more financial resources to identify people with TB than PCF. Program managers should take this into account when planning and evaluating, and the cost of the tests and the logistics around the activity must be properly planned. Similarly, the number needed to test is highly dependent on the screening criteria, as well as the diagnostic test used, and must be interpreted with caution. Other cost issues to consider are the ease of access to the population that is to be screened, the testing algorithm to be used, extra human resources needed, data monitoring and the periodicity of the interventions, whether they should be repeated or one-off events. Although the lack of proper cost-effectiveness analysis of ACF interventions has been raised as an important gap,18,56 only recently has any study been published,57 and more are needed. Calculating an adjusted ‘number needed to screen to find an additional case’ and related cost may provide a better measure of return on effort as it takes into account the fact that not all cases found through ACF are additional.
Replicating and scaling-up
A number of implementation-related and external factors are likely to influence the success of any given approach: the potential benefits and risks to the patients, acceptability of screening, level of effort and cost.14 Readiness of health systems to deal with increased demands on diagnostic and care services, interpretation and acceptance of new guidelines and algorithms by the staff and adherence to quality assurance mechanisms may also impact effectiveness. Most published studies lack a critical description of program implementation and analysis of factors that may be considered essential for success or failure of an intervention, limiting the ability to predict replication of success in a different setting.58
Interventions to improve TB case finding are often introduced as a compound package of activities, building on assumptions of how best to address various identified barriers to access and early diagnosis. Elements such as staff support, incentives, patient enablers and system strengthening are often added to support active case finding activities. The level of synergy with the wider health system is also likely to influence performance.54 Therefore, when evaluating interventions it is important to unpack them, identifying the components that are essential to their success and reviewing intervention delivery and implementation issues that may explain effectiveness.59 Combining the analysis of case detection with a systematic description of the implementation processes will contribute to explaining differences in success levels of an ACF strategy when implemented in different settings and may assist in predicting success when replicated elsewhere.58
Conclusions
Increased interest in better ways to diagnose and successfully treat people with previously undetected TB has led to a myriad of new interventions and approaches. Introduction of these innovations requires a robust yet practical system to evaluate progress and their effect on case notification and treatment outcomes. When randomized controlled trials are not feasible, well designed, trend-adjusted before-after studies are a reasonable option and provide important evidence for what happens under routine conditions. Using these studies with a standardized process evaluation, and a systematic description of program implementation design, cost and context will help to identify the conditions needed for success. The robust TB surveillance system can support monitoring and evaluating of the impact of interventions on case detection. Continuous monitoring of both project and notification data during the introduction and maintenance of an intervention may be timely, uncomplicated and informative enough to indicate where fine-tuning of strategy or incremental changes in operational management lead to improvements in TB case detection. Moreover, periodic evaluation of the impact of an intervention on disease registration may provide evidence that is sufficiently persuasive to attempt to reproduce it in other settings, scale-up the intervention, or even draw tentative policy conclusions. Finally, the usage of disease registration systems for monitoring and evaluation increases skills in evidence-based reflection and decision-taking among TB professionals, and its continual application increases the relevance of data in the same operational settings that collect it. The resulting opportunity to improve data quality is beneficial to policy makers and researchers in addition to disease control programs themselves. Findings can then guide decisions on scale-up and replication in different target populations and settings.
Acknowledgments
Authors' disclaimers: JC and SS are WHO staff members. The views expressed in this article are their own and do not necessarily represent WHO policies.
Authors' contributions: LB and JC both contributed equally to this paper. RS, MIB, SS, LB and JC conceived the approach; JC and LB drafted the manuscript; LB, JC, MIB, MB, RS, OR, OW, PK and SS critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. JC and LB are guarantors of the paper.
Acknowledgements: We thank Pamela Scofield and Christina Mergenthaler for their review and editing of the manuscript for clarity and message. We acknowledge Knut Lonnroth for his review and technical input and Miguel Bernal for the manuscript preparation and the tables and figures.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.WHO. Geneva: World Health Organization; 2013. Global tuberculosis report 2013. [Google Scholar]
- 2.WHO. Geneva: World Health Organization; 2006. Revised TB recording and reporting forms and registers - version 2006. [Google Scholar]
- 3.WHO. Definitions and Reporting Framework for Tuberculosis - 2013 revision. Geneva: World Health Organization; 2013. World Health Organization. [Google Scholar]
- 4.Uplekar M, Creswell J, Ottmani SE, et al. Programmatic approaches to screening for active tuberculosis. Int J Tuberc Lung Dis. 2013;17:1248–56. doi: 10.5588/ijtld.13.0199. [DOI] [PubMed] [Google Scholar]
- 5.Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–29. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 6.Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012;379:1902–13. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 7.Creswell J, Sahu S, Blok L, et al. A multi-site evaluation of innovative approaches to increase tuberculosis case notification: summary results. PLoS One. 2014;9:e94465. doi: 10.1371/journal.pone.0094465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinderaker SG, Rusen ID, Chiang CY, et al. The FIDELIS initiative: innovative strategies for increased case finding. Int J Tuberc. 2011;15:71–6. [PubMed] [Google Scholar]
- 9.WHO. Geneva: World Health Organization; 2013. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. [PubMed] [Google Scholar]
- 10.WHO. Geneva: World Health Organization; 2013. Systematic screening for active tuberculosis - principles and recommendations. [PubMed] [Google Scholar]
- 11.WHO. Geneva: World Health Organization; 2004. Interim policy on collaborative TB/HIV activities. [Google Scholar]
- 12.WHO. Geneva: World Health Organization; 2011. Fluorescent light-emitting diode (LED) microscopy for diagnosis of tuberculosis: policy statement. [PubMed] [Google Scholar]
- 13.WHO. Policy update. Geneva: World Health Organization; 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. [PubMed] [Google Scholar]
- 14.Lonnroth K, Corbett E, Golub J, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17:289–98. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 15.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–94. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 16.Cavalcante SC, Durovni B, Barnes GL, et al. Community-randomized trial of enhanced DOTS for tuberculosis control in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2010;14:203–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–53. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub JE, Dowdy DW. Screening for active tuberculosis: methodological challenges in implementation and evaluation. Int J Tuberc Lung Dis. 2013;17:856–65. doi: 10.5588/ijtld.13.0059. [DOI] [PubMed] [Google Scholar]
- 19.Cobelens F, van den Hof S, Pai M, et al. Which new diagnostics for tuberculosis, and when? J Infect Dis. 2012;205(Suppl 2):S191–8. doi: 10.1093/infdis/jis188. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Geneva: World Health Organization; 2014. Sixty-Seventh World Health Assembly. A67/11. Draft global strategy and targets for tuberculosis prevention, care and control after 2015. [Google Scholar]
- 21.Datiko DG, Lindtjørn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomised trial. PLoS One. 2009;4:e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shargie EB, Mørkve O, Lindtjørn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomized trial. Bull World Health Organ. 2006;84:112–9. doi: 10.2471/blt.05.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AC, Golub JE, Cavalcante SC, et al. Controlled trial of active tuberculosis case finding in a Brazilian favela. Int J Tuberc Lung Dis. 2010;14:720–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Eang MT, Satha P, Yadav RP, et al. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;(12):469. doi: 10.1186/1471-2458-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yassin MA, Datiko DG, Tulloch O, et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in southern Ethiopia. PLoS One. 2013;8:e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sendagire I, Schim Van der Loeff M, Mubiru M, et al. Long delays and missed opportunities in diagnosing smear-positive pulmonary tuberculosis in Kampala, Uganda: a cross-sectional study. PLoS One. 2010;5:e14459. doi: 10.1371/journal.pone.0014459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 28.Cuevas LE, Al-Sonboli N, Lawson L, et al. LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis: a multi-country cross-sectional evaluation. PLoS Med. 2011;8:e1001057. doi: 10.1371/journal.pmed.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MS, Dar O, Sismanidis C, et al. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007;369:1955–60. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 30.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reza LW, Satyanarayna S, Enarson DA, et al. LED-fluorescence microscopy for diagnosis of pulmonary tuberculosis under programmatic conditions in India. PLoS One. 2013;8:e75566. doi: 10.1371/journal.pone.0075566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lönnroth K, Uplekar M, Blanc L. Hard gains through soft contracts: productive engagement of private providers in tuberculosis control. Bull World Health Organ. 2006;84:876–83. doi: 10.2471/blt.06.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maung M, Kluge H, Aye T, et al. Private GPs contribute to TB control in Myanmar: evaluation of a PPM initiative in Mandalay Division. Int J Tuberc Lung Dis. 2006;10:982–7. [PubMed] [Google Scholar]
- 34.Khan AJ, Khowaja S, Khan FS, et al. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. Lancet Infect Dis. 2012;12:608–16. doi: 10.1016/S1473-3099(12)70116-0. [DOI] [PubMed] [Google Scholar]
- 35.Jaramillo E. The impact of media-based health education on tuberculosis diagnosis in Cali, Colombia. Health Policy Plan. 2001;16:68–73. doi: 10.1093/heapol/16.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Claassens MM, Jacobs E, Cyster E, et al. Tuberculosis cases missed in primary health care facilities: should we redefine case finding? Int J Tuberc Lung Dis. 2013;17:608–14. doi: 10.5588/ijtld.12.0506. [DOI] [PubMed] [Google Scholar]
- 37.Sekandi JN, List J, Luzze H, et al. Yield of undetected tuberculosis and human immunodeficiency virus co-infection from active case finding in urban Uganda. Int J Tuberc Lung Dis. 2014;18:13–9. doi: 10.5588/ijtld.13.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ministry of Health, Department of Health, Government of Myanmar. Yangon, Myanmar: Ministry of Health; 2011. Report on National TB Prevalence Survey 2009–2010, Myanmar. [Google Scholar]
- 39.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–68. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 41.Baussano I, Williams BG, Nunn P, et al. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7:e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kranzer K, Houben RM, Glynn JR, et al. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rie A, Hanrahan C. Active case finding for tuberculosis: what is the most informative measure for policy makers? Int J Tuberc Lung Dis. 2014;18:377. doi: 10.5588/ijtld.13.0924. [DOI] [PubMed] [Google Scholar]
- 44.Botha E, Den Boon S, Verver S, et al. Initial default from tuberculosis treatment: how often does it happen and what are the reasons? Int J Tuberc Lung Dis. 2008;12:820–3. [PubMed] [Google Scholar]
- 45.Botha E, den Boon S, Lawrence KA, et al. From suspect to patient: tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis. 2008;12:936–41. [PubMed] [Google Scholar]
- 46.Sai Babu B, Satyanarayana AV, Venkateshwaralu G, et al. Initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Andhra Pradesh, India. Int J Tuberc Lung Dis. 2008;12:1055–8. [PubMed] [Google Scholar]
- 47.Kranzer K, Lawn SD, Meyer-Rath G, et al. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9:e1001281. doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kranzer K, Afnan-Holmes H, Tomlin K, et al. The benefits to communities and individuals of screening for active tuberculosis, a systematic literature review. Int J Tuberc Lung Dis. 2013;17:432–46. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 49.den Boon S, Verver S, Lombard CJ, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect. 2008;136:1342–9. doi: 10.1017/S0950268807000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storla DG, Yimer S, Gunnar AB. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapoor SK, Raman AV, Sachdeva KS, Satyanarayana S. How did the TB patients reach DOTS services in Delhi? A study of patient treatment seeking behavior. PLoS One. 2012;7:e42458. doi: 10.1371/journal.pone.0042458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell EMH, den Boon S, Lönnroth K. Geneva: World Health Organization; 2012. Acceptability of household and community-based TB screening in high burden communities: a systematic literature review. http://www.who.int/tb/Review4bAacceptabilityHousehold_CommunityScreening.pdf. [accessed 20 June 2014] [Google Scholar]
- 53.Shah SA, Qayyum S, Arbo R, et al. Active contact investigation and treatment support: an integrated approach in rural and urban Sindh, Pakistan. Int J Tuberc Lung Dis. 2013;17:1569–74. doi: 10.5588/ijtld.13.0169. [DOI] [PubMed] [Google Scholar]
- 54.Adam T, de Savigny D. Systems thinking for strengthening health systems in LMICs: need for a paradigm shift. Health Policy Plan. 2012;27(Suppl 4):iv1–3. doi: 10.1093/heapol/czs084. [DOI] [PubMed] [Google Scholar]
- 55.TBC India. India's Revised National TB Control Programme. http://tbcindia.nic.in/home.html. [accessed 6 May 2014] [Google Scholar]
- 56.Sekandi JN, List J, Luzze H, et al. In reply to ‘Active case finding for tuberculosis: what is the most informative measure for policy makers? Int J Tuberc Lung Dis. 2014;18:377–8. doi: 10.5588/ijtld.13.0924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadav RP, Nishikiori N, Satha P, et al. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighborhood contacts in Cambodia. Am J Trop Med Hyg. 2014;90:866–72. doi: 10.4269/ajtmh.13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rychetnik L, Frommer M, Hawe P, Shiell A. Criteria for evaluating evidence on public health interventions. J Epidemiol Community Health. 2002;56:119–27. doi: 10.1136/jech.56.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waters E, Hall BJ, Armstrong R, et al. Essential components of public health evidence reviews: capturing intervention complexity, implementation, economics and equity. J Public Health. 2011;33:462–5. doi: 10.1093/pubmed/fdr064. [DOI] [PubMed] [Google Scholar]