Description
Platinum (Pt)-containing drugs are currently used in the clinic for treating various types of cancers, including testicular germ-cell tumors, small cell lung cancer, colon carcinoma, ovarian cancer, head and neck cancer, bladder cancer, lymphomas, and sarcomas [1,2]. Pt is the 78th element in the periodic table and has been used in medicine since the mid 1960’s. The major Pt-containing drugs are cisplatin, carboplatin, and oxaliplatin. They destroy cancerous cells by interfering with the DNA, through interstrand and intrastrand crosslinks, and DNAprotein crosslinks, thereby preventing cell division and growth [3,4].
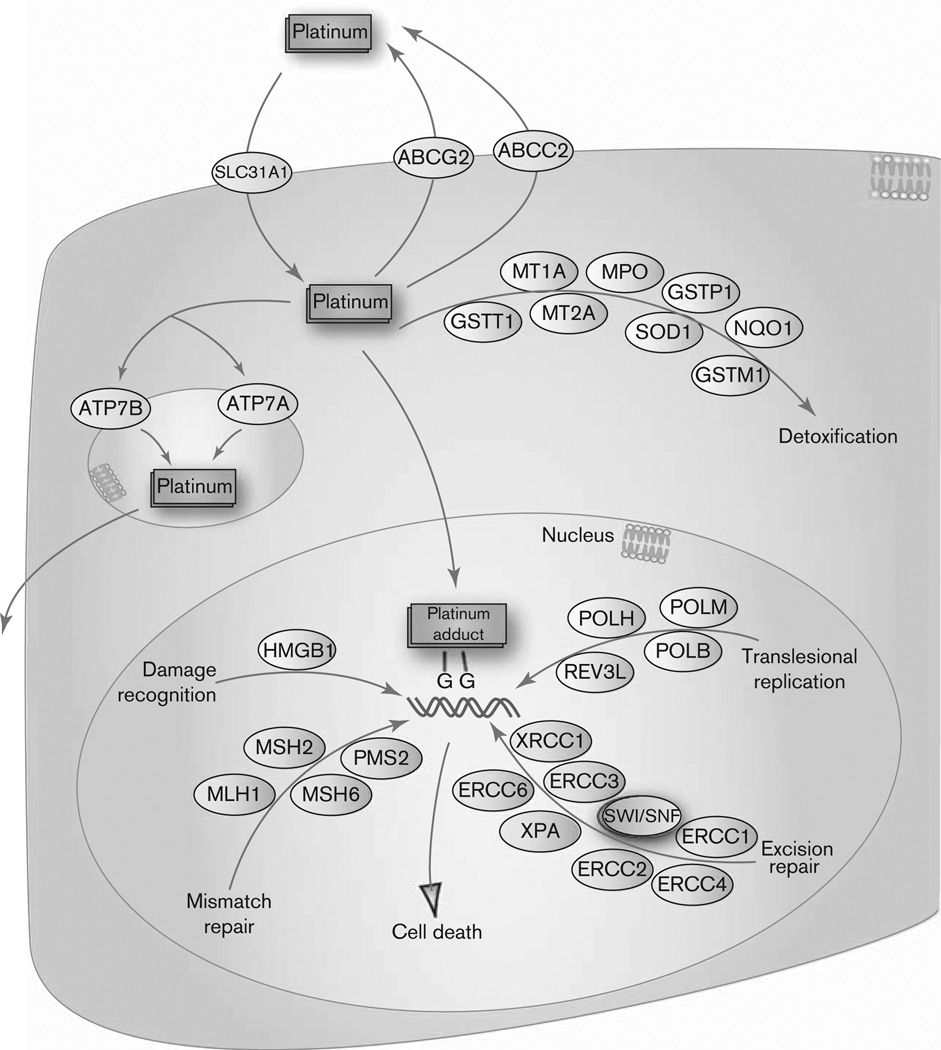
Although Pt-based drugs are the most widely used in cancer treatment, many tumors are completely resistant to these drugs and no clinical response is attained. The difference in clinical response is thought to be due, in part, to the pharmacokinetics of these drugs. The influx of Pt drugs into the cell is regulated by SLC31A1 (CTR1) and the efflux by ABCC2, ABCG2, ATP7A, and ATP7B [5–10] (Fig. 1). ATP7A is involved in the copper transport from cytoplasm into trans-Golgi network, where it serves to export copper from the cell through the vesicular secretory pathway. ATP7B is also an exporter of copper and is localized to the trans-Golgi network. When the copper content of the cell increases, ATP7A moves from trans-Golgi network to the plasma membrane and ATP7B relocates to intracellular vesicular compartments, presumably involved in the export pathway. Once Pt is inside the cell, the primary antitumor mechanism is the formation of Pt-DNA adducts which lead to cell-cycle arrest and apoptosis [3]. HMGB1 is important in the cell recognition of these Pt-DNA adducts, and therefore signals cellular response to these adducts [11,12]. Genes involved in mismatch repair, such as MSH6 and MLH1 [12], decrease the cell-sensitivity to these drugs. In addition, nucleotide excision repair is mediated by XRCC1, ERCC1, ERCC2, and XPA, and known variants in these genes affect patient’s response to Pt-based drugs [13–19]. These genes act by detecting single-strand breaks and removing proteins from the DNA helix, which then becomes more accessible to repair enzymes. POLH and POLB variants have been shown to provide tolerance to Pt-based drugs, and therefore represent an important determinant of the cellular response to Pt drugs [12–21]. In addition, there are several genes, such as MPO, SOD1, GSTM1, NQO1, GSTP1, and MT that are responsible for lowering the intracellular concentration of Pt drugs and therefore play a key role in cellular resistance to these drugs [22–26]. Patients who carry certain alleles of these detoxification-related genes have been shown to have differences in survival due to variation in drug sensitivity and adverse drug reactions [27–29].
Fig. 1.
Representation of the candidate genes involved in the metabolism, transport, and cellular effect of platinum containing drugs.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://www.pharmgkb.org/do/serve?objId=PA150642262&objCls=Pathway#).
References
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Kostova I. Platinum complexes as anticancer agents. Recent Pat Anticancer Drug Discov. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 3.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Surrah AS. Development and current status of unconventional platinum anticancer complexes. Mini Rev Med Chem. 2007;7:203–211. doi: 10.2174/138955707779802615. [DOI] [PubMed] [Google Scholar]
- 5.Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res. 2003;9:5853–5859. [PubMed] [Google Scholar]
- 6.Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, et al. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 7.Lin X, Okuda T, Holzer A, Howell SB. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol. 2002;62:1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 8.Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 9.Liedert B, Materna V, Schadendorf D, Thomale J, Lage H. Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol. 2003;121:172–176. doi: 10.1046/j.1523-1747.2003.12313.x. [DOI] [PubMed] [Google Scholar]
- 10.Ceckova M, Vackova Z, Radilova H, Libra A, Buncek M, Staud F. Effect of ABCG2 on cytotoxicity of platinum drugs: interference of EGFP. Toxicol In Vitro. 2008;22:1846–1852. doi: 10.1016/j.tiv.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Pasheva EA, Ugrinova I, Spassovska NC, Pashev IG. The binding affinity of HMG1 protein to DNA modified by cis-platin and its analogs correlates with their antitumor activity. Int J Biochem Cell Biol. 2002;34:87–92. doi: 10.1016/s1357-2725(01)00096-6. [DOI] [PubMed] [Google Scholar]
- 12.Chaney SG, Vaisman A. Specificity of platinum-DNA adduct repair. J Inorg Biochem. 1999;77:71–81. doi: 10.1016/s0162-0134(99)00149-x. [DOI] [PubMed] [Google Scholar]
- 13.Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer. 2003;39:112–119. doi: 10.1016/s0959-8049(02)00411-2. [DOI] [PubMed] [Google Scholar]
- 14.Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei DD, Groshen S, Lenz HJ. A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res. 2001;61:8654–8658. [PubMed] [Google Scholar]
- 15.Gurubhagavatula S, Liu G, Park S, Zhou W, Su L, Wain JC, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol. 2004;22:2594–2601. doi: 10.1200/JCO.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 16.Stoehlmacher J, Ghaderi V, Iobal S, Groshen S, Tsao-Wei D, Park D, Lenz HJ. A polymorphism of the XRCC1 gene predicts for response to platinum based treatment in advanced colorectal cancer. Anticancer Res. 2001;21:3075–3079. [PubMed] [Google Scholar]
- 17.Van der Straaten T, Kweekel D, Tiller M, Bogaartz J, Guchelaar HJ. Multiplex pyrosequencing of two polymorphisms in DNA repair gene XRCC1. J Mol Diagn. 2006;8:444–448. doi: 10.2353/jmoldx.2006.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakano S, Wada T, Matsumoto H, Sugiyama S, Inoue R, Eguchi S, et al. Single nucleotide polymorphisms in DNA repair genes might be prognostic factors in muscle-invasive bladder cancer patients treated with chemoradiotherapy. Br J Cancer. 2006;95:561–570. doi: 10.1038/sj.bjc.6603290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Li F, Sun N, Shukui Q, Baoan C, Jifeng F, et al. Polymorphisms in XRCC1 and XPG and response to platinum-based chemotherapy in advanced non-small cell lung cancer patients. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2008.11.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya R, Beck DJ. Survival and SOS induction in cisplatin-treated Escherichia coli deficient in Pol II, RecBCD and RecFOR functions. DNA Repair (Amst) 2002;1:955–966. doi: 10.1016/s1568-7864(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 21.Vaisman A, Masutani C, Hanaoka F, Chaney SG. Efficient translesion replication past oxaliplatin and cisplatin GpG adducts by human DNA polymerase beta. Biochemistry. 2000;39:4575–4580. doi: 10.1021/bi000130k. [DOI] [PubMed] [Google Scholar]
- 22.Stoehlmacher J, Park DJ, Zhang W, Groshen S, Tsao-Wei DD, Yu MC, Lenz HJ. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94:936–942. doi: 10.1093/jnci/94.12.936. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros R, Pereira D, Afonso N, Palmeira C, Faleiro C, Afonso-Lopes C, et al. Platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma: glutathione S-transferase genetic polymorphisms as predictive biomarkers of disease outcome. Int J Clin Oncol. 2003;8:156–161. doi: 10.1007/s10147-003-0318-8. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Yagi T. Expression of myeloperoxidase in the inner ear of cisplatin-treated guinea pigs. Anticancer Drugs. 2000;11:727–730. doi: 10.1097/00001813-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Kolesar JM, Pritchard SC, Kerr KM, Kim K, Nicolson MC, McLeod H. Evaluation of NQO1 gene expression and variant allele in human NSCLC tumors and matched normal lung tissue. Int J Oncol. 2002;21:1119–1124. [PubMed] [Google Scholar]
- 26.Meijer C, Timmer A, De Vries EG, Groten JP, Knol A, Zwart N, et al. Role of metallothionein in cisplatin sensitivity of germ-cell tumours. Int J Cancer. 2000;85:777–781. doi: 10.1002/(sici)1097-0215(20000315)85:6<777::aid-ijc6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Peters U, Preisler-Adams S, Hebeisen A, Hahn M, Seifert E, Lanvers C, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Okcu MF, Selvan M, Wang LE, Stout L, Erana R, Airewele G, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 29.Lu C, Spitz MR, Zhao H, Dong Q, Truong M, Chang JY, et al. Association between glutathione S-transferase pi polymorphisms and survival in patients with advanced nonsmall cell lung carcinoma. Cancer. 2006;106:441–447. doi: 10.1002/cncr.21619. [DOI] [PubMed] [Google Scholar]