Abstract
Aberrant protein glycosylation is known to be associated with the development of cancers. The aberrant glycans are produced by the combined actions of changed glycosylation enzymes, substrates and transporters in glycosylation synthesis pathways in cancer cells. To identify glycosylation enzymes associated with aggressive prostate cancer (PCa), we analyzed the difference in the expression of glycosyltransferase genes between aggressive and non-aggressive PCa. Three candidate genes encoding glycosyltransferases that were elevated in aggressive PCa were subsequently selected. The expression of the three candidates was then further evaluated in androgen-dependent (LNCaP) and androgen-independent (PC3) PCa cell lines. We found that the protein expression of one of the glycosyltransferases, α (1,6) fucosyltransferase (FUT8), was only detected in PC3 cells, but not in LNCaP cells. We further showed that FUT8 protein expression was elevated in metastatic PCa tissues compared to normal prostate tissues. In addition, using tissue microarrays, we found that FUT8 overexpression was statistically associated with PCa with a high Gleason score. Using PC3 and LNCaP cells as models, we found that FUT8 overexpression in LNCaP cells increased PCa cell migration, while loss of FUT8 in PC3 cells decreased cell motility. Our results suggest that FUT8 may be associated with aggressive PCa and thus is potentially useful for its prognosis.
Keywords: aggressive prostate cancer; α (1,6) fucosyltransferase
Introduction
Prostate cancer (PCa) is the most common cancer in men and the second leading cause of cancer death in men in the United States with an estimated 238,590 newly diagnosed cases and 29,720 deaths in 2012 (Siegel et al. 2013). Prostate-specific antigen (PSA), a marker used to screen for PCa, has obvious limitations in sensitivity and specificity (Nadler et al. 1995; Thompson et al. 2004). There is now significant controversy as to whether PSA screening is associated with mortality reduction and whether or not the test results in overtreatment (Schroder et al. 2009; Schroder 2011; Tosoian et al. 2011). In present, the best indicator for outcome of PCa is the Gleason patterns determined by PCa histopathology, characterized primarily by morphological and architectural attributes of histological structures of surgically resected prostate tumors, which are highly correlated with disease aggressiveness and patient outcome. A high Gleason score of 8, 9 or 10 commonly indicates poor prognosis; however, the clinical course of patients with a Gleason scores of 7 is available (Markert et al. 2011). Thus, methods to reliably distinguish aggressive or lethal PCa from non-aggressive or indolent PCa are needed.
Aggressive cancers often derive from molecular alterations in cell survival pathways, and these types of cancer tend to metastasize from the primary tumor site to other organ sites. Metastasis is a multi-step process in which cancerous cells separate from the primary tissue and enters the circulatory system where they interact with various host cells before they migrate and lodge in the target organ to form secondary metastatic colonies. Mounting evidence suggests the roles of glycoproteins and their interactions with extracellular matrix proteins, such as interaction of selectins with glycan ligands, in metastasis (Hakomori 1996).
As one of the most abundant protein modifications, aberrant glycosylation has long been known to play an important role in the development and progression of many human diseases (Ohtsubo and Marth 2006; Taniguchi et al. 2006). Aberrant glycosylation is the result of alterations in glycosylation genes that may lead to cancer development. For this reason, analysis of expression of glycogenes can facilitate the discovery of molecular changes associated with cancer development. Currently, many clinical cancer biomarkers are glycoproteins (Dube and Bertozzi 2005), such as PSA for PCa, α-fetoprotein (AFP) for hepatocellular carcinoma (HCC) and CA125 for ovarian cancer.
In this study, we investigated the expression of glycosyltransferases in PCa. From the gene expression profiles (Pascal et al. 2009), we found differences in the gene expression of three glycosyltransferases between samples from patients with aggressive PCa (Gleason grade 4) and those with non-aggressive PCa (Gleason grade 3). In addition, we found that the protein expression of α (1,6) fucosyltransferase (FUT8) was detected predominantly in androgen-independent PCa cell line PC3 but not in androgen-dependent LNCaP cells. We also found that FUT8 protein was increased in tumor tissue from patients with metastatic and aggressive primary PCa. More importantly, tissue microarray (TMA) analysis for FUT8 showed that the overexpression of FUT8 was positively correlated with PCa with high Gleason score. Further functional assays showed that FUT8 regulated cancer cell migration in both PC3 and LNCaP cells. Our results suggested that FUT8 may be associated with aggressive PCa.
Results
Altered expression of glycosyltransferase genes in aggressive and non-aggressive prostate cancer
Altered glycosylation in prostate tumor have been shown to be associated with PCa and may be responsible for cancer progression (de Leoz et al. 2008; Li et al. 2011). To identify glycosyltransferase genes associated with aggressive PCa, we analyzed the previously published gene expression data (Pascal et al. 2009) (Supplementary data, Table S1) and identified three genes encoding glycosylation enzymes with significant differences in the expression level between Gleason 3 and Gleason 4 tumors. These three candidate genes met two criteria: (i) they encode N-glycan synthesis enzymes and (ii) their expression was up-regulated in high-grade prostate cancer (Gleason 4). These three genes are β (1,4) N-acetylglucosaminyltransferase isozyme A (MGAT4A), α (1,6) fucosyltransferase (FUT8) and β (1,2) N-acetylglucosaminyltransferase (MGAT2).
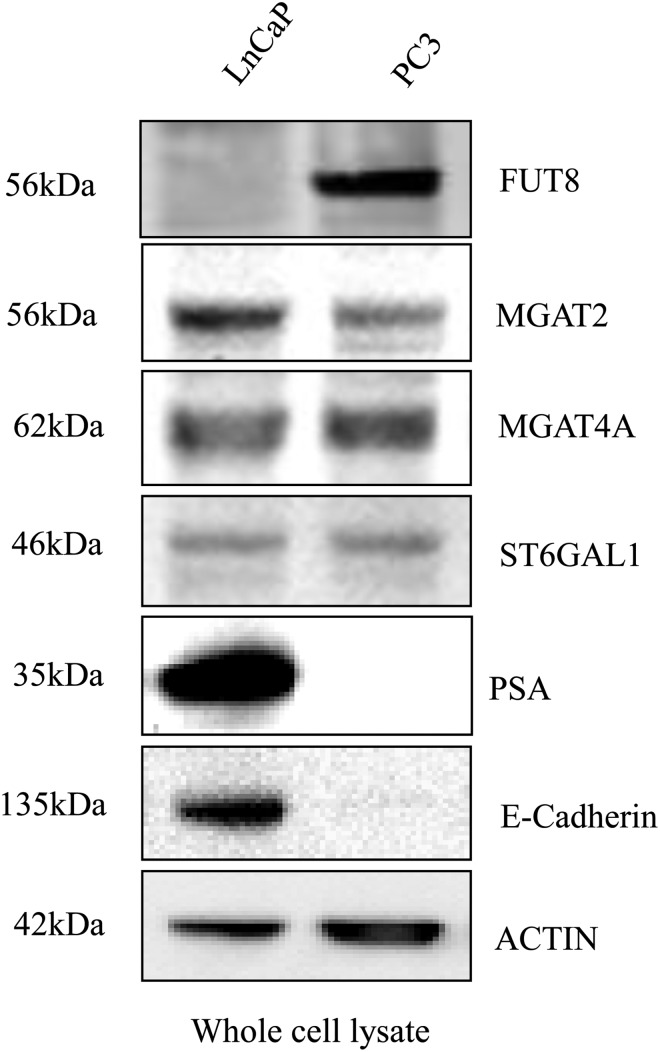
Subsequently, we examined the protein expression of these genes in androgen-dependent (less aggressive) and androgen-independent (more aggressive) PCa cell lines, LNCaP and PC3, respectively (Figure 1). Our results showed that FUT8 was expressed at high level in PC3 cells, but was almost undetectable in LNCaP cells. The expression level of the other two genes, MGAT4A and MGAT2, were similar between LNCaP and PC3 cells. To further investigate FUT8 expression in PCa cell lines, FUT8 expression was compared in a panel of prostate cell lines. FUT8 was clearly detected in PC3 cells, weakly detected in DU145 and LAPC4 cells, and not detected in 22RV1, MDA Pca2b and LNCaP cells (Supplementary data, Figure S1).
Fig. 1.
Altered expression of glycosyltransferases in androgen-dependent and -independent PCa cell lines LNCaP and PC3. Representative western blots of FUT8, MGAT2, MGAT4A, ST6GAL1, PSA and E-cadherin in whole cell lysates; actin blot shows the loading amount of protein.
PSA, a glycoprotein that is the current PCa screening marker and a gene known to be regulated by androgen, was expressed in androgen-dependent LNCaP cells, but not in androgen-independent PC3 cells. α (2,6)-Sialyltransferase 1 (ST6GAL1), whose expression is increased in colon cancer (Seales et al. 2005; Swindall and Bellis 2011), was not differentially expressed in LNCaP and PC3 cells in this study. We also detected the loss of E-cadherin expression in PC3 cells (Figure 1). E-cadherin plays a crucial role in epithelial cell–cell adhesion and in the maintenance of tissue architecture. Loss of the expression of E-cadherin results in loss of intercellular adhesion, with possible consequent cell transformation and tumor progression (van Roy and Berx 2008). Thus, the loss of E-cadherin expression in PC3 cells suggests that PC3 is a more invasive PCa cells.
Up-regulation of FUT8 expression in metastatic tissue of prostate cancer
To investigate the expression of FUT8 in PCa tissue, we analyzed prostate tissue from 10 normal individuals and 10 metastatic PCa tumor using FUT8 antibody (Figure 2A). The intensities of FUT8 was quantified and normalized by Coomassie Brilliant Blue (CBB) staining of total proteins using NIH ImageJ (Figure 2B). FUT8 expression was high in 6 of 10 metastatic prostate tumor tissue and was significantly elevated in comparison to normal prostate tissue (P ≤ 0.001) (Figure 2B). An additional two normal prostate tissue and nine metastatic PCa tissue were analyzed. The expression of FUT8 was markedly increased in metastatic PCa (Supplementary data, Figure S2).
Fig. 2.
Upregulation of FUT8 in metastatic tissue of PCa. (A) Proteins extracted from normal prostate tissue and metastatic prostate tumor tissue were subjected to western blot using FUT8 antibody (top panel). CBB staining (low panel) was used as loading control. (B) FUT8 optical density was normalized by CBB staining using NIH ImageJ. Statistically significant differences (P) between normal and metastatic groups are indicated.
Immunohistochemical staining of FUT8 expression in primary prostate tumor tissue and tissue microarray
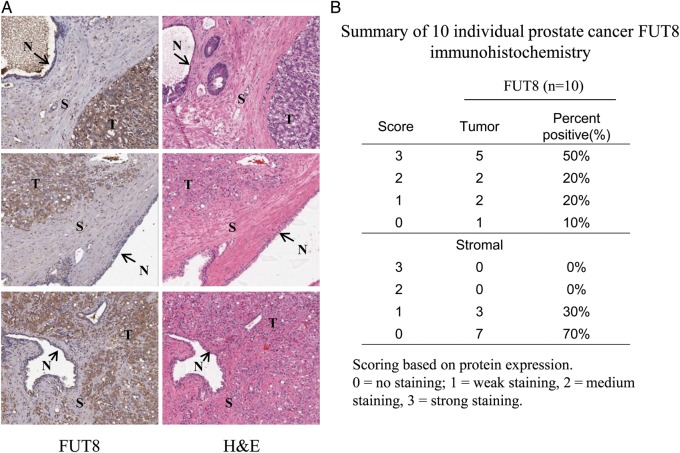
To explore the expression of FUT8 in primary prostate tumors, we performed immunohistochemical (IHC) staining on 10 slides with primary prostate tumor tissue. Increased expression of FUT8 was detected in the tumor epithelial compartments, but not in stromal areas and normal areas (Figure 3A). Strong and medium staining of FUT8 was detected in 7 of 10 primary prostate tumors (Figure 3B). These results revealed that FUT8 overexpression was a feature of tumor epithelial cells but not adjacent normal and stromal tissue.
Fig. 3.
IHC analysis of FUT8 expression in primary PCa tissue. (A) Representative images of IHC analyses of FUT8 expression from primary PCa tissue. Hematoxylin and eosin (H&E) staining were obtained to confirm the tumor area. T, tumor; S, stromal; N, normal. (B) Summary of FUT8 staining. Slides were evaluated independently by a pathologist (Q.K.L.). The intensity of staining was graded visually as no staining (0), weak staining (1), medium staining (2) and strong staining (3). Strong and medium staining of FUT8 were detected in 7 of 10 primary prostate tumors.
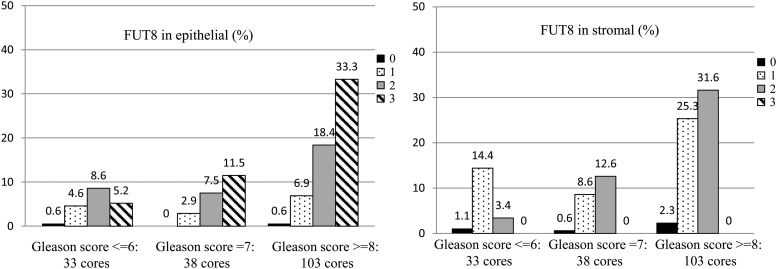
To further evaluate FUT8 expression in prostate tissue, we conducted IHC staining with a TMAs that contained two tissue cores for each of 87 prostate adenocarcinoma cases (174 cores in total). The majority of cases (73.6%) contained a consistent Gleason score for the two cores, including 44 cases (50.6%) with both cores showing a Gleason score of 8 or above (8+), 10 cases (11.5%) with both cores showing a Gleason score of 7, and 10 cases (11.5%) with both cores showing a Gleason score of 6 or less. The remaining cases (26.4%) showed different Gleason scores for the 2 cores. The prostate tumor cores were classified into three categories with 33 cores (19.0%) of Gleason 6 or less, 38 cores (21.8%) of Gleason 7 and 103 cores (59.2%) of Gleason 8 or above (Figure 4).
Fig. 4.
Proportion of staining intensity in stromal and epithelial cells of PCa TMA). The prostate tumor cores were classified by pathologist into three categories with 33 cores (19.0%) from Gleason 6 or less, 38 cores (21.8%) from Gleason 7 and 103 cores (59.2%) from Gleason 8 or above. A four-tired scoring system was used: 0 = no staining, 1 = weak staining, 2 = medium staining and 3 = strong staining.
The staining intensity in the stromal and epithelial area was then evaluated with the intensity of each core scored independently by a board certified pathologist without the identification of tissue information, FUT8 staining was observed in most of the prostate adenocarcinoma tissue sections (Figure 4). A subset of prostate adenocarcinoma cores exhibited strong staining (value of 3) for FUT8 (50.0%, 87 cores) in epithelial compartment, while no strong FUT8 staining was observed in stromal compartments (Table I, Test 1a). Prostate adenocarcinomas with two cores of Gleason 8 or above (88 cores) exhibited high percentage of strong staining FUT8 (56.8%, 50 cores) (Table I, Test 1b). These data indicated strong staining for FUT8 in epithelial compartments compared with stromal compartments and suggested that increased expression of FUT8 might be associated with high Gleason score in primary PCas.
Table I.
Statistical analysis of TMA
| Test 1a. Compare proportion of strong staining intensity (value of 3) in epithelial and stromal compartments (all men: 174 cores) | |||||
| Stain | Proportion strong staining (intensity = 3) |
||||
| Stromal | Epithelial | ||||
| FUT8 | 0 | 87 (50.0%) | |||
| Test 1b. Compare proportion of strong staining intensity (value of 3) in epithelial and stromal compartments (men with two cores 8+ Gleason: 88 cores) | |||||
| Stain | Proportion strong staining (intensity = 3) |
||||
| Stromal | Epithelial | ||||
| FUT8 | 0 | 50 (56.8%) | |||
| Test 2. Agreement in staining intensity among all men (N = 87 cases) | |||||
| Stain | Staining intensity |
Strong staining |
|||
| Kappa | Weighted kappa | Kappa | |||
| FUT8 | |||||
| Stromal | 0.488 (0.320, 0.657) | 0.523 (0.366, 0.680) | – | ||
| Epithelial | 0.568 (0.424, 0.711) | 0.620 (0.488, 0.752) | 0.702 (0.554, 0.851) | ||
| Test 3. Odds ratios of higher Gleason score (4 + 3 or higher vs.3 + 4 or lower) and 95% CI by strong stating in epithelial compartments | |||||
| Gleason 4 + 3 or higher | Gleason 4 + 4 or higher | ||||
| Model | Staining | OR (95% CI) | P | OR (95% CI) | P |
| FUT8 | Weak staining | 1.00 Ref |
1.00 Ref |
||
| Strong staining | 2.43 (0.97, 6.08) | 0.06 | 1.87 (0.88, 3.95) | 0.10 | |
Cohen's kappa coefficient (Poor: <0.20, Fair: 0.21–0.40, Moderate: 0.41–0.60, Good: 0.61–0.80, Very Good: 0.81–1.00).
The unweighted and weighted kappa statistics were then calculated to test the agreement of FUT8 staining in the epithelial compartment between two cores per case (staining intensity and strong intensity with a value of 3 vs. all others) for 87 men (174 cancer tissue cores). The kappa statistic for strong FUT8 staining (value of 3 vs. all others) indicated good agreement (0.702) in the epithelial compartment among all cases (Table I Test 2).
Next, the association of FUT8 overexpression with Gleason score was tested by calculating the odds ratios (ORs) and 95% confidence intervals (CIs) of strong FUT8 staining (value of 3) in the epithelial compartments using logistic regression (Table I, Test 3). The OR of strong staining in cases with a Gleason score of 4 + 3 or higher over that with a Gleason score of 3 + 3 and lower was 2.43; the OR of cases with a Gleason score of 4 + 4 or higher over that with a Gleason score of 4 + 3 and lower was 1.87. This result suggested that the overexpression of FUT8 was associated with high Gleason score PCa.
FUT8 knockdown inhibits PC3 cells motility
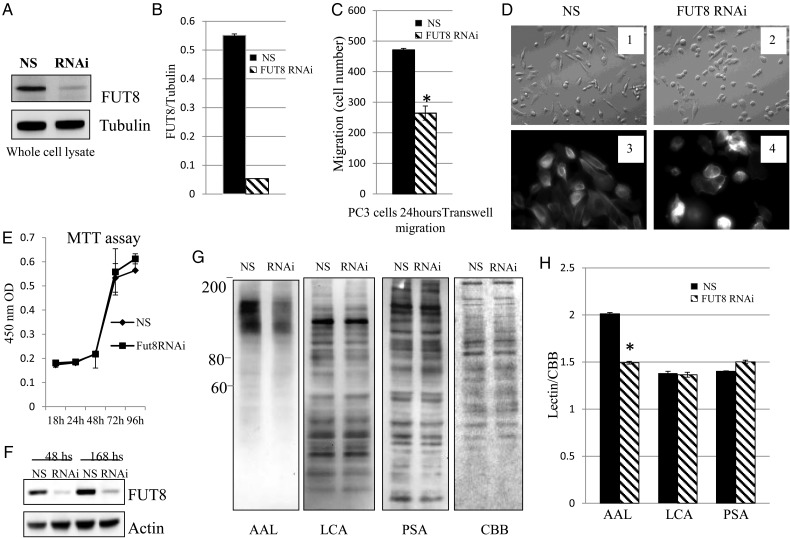
FUT8 has been reported to regulate α3β1 integrin-mediated cell migration (Zhao et al. 2006). Therefore, we investigated the role of FUT8 in PCa progression. The effect of FUT8 on PCa cell invasiveness was evaluated using multiporous polycarbonate membrane in transwell Boyden chambers. We found that silence of FUT8 expression by FUT8 siRNA significantly decreased FUT8 expression (Figure 5A). The expression level of FUT8 was quantified by densitometry using tubulin as the loading control (Figure 5B).
Fig. 5.
FUT8 knockdown inhibits PC3 cells motility. (A) Representative western blots of endogenous FUT8 in PC3 cells with and without FUT8 knockdown. NS: non-silencing control siRNA; RNAi: FUT8 siRNA. (B) Quantification of FUT8 knockdown by optical densitometry in PC3 cell using NIH ImageJ. (C) Transwell migration assays of PC3 cells transfected with either non-silencing control siRNA or FUT8 siRNA. Cell numbers at the bottom of transwell chamber were counted 24 h after migration. * P < 0.05. (D) Morphological changes in PC3 cells after FUT8 silencing by siRNA 48 h. 1 and 2: representative field photographed using a phase-contrast microscope. 3 and 4: immunofluorescent staining of F-actin showing disruption of cell protrusion as the results of interfering with FUT8 expression. (E) Cell proliferation was determined by MTT assay using Cell Counting Kit-8. PC3 cells were transfected with either control or FUT8 siRNA. Data represented mean ± SD (n = 3). (F) FUT8 expression remains low 168 h after FUT8 siRNA transfection. The expression of FUT8 in whole cell lysates was detected by western blot. (G) Whole cell lysates from either non-silencing control siRNA or FUT8 siRNA transfected PC3 cells were subjected to lectin blot analysis using AAL, LCA and PSA. CBB staining of gels shows comparable amounts proteins in each lane. (H) Optical density of lectins staining was quantified by CBB staining using NIH ImageJ (*P < 0.01).
We also found that FUT8 knockdown dramatically decreased the migration of PC3 cells compared with that of cells transfected with non-silencing control siRNA (Figure 5C). Furthermore, we observed that reduction in FUT8 expression induced cell rounding and loss of membrane protrusion (Figure 5D). These results suggest that expression of FUT8 may be required for cancer cells to migrate.
Next, the effect of FUT8 on cell proliferation was evaluated. We found that non-silencing control siRNA and FUT8 siRNA transfected PC3 cells exhibited equal growth at several time points (Figure 5E). This result suggests that knockdown of FUT8 expression does not affect PC3 cell proliferation. The reduction in FUT8 expression was confirmed by western blot, and we verified that the expression of FUT8 remained low even 168 h after FUT8 knockdown (Figure 5F). To examine whether FUT8-catalyzed fucosylation was affected by FUT8 silencing, fucosylated N-glycans were analyzed by Aleuria aurantia lectin (AAL) blot (Figure 5G), which preferentially recognizes the core fucose (Matsumura et al. 2007). We found that several bands migrating at ∼80–200 kDa in molecular mass were faintly stained with AAL in FUT8 knockdown cells; the non-silencing control siRNA transfected cells were strongly stained. Staining with lens culinaris agglutinin (LCA) (Kornfeld et al. 1981) which recognize α-mannosyl core of biantennary, and Pisum sativum agglutinin (PSA) (Kaku et al. 1991), which recognize α-mannose moieties, were similar in FUT8 siRNA and non-silencing siRNA transfected cells. Coomassie blue staining of gel showed even protein loading in each sample. This result indicated that silencing of FUT8 expression mainly affected core fucosylation of N-glycans. Because AAL lectin also binds to other branch fucose, silence of FUT8 only affects the structure of core fucose, so we still observed the binding of AAL lectin. The staining of lectins between non-silencing siRNA and FUT8 siRNA was quantified by densitometry (Figure 5H). AAL lectin was significantly decreased in FUT8 siRNA PC3 cells (P < 0.01).
FUT8 overexpression increases LNCaP cell motility
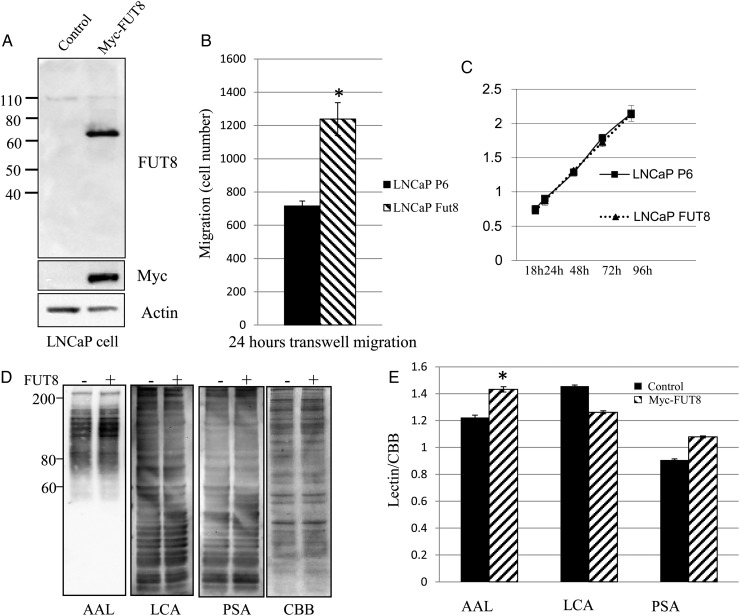
Subsequently, we established LNCaP cells that stably expressed Myc-tagged FUT8 to investigate whether FUT8 increases PCa cells motility. FUT8 and Myc-tag were not detected in empty vector transfected cells compared with Myc-tagged FUT8 transfected cells (Figure 6A). We found that introduction of FUT8 greatly increased LNCaP cell migration compared with empty control vector transfected LNCaP cells (Figure 6B). Furthermore, the effect of overexpression of FUT8 on cell proliferation was analyzed by MTT assay (Figure 6C). LNCaP cells with overexpression of FUT8 exhibited equal growth at several time points compared with empty control vector transfected LNCaP cells. Finally, the abundance of core fucosylation, regulated by FUT8, was measured by AAL lectin, which recognizes focosylated glycans (Figure 6D). As expected, an increase in AAL staining was observed in LNCaP cells with FUT8 overexpression. On the other hand, LCA and PSA have specificity against mannose, and the staining of FUT8 overexpressed LNCaP cells with LCA and PSA was not affected by FUT8 overexpression (Figure 6E).
Fig. 6.
FUT8 overexpression increases LNCaP cell motility. (A) LNCaP cells stably expressed Myc-FUT8. LNCaP cells were transfected with empty vector control P6 (Control P6) or Myc-FUT8. After screening and expanding, whole cell lysates were subjected to western blot using anti-FUT8 and anti-Myc monoclonal antibody. (B) Transwell migration assay of LNCaP cells transfected with either empty vector control P6 (LNCaP P6) or Myc-FUT8 (LNCaP FUT8). Cell numbers at the bottom of transwell chamber were counted 24 h after migration. * P < 0.05, data represented mean ± SD (n = 3). (C) Cell proliferation was determined by MTT assay using Cell Counting Kit-8. LNCaP cells were transfected with either empty vector control P6 (LNCaP P6) or Myc-FUT8 (LNCaP FUT8). Data represented mean ± SD (n = 3). (D) Whole cell lysates from either empty vector control P6 (P6) or Myc-FUT8 transfected LNCaP cells were subjected to lectin blot analysis using AAL, LCA and Arachis hypogaea lectin (PSA). CBB staining of gels shows comparable amounts proteins in each lane. (−) empty vector control; (+) Myc-FUT8. (E) Optical density of lectins staining was quantified by CBB staining using NIH ImageJ. In AAL lectin staining result, the P-value is <0.05 (*).
Discussion
FUT8 catalyzes the transfer of a fucose from GDP-fucose to the innermost GlcNAc residue of hybrid and complex N-linked oligosaccharides in glycoproteins via α (1,6)-linkage to form the core fucosylation in mammals. FUT8 has marked functions on signal transduction (Wang et al. 2005, 2006), cell adhesion (Zhao et al. 2006) and intracellular signaling (Li et al. 2012). Core fucosylated N-glycans are widely distributed in a variety of glycoproteins and are altered under some pathological conditions (Taniguchi et al. 2006). Core fucosylated AFP, but not AFP alone, is used clinically to distinguish patients with HCC from those with chronic hepatitis and liver cirrhosis (Sato et al. 1993). Increased fucosylation is also observed in other types of cancers, including lung cancer, colorectal cancer, breast cancer and pancreatic cancer (Kyselova et al. 2008; Liu et al. 2011; Miyoshi et al. 2010). In PCa, increased fucosylation of PSA and haptoglobin is detected in serum of PCa patients (Kosanovic and Jankovic 2005; Fujimura et al. 2008), and elevated core fucosylation and a 2–3 sialylation were reported in serum proteins from PCa patients but not those with benign prostate hyperplasia by mass spectrometer analysis (Saldova et al. 2011). However, the function and expression of glycosyltransferases such as FUT8 in PCa progression has not been demonstrated. In this study, we found that FUT8 modulated PCa cell migration and the overexpression of FUT8 was associated with aggressive PCa.
Two PCa cell lines were selected as model systems for initial glycobiology analysis to determine the expression and function of glycosyltransferase in cancer progression. The PC3 cell line is an androgen-independent prostate cell that does not express PSA (Kaighn et al. 1979) and it is a more aggressive PCa cell line that resists TNF (Sherwood et al. 1990). LNCaP cell line is androgen dependent and is commonly used as a cell model of less aggressive PCa (Horoszewicz et al. 1980; Sensibar et al. 1995; Navone et al. 1997). The androgen receptor pathway is related to the invasive behavior of PCa cells (Baldi et al. 2003). In our studies, the expression of the four glycosylation enzymes FUT8, MGAT2, MGAT4A, and ST6GAL1 were analyzed in both androgen-dependent and -independent cell lines LNCaP and PC3, respectively. Of the four candidates, FUT8 was expressed at a significantly higher level in PC3 cells than in LNCaP cells. FUT8 has a functional role in PCa cell migration upon loss and gain of FUT8 expression in PC3 cells and LNCaP cells, respectively.
From western blot analysis of 10 normal and 10 metastatic PCa tissue, as well as IHC analysis of 10 cases of primary PCa tissue, we found that FUT8 expression was elevated in metastatic cancer tissue and primary cancer tissues. The results were further verified by staining of a PCa TMA for FUT8 expression. A significant correlation was seen between strong staining of FUT8 in the epithelial compartment and the Gleason scores of the tumor tissues.
Alterations in glycosyltransferases expression may provide valuable information with regard to the molecular changes that occur in aggressive cancer. For instance, N-glycan GlcNAc transferase V and GlcNAc transferase III, which respectively catalyze synthesis of the β (1,6)-GlcNAc and bisecting branch of N-glycans, are upregulated in numerous cancers, and the overexpression of these enzymes results in changes of tumor metastasis (Yoshimura et al. 1995; Granovsky et al. 2000). Sialyltransferases, such as ST3Gal I, ST6GalNAc I and ST6Gal I, are overexpressed in breast and colon cancer (Burchell et al. 1999; Chiricolo et al. 2006; Sewell et al. 2006). FUT8 expression is upregulated in thyroid carcinoma and associated with tumor size (Ito et al. 2003). In a recent study, FUT8 was identified as a functional regulator in non-small cell lung cancer and suggested that FUT8 is a promising target for prognosis and therapy of lung cancer (Chen et al. 2013). Our data showed that FUT8 was overexpressed in androgen-independent PC3 cell and clinical PCa tissue. The TMA analysis showed that 50% (87 cores) of the epithelial compartment and 0% (0 cores) of the stromal compartment were stained strongly for FUT8. Higher percentage of cores (56.8%) showed strong FUT8 staining in cases with a Gleason score of 4 + 4 and higher (88 cores, 44 cases) (Table I). Importantly, FUT8 in epithelial compartments is also associated with a higher Gleason score (Table I). These results suggest that FUT8 may act as an indicator in distinguishing aggressive from non-aggressive PCa.
In summary, overexpression of FUT8 was found to be associated with aggressive PCa. FUT8 is a promising target that appears to differentiate between aggressive and non-aggressive PCa. By measuring enzyme expression, the value of FUT8 in the diagnosis of aggressive PCa in tissues and body fluids may be determined in future studies.
Materials and methods
Cell lines and culture conditions
Human PCa cell lines LNCaP and PC3 were purchased from ATCC (Manassas, VA). LNCaP cells were maintained in RPMI 1640 media supplemented with 10% HyClone FBS (Thermo, Rockford, IL). PC3 cells were maintained in F-12K media with 10% HI FBS (Gibco, Grand Island, NY).
Prostate cancer samples
Clinical samples and slides for protein extraction and immunohistochemistry were obtained with approval of the Institutional Review Board of the Johns Hopkins University. Metastatic prostate tumors were from men who died of metastatic PCa between 1995 and 2004. The tissue wad microdissected to enrich tumor content. Normal prostate tissue was obtained from surgically removed prostates of transplant tissue donors and was stained with H&E for histological review by a board certified pathologist. No tumor was present as assessed. The age of the transplant donors were between 18 and 41 years old with a mean age of 31 years old. At this age, the presence of low grade PCa is not prevalent. The samples were flash frozen after collection, embedded in OCT and stored at −80° until use. The collection process was performed with support from the Transplant Resource Center of Maryland. Formalin fixed paraffin embedded slides were obtained for primary prostate tumors from radical prostatectomy. The Gleason scores were 6 (3 patients), 7 (3 patients), 8 (2 patients) and 9 (2 patients).
Western and lectin blotting
Methods used for western and lectin blotting were described as previously (Wang et al. 2006). Cells and clinical tissue were lysed in 1× RIPA buffer (Millipore, Temecula, CA) and incubated on ice for 10 min. After the incubation, the samples were centrifuged at 15,000 × g for 15 min. Protein concentration was determined using a BCA protein assay kit (Thermo, Rockford, IL). For each sample, equal amounts of protein (10–20 µg) were run on 4–12% NuPAGE gel (Invitrogen, Carlsbad, CA) and then to nitrocellulose membrane (Invitrogen). The following antibodies were used: MGAT2 (GnT-II S-21, 1:500), MGAT4A (GnT-IVA M-71, 1:500), FUT8 (B-10, 1:500), Myc (9E10, 1:1000) and ST6Gal1(LN-1, 1:500) (Santa Cruz Biotech, Dallas, TX); PSA (MP077, 1:1000Scripps Lab, San Diego, CA); E-cadherin (24E10, 1:2000), α-tubulin (2144S, 1:2000) and actin (1:2000) (Cell Signaling, Beverly, MA). The secondary antibodies conjugated to HRP were used to probe the membranes for 1 h at room temperature. Biotinylated lectins (Vector Labs, Burlingame, CA) were used for glycan detection followed by incubating with High Sensitivity Streptavidin-HRP for 30 min at room temperature. The HRP conjugates were visualized by reagents supplied in the SuperSignal West Pico or Femto Chemiluminescent Kit (Thermo, Waltham, MA).
Immunochemical staining and tissue microarrays
Staining was performed on 10 slides with individual primary prostate tumors and a commercial PCa TMA (PR2085b US Biomax, Rockville, MD). Briefly, sections of tissue were deparaffinized and rehydrated. Tissue was incubated in antigen retrieval buffer (R&D Systems, Minneapolis, MN) at 92–95°C for 10 min. Tissue was blocked by dual endogenous enzyme, avidin and biotin blocking buffer (Dako, Carpinteria, CA) for 10 min, respectively, at room temperature. FUT8 staining was evaluated using the Cell and Tissue Staining Sheep Kit (R&D Systems) according to the manufacturer's protocol. FUT8 antibody (R&D Systems) was diluted 1:50 in antibody dilution buffer (Dako) and incubated overnight at 4°C. After washing three times with PBS, tissue was incubated in biotinylated sheep secondary antibody for 1 h at room temperature. Next, tissue was incubated with High Sensitivity Streptavidin-HRP for 30 min. The immunoreaction was detected using the Dako DAB kit.
Statistical analyses of tissue microassay
The intensity of all immunostaining was visually graded by a board certified pathologist to 0 (no staining), 1 (weak staining), 2 (medium staining) and 3 (strong staining). A total of 87 cases (two cores from each case) were graded. Scored cores are summarized in Figure 4. The proportion of strong FUT8 staining intensity was compared in the stromal and epithelial compartments for all 88 men and the 44 men for whom both cores were Gleason 8+. To determine agreement in FUT8 staining intensity by compartment, unweighted and weighted kappa statistics were calculated. Using logistic regression, the OR of higher Gleason score (8 or higher vs. 7 or lower) was calculated for FUT8 staining.
RNAi experiments
Oligofectamine (Invitrogen) was used to transfect siRNA into cells. Briefly, for 1 well on a 6-well plate, 12 μL of 20 μmol/L negative control siRNA (QIAGEN 1027310, Forster city, CA) or Hs_FUT8_5 siRNA (QIAGEN SI03149118) were mixed in 88 μL of Opti-MEM with 12 μL Oligofectamine in 88 μL Opti-MEM. After incubating for 25 min at room temperature, the mixture was added to cultured cells (40−50% confluent) in 800 μL of Opti-MEM. Regular media (1000 μL) containing 20% fetal bovine serum (FBS) was added to the transfected cells 3 h after transfection. Two days post-transfection, cells were harvested for western blot or various assays.
Establishment of stable expression FUT8 LNCaP cells
Myc-DDK-tagged ORF clone of Homo sapiens FUT8 (RC223075) (Origene, Rockville, MD) or pCMV6-Entry (PS100001) (Origene) vector was transfected to LNCaP cell using Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications. G418 (Invitrogen) was used to screen the positive clones at 400 to 800 µg/mL. The cell culture media was changed every 3 days for 2 weeks. Once the cells formed colonies, the cells from each clone were expanded and used for western blot or other assay.
Immunofluorescence
PC3 cells transfected with non-silence siRNA or FUT8siRNA for 48 h were seeded on four-chamber vessel (BD Falcon, San Jose, CA) for staining after 4–6 h. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 20 min and permeabilized with 0.5% Triton X-100 for 5 min at room temperature. After washing three times with phosphate-buffered saline, cells were blocked in 5% BSA for 1 h at room temperature and then incubated with Texas Red-Phalloidin (Invitrogen) at a 1:500 dilution for 1 h at room temperature and mounted with DAPI (Vector Lab H-1200) to visualize nuclei. Cells were examined by Nikon super high pressure mercury lamp and Nikon eclipse TE 200 microscope.
Migration assay
The cell migration assay was performed in 6-mm Biocoat cell culture control inserts (BD Biosciences, San Jose, CA). Cells were starved overnight in 0.2% FBS medium. PC3 cells (2 × 105) in 0.2 mL 0.2% FBS medium were seeded to the upper well of transwell chamber. Ten percent of FBS medium 0.6 mL were added to the bottom of the well. After the incubation for 24 h at 37°C, migrated cells were stained using the Diff-Quik stain kit (Fisher Scientific, Pittsburg, PA). All cells on the membrane were counted under a microscope.
Proliferation assay
The proliferation of PC3 cell and LNCaP cell was measured using an MTT assay and a Cell Counting Kit-8 (Dojindo, Rockville, MD) as described by the manufacturer. Briefly, non-silence control siRNA or FUT8 siRNA transfected PC3 cells (2000/well) and LNCaP vector control or FUT8 overexpression cells (5000/well) were cultured in a 96-well plate using regular culture medium in triplicate for 18–96 h. CCK-8 solution (10 µL) was added to each well of the plate. After 4 h incubation, the absorbance was measured at 450 nm using a Bio-Tek's µQuant microplate spectrophotometer.
Supplementary data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health, the National Cancer Institute (by grantsU01CA152813, 1U24CA160036, U24CA115102 and 2R01CA112314) and by the National Institutes of Health, National Heart Lung and Blood Institute (by grant P01HL107153 and contract N01-HV-00240).
Conflict of interest statement
None declared.
Abbreviations
AAL, Aleuria aurantia lectin; AFP, α-fetoprotein; CBB, Coomassie Brilliant Blue; CIs, confidence intervals; FBS, fetal bovine serum; FUT8, α (1,6) fucosyltransferase; HCC, hepatocellular carcinoma; IHC, immunohistochemical; LCA, lens culinaris agglutinin; MGAT2, β (1,2) N-acetylglucosaminyltransferase; MGAT4A, β (1,4) N-acetylglucosaminyltransferase isozyme A; OR, odds ratio; PCa, prostate cancer; ST6GAL1, α (2,6)-sialyltransferase 1; TMA, tissue microarrays;
Supplementary Material
References
- Baldi E, Bonaccorsi L, Forti G. Androgen receptor: Good guy or bad guy in prostate cancer invasion? Endocrinology. 2003;144:1653–1655. doi: 10.1210/en.2003-0234. [DOI] [PubMed] [Google Scholar]
- Burchell J, Poulsom R, Hanby A, Whitehouse C, Cooper L, Clausen H, Miles D, Taylor-Papadimitriou J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology. 1999;9:1307–1311. doi: 10.1093/glycob/9.12.1307. [DOI] [PubMed] [Google Scholar]
- Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, Wong CH. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci USA. 2013;110:630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricolo M, Malagolini N, Bonfiglioli S, Dall'Olio F. Phenotypic changes induced by expression of beta-galactoside alpha2,6 sialyltransferase I in the human colon cancer cell line SW948. Glycobiology. 2006;16:146–154. doi: 10.1093/glycob/cwj045. [DOI] [PubMed] [Google Scholar]
- de Leoz ML, An HJ, Kronewitter S, Kim J, Beecroft S, Vinall R, Miyamoto S, de Vere White R, Lam KS, Lebrilla C. Glycomic approach for potential biomarkers on prostate cancer: Profiling of N-linked glycans in human sera and pRNS cell lines. Dis Markers. 2008;25:243–258. doi: 10.1155/2008/515318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation – Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Shinohara Y, Tissot B, Pang PC, Kurogochi M, Saito S, Arai Y, Sadilek M, Murayama K, Dell A, et al. Glycosylation status of haptoglobin in sera of patients with prostate cancer vs. benign prostate disease or normal subjects. Int J Cancer. 2008;122:39–49. doi: 10.1002/ijc.22958. [DOI] [PubMed] [Google Scholar]
- Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, et al. The LNCaP cell line – A new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- Ito Y, Miyauchi A, Yoshida H, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, et al. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: Its linkage to biological aggressiveness and anaplastic transformation. Cancer Lett. 2003;200:167–172. doi: 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Kaku H, Goldstein IJ, Oscarson S. Interactions of five D-mannose-specific lectins with a series of synthetic branched trisaccharides. Carbohydrate Res. 1991;213:109–116. doi: 10.1016/s0008-6215(00)90602-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld K, Reitman ML, Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981;256:6633–6640. [PubMed] [Google Scholar]
- Kosanovic MM, Jankovic MM. Sialylation and fucosylation of cancer-associated prostate specific antigen. J Buon. 2005;10:247–250. [PubMed] [Google Scholar]
- Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge GW, Schnaper L, Hickey RJ, Malkas LH, Novotny MV. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54:1166–1175. doi: 10.1373/clinchem.2007.087148. [DOI] [PubMed] [Google Scholar]
- Li W, Liu Q, Pang Y, Jin J, Wang H, Cao H, Li Z, Wang X, Ma B, Chi Y, et al. Core fucosylation of mu heavy chains regulates assembly and intracellular signaling of precursor B cell receptors. J Biol Chem. 2012;287:2500–2508. doi: 10.1074/jbc.M111.303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tian Y, Rezai T, Prakash A, Lopez MF, Chan DW, Zhang H. Simultaneous analysis of glycosylated and sialylated prostate-specific antigen revealing differential distribution of glycosylated prostate-specific antigen isoforms in prostate cancer tissues. Anal Chem. 2011;83:240–245. doi: 10.1021/ac102319g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, Chen CH, Khoo KH, Yu CJ, Yang PC, et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci USA. 2011;108:11332–11337. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert EK, Mizuno H, Vazquez A, Levine AJ. Molecular classification of prostate cancer using curated expression signatures. Proc Natl Acad Sci USA. 2011;108:21276–21281. doi: 10.1073/pnas.1117029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Higashida K, Ishida H, Hata Y, Yamamoto K, Shigeta M, Mizuno-Horikawa Y, Wang X, Miyoshi E, Gu J, et al. Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: A novel probe for core fucose. J Biol Chem. 2007;282:15700–15708. doi: 10.1074/jbc.M701195200. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Shinzaki S, Moriwaki K, Matsumoto H. Identification of fucosylated haptoglobin as a novel tumor marker for pancreatic cancer and its possible application for a clinical diagnostic test. Methods Enzymol. 2010;478:153–164. doi: 10.1016/S0076-6879(10)78006-X. [DOI] [PubMed] [Google Scholar]
- Nadler RB, Humphrey PA, Smith DS, Catalona WJ, Ratliff TL. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154:407–413. doi: 10.1097/00005392-199508000-00023. [DOI] [PubMed] [Google Scholar]
- Navone NM, Olive M, Ozen M, Davis R, Troncoso P, Tu SM, Johnston D, Pollack A, Pathak S, von Eschenbach AC, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–2500. [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Pascal LE, Vencio RZ, Page LS, Liebeskind ES, Shadle CP, Troisch P, Marzolf B, True LD, Hood LE, Liu AY. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer. 2009;9:452. doi: 10.1186/1471-2407-9-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldova R, Fan Y, Fitzpatrick JM, Watson RW, Rudd PM. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2011;21:195–205. doi: 10.1093/glycob/cwq147. [DOI] [PubMed] [Google Scholar]
- Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802–1806. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- Schroder FH. Stratifying risk – The U.S. Preventive Services Task Force and prostate-cancer screening. N Engl J Med. 2011;365:1953–1955. doi: 10.1056/NEJMp1112140. [DOI] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin) Cancer Res. 1995;55:2431–2437. [PubMed] [Google Scholar]
- Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, Gatgens J, Clausen H, Hansson GC, Burchell J, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- Sherwood ER, Pitt Ford TR, Lee C, Kozlowski JM. Therapeutic efficacy of recombinant tumor necrosis factor alpha in an experimental model of human prostatic carcinoma. J Biol Response Modif. 1990;9:44–52. [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286:22982–22990. doi: 10.1074/jbc.M110.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N, Miyoshi E, Gu J, Honke K, Matsumoto A. Decoding sugar functions by identifying target glycoproteins. Curr Opin Struct Biol. 2006;16:561–566. doi: 10.1016/j.sbi.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, Walsh PC, Carter HB. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 2006;281:2572–2577. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, et al. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci USA. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci USA. 1995;92:8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J Biol Chem. 2006;281:38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.