Abstract
Radiation therapy is a main stay in treating solid tumors and plays a significant role in definitive and adjuvant therapy. Unfortunately, local control remains a challenge, in which the success of radiotherapy is largely dictated by tumor hypoxia, DNA damage repair and the antitumor immune response. Extensive efforts have therefore been devoted to targeting the factors that attenuate tumor radiosensitivity, although with limited success. Mounting evidence suggests that tumor and endothelial cells may utilize galectin-1 (Gal-1) for protection against radiation through several mechanisms. Targeting Gal-1 in combination with radiotherapy provides an exciting approach to address several radiation-prohibitive mechanisms.
Keywords: galectin-1, radiotherapy, tumor hypoxia
Introduction
Despite advances in radiotherapy, disease control in many solid tumors remains a challenge in the clinic. Past approaches to target hypoxia in radiotherapy included modification of tumor oxygen consumption and vasculature as well as development of agents that sensitize hypoxic cancer cells to radiation. These approaches were met with limited success; however, establishing hypoxia markers to properly identify patients best suited for hypoxia-targeted therapies and employing novel therapies that simultaneously address factors affecting tumor radiosensitivity may impart a greater impact on the therapeutic ratio.
Galectin-1 (Gal-1) is the prototype member of the Galectin superfamily, characterized by high affinity binding to β-galactosides through a well-conserved carbohydrate-recognition domain (Barondes et al. 1994). Gal-1 has been shown to regulate a variety of biological processes, including T-cell homeostasis, resolution of inflammatory responses, host–pathogen interactions, selective deletion of specific thymocytes during T-cell development, fetomaternal tolerance and embryogenesis (Perillo et al. 1997; Van den Brule et al. 1997; Rabinovich et al. 1999, 2002; Sotomayor and Rabinovich 2000; Zuniga et al. 2001; Blois et al. 2007)
Gal-1 is overexpressed in most cancers, promotes an aggressive phenotype and is associated with poor survival (Cindolo et al. 1999; Camby et al. 2001; Rorive et al. 2001; van den Brule et al. 2001; Szoke et al. 2005; Le et al. 2007; Saussez et al. 2007; Croci et al. 2012; Dalotto-Moreno et al. 2013). Gal-1 is also upregulated in response to both hypoxia (Le et al. 2005; Zhao et al. 2010, 2011) and radiation (Strik et al. 2007; Upreti et al. 2013), in which its enrichment after radiation may boost tumorigenicity. Targeting Gal-1 and, in turn its modulation of DNA damage repair, vasculature normalization and antitumor immune response may address multiple pathways, potentially rendering tumor and endothelial cells (ECs) more radiosensitive (Rubinstein et al. 2004; Huang et al. 2012; Jain 2013; Croci et al. 2014).
Hypoxia impact on tumor radiosensitivity and patient prognosis
Hypoxia, a consequence of rapid cancer cell proliferation with increased oxygen and nutrient demands that cannot be met by the surrounding vasculature, is a common occurrence in solid tumors and is well accepted as a deleterious factor in cancer therapies, compromising radiotherapy and driving malignant progression (Gray et al. 1953; Nordsmark et al. 2005). Radiation-induced DNA damage through the generation of H2O2 and hydroxyl radicals requires oxygen (Massie et al. 1972; Lesko et al. 1982; Prise et al. 1989). In turn, the radiation dose required to achieve the same effect as a hypoxic tumor is approximately three times higher than in the presence of normal oxygen levels, described by the oxygen enhancement ratio (Gray et al. 1953; Deschner and Gray 1959).
Studies relating treatment outcomes to direct tumor pO2 measurements have shown that tumor hypoxia is a major contributor to poor prognosis after radiotherapy (Brizel et al. 1997; Nordsmark and Overgaard 2000). The fraction of tumor pO2 measurements below 2.5 mm Hg was shown to be an independent predictor for radiation response and locoregional tumor control in a large cohort of HNSCC on multivariate analysis (Nordsmark et al. 2005). A significantly lower 12-month disease free survival was reported in radiotherapy patients with median tumor pO2 <10 mmHg vs. those with higher tumor pO2 (Brizel et al. 1997). The relationship between tumor oxygenation and prognosis has also been demonstrated with endogenous and PET-based hypoxia tracers (Rajendran et al. 2006; Ferreira et al. 2011; Zips et al. 2012).
The impact of hypoxia on radiosensitivity is also attributed to proteomic and genomic changes that increase tumor cell proliferation while decreasing their apoptotic potential (Loeb 1991; Graeber et al. 1996; Vaupel 2004). The adaptive responses to match oxygen supply with metabolic and bioenergetics demands under hypoxia involve various cellular pathways including gene regulation by hypoxia-inducible factors (HIFs). Therefore, targeting HIF downstream effectors, such as Gal-1, may render the tumor microenvironment more vulnerable to radiation.
Gal-1: a hypoxia-responsive protein
Gal-1 was first shown to be a hypoxia-responsive protein by Le and colleagues using SELDI-TOF-MS to identify molecular markers secreted under hypoxia in a head and neck cancer cell line (Le et al. 2005). Their analysis revealed that a 15 kDa protein that reliably increased with hypoxia treatment in vitro was a B-galactoside-binding protein, Gal-1. This induction was also observed in tongue (Scc4) and head and neck (SQB20) squamous cell carcinomas, pancreatic adenocarcinoma (Panc1) and an immortalized B-cell line (V2P3). Increased circulating Gal-1 was observed in HNC tumor bearing mice after breathing 10% oxygen, which effectively increased tumor hypoxia (Le et al. 2005). Gal-1 expression also correlated with the hypoxia marker, carbonic anhydrase IX (CAIX) (Le et al. 2007). Gal-1 upregulation by hypoxia has since been documented in colorectal and prostate cancer cells as well as Kaposi's sarcoma and acute myeloid leukemia cells (Zhao et al. 2010, 2011; Croci et al. 2012; Laderach et al. 2013).
Hypoxia driven Gal-1 expression occurs through two HREs 441 and 423 base pairs upstream of the transcriptional start site. Overexpression of HIF1α ectopically potently increases Gal-1 expression while its inhibition attenuates hypoxia induction of Gal-1 (Zhao et al. 2010) in colorectal cell lines. CoCl2 treatment, which mimics hypoxia by stabilizing HIF1α, also increased Gal-1 expression. Gal-1 is also regulated by an HIF interacting transcription factor for hematopoietic cell differentiation (C/EPBa), which works in synergism with HIF1α, enhancing its transcriptional activity in acute myeloid leukemia (Zhao et al. 2011).
Hypoxia induction of Gal-1 has also been shown to occur through an NFKB-dependent and HIF-independent mechanism in Kaposi's sarcoma; Inhibition of HIF1α and HIF2α in these tumor cells did not prevent Gal-1 upregulation by hypoxia (Croci et al. 2012). Human LGALS1 contains several putative NFKB consensus sites and the expression of a super-repressor (IkB-a-SR) or pharmacological inhibitor (BAY-117802) prevented hypoxia induction of Gal-1. Furthermore, NFKB-mediated Gal-1 expression under hypoxia was activated by ROS and N-acetylcysteine scavenging of ROS inhibited hypoxia induction of Gal-1 (Croci et al. 2012).
Gal-1 upregulation by radiation and modulation of tumor radiation response
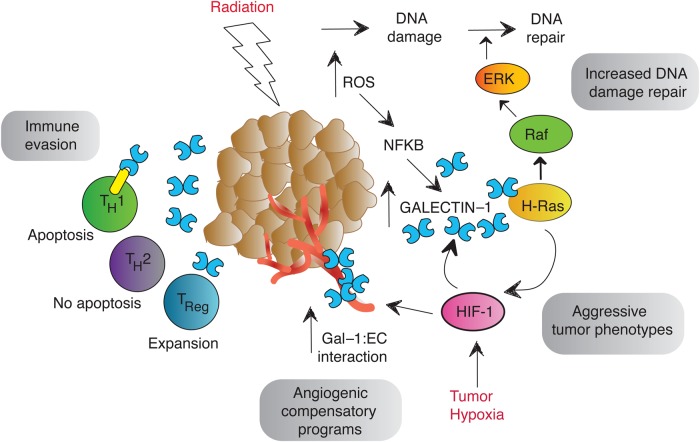
In addition to hypoxia, radiation is also a potent inducer of Gal-1 in glioma and ECs in vitro (Strik et al. 2007; Upreti et al. 2013). Mounting evidence suggests that tumor and ECs may utilize Gal-1 to attenuate tumor radiation response (Figure 1).
Fig. 1.
Gal-1 modulation of tumor radiation response. Gal-1 expression increases in the presence of hypoxia and after tumor irradiation. This elevation of Gal-1 increases repair of radiation-induced DNA damage through H-Ras signaling to promote cell survival. The HIF1/Gal-1/H-Ras interaction may form a positive feedback loop promoting HIF1 transcriptional activity to drive aggressive tumor phenotypes and radiation resistance. Hypoxia also increases Gal-1 to EC binding to mediate proangiogenic signaling. Gal-1 also induces the apoptosis of effector T cells while promoting Treg expansion to create tumor immune privilege.
Anginex, which recognizes Gal-1 overexpressed by the tumor neovasculature, is a 33 amino acid antiangiogenic peptide that was designed based on known features of endogenous inhibitors of angiogenesis (Griffioen et al. 2001; Thijssen et al. 2006; Upreti et al. 2013). Anginex infusion combined with suboptimal radiation dose caused human ovarian tumor xenografts to regress and combined Anginex and a single radiation dose of 25 Gy reduced SCK mammary tumor growth synergistically compared with either therapy alone (Dings et al. 2005). Anginex improvement of radiation response was also observed in syngeneic SCCVII squamous cell carcinoma and focal multiple myeloma SCID-rab mouse models (Amano et al. 2007; Jia et al. 2010). In a microbeam radiation therapy study, Anginex radiosensitized tumors when combined with wider beam spacing and lower radiation doses that did not affect tumor growth alone. Beam geometries and doses capable of slowing tumor growth were also more effective when combined with Anginex (Griffin et al. 2012).
Antiangiogenic agents have been shown to transiently normalize tumor vasculature, which can alleviate hypoxia, increase drug and antitumor immune cell delivery and improve response with various therapies (Jain 2013). Croci and colleagues found that mAb inhibition of Gal-1, similar to other antiangiogenic agents, resulted in transient vessel normalization as evidenced by vasculature remodeling, increased pericyte coverage of vessels and T-cell infiltration, as well as reduced tumor hypoxia (Croci et al. 2014). Interestingly, hypoxia influences the EC surface glycome by increasing B-6GlcNAc-branched N-glycans and poly-LacNAc structures, resulting in enhanced Gal-1 to EC binding and proangiogenic signaling, which may contribute to hypoxia-mediated angiogenic rescue programs (Potente et al. 2011; Croci et al. 2014). Targeting Gal-1 to transiently normalize tumor vasculature may create a window of tumor vulnerability to radiation and enhance therapeutic response.
Ionizing radiation induces cell death through apoptosis, mitotic catastrophe, autophagy and senescence when radiation-induced lesions, such as DNA single- and double-strand breaks, base alterations and DNA-to-DNA and DNA-to-protein cross-linking, are left unrepaired. Interestingly, Gal-1 overexpression reduced DNA damage after C33A irradiation, while shGal-1 HeLa cells exhibited greater DNA damage (Huang et al. 2012). DNA damage repair has been shown to occur through Raf-1 signaling (Golding et al. 2007) and Gal-1 interacts with H-ras and its overexpression enhanced Raf-1 and ERK1 phosphorylation to promote repair of radiation-induced DNA damage. Radiation stimulation of tumor Gal-1 expression can theoretically increase the efficiency of DNA damage repair, promoting tumor and EC survival through H-ras signaling (Huang et al. 2012).
Interestingly, Chen and colleagues reported that an interaction exists between H-ras and HIF1, providing a possible HIF1/Gal-1-positive feedback loop where HIF1 signaling under hypoxia can enhance Gal-1, which acts through H-ras to further promote HIF1 transcriptional activity (Chen et al. 2001; Huang et al. 2012). Tumors may utilize this positive feedback loop to maintain elevated Gal-1 expression and HIF1 signaling to drive radioresistance and aggressive tumor phenotypes.
Another avenue in which Gal-1 promotes tumor radioresistance involves its immunomodulatory functions. Radiotherapy can enhance the antitumor immune response, increasing the peptide repertoire to promote the antigen presentation pathway and recruit cytotoxic T lymphocytes to lyse tumor cells (Lugade et al. 2005; Liang et al. 2013). However, radiotherapy does not always result in protective immunity as relapse occurs. Gal-1 potently induces activated T-cell apoptosis and enforces an Th2 cytokine profile to block immune effector functions while promoting IL-10-producing T regulatory cells to create an immune privileged site at the tumor (Rubinstein et al. 2004; Toscano et al. 2006). The enhancement of Gal-1 expression after radiation may promote tumor immune evasion, limiting therapeutic response.
Patient selection for hypoxia-targeted therapies: hypoxia markers with prognostic value
Aside from targeting hypoxia through its downstream effectors, significant effort has also been devoted to developing tools to select patients who may benefit the most from hypoxia-targeted therapies. In 2007, Le and colleagues sought out to establish a panel of hypoxia markers having prognostic value in head and neck squamous cell carcinoma (HNSCC) treated with concurrent chemoradiation or adjuvant radiation therapy to fulfill this very need. In a cohort of 101 HNSCC patients, Gal-1 stained strongly in approximately half of the tumors and significantly correlated with the CAIX hypoxia marker. Furthermore, tumors with strong Gal-1 staining showed a trend for increasing tumor pO2 measurements <5 mgHg, although the correlation was not statistically significant. Most notably, Gal-1 tumor staining achieved a statistically significant difference for overall survival (Le et al. 2007).
Huang et al. (2013) recently published Gal-1 as an independent prognostic factor for local recurrence uterine cervix squamous cell carcinoma treated with definitive radiotherapy. Local recurrence rates were higher in tumors staining higher for Gal-1 and tumor-specific Gal-1 was also associated with reduced cancer-specific survival rates (Huang et al. 2013).
Conclusions
In summary, Gal-1 has been shown to be highly expressed in tumors that are particularly radioresistant, including prostate cancer, glioma and melanoma, which require large radiation doses to establish control (Rorive et al. 2001; Mathieu et al. 2012; Laderach et al. 2013). In contrast, Gal-1 level is not detectable in nodular lymphocyte-predominant Hodgkin's lymphoma, which is a group of radioresponsive Hodgkin's lymphoma with better prognosis (Gandhi et al. 2007). Preclinical and clinical studies suggest that Gal-1 expression in many cancer cells can be upregulated by hypoxia, which has been intimately linked to radiation resistance and tumor aggressiveness. Mechanistically, Gal-1 affects the tumor vasculature, DNA damage repair and antitumor immunity, all of which have been implicated in modulation of radiation-induced cell kill and tumor growth. Based on these data, targeting Gal-1 with radiotherapy provides a compelling multifaceted approach to increasing radiation effectiveness, which in turn will result in a higher cure rate for certain solid tumors.
Abbreviations
CAIX, carbonic anhydrase IX; Gal-1, galectin-1; HIFs, hypoxia-inducible factors; HNSCC, head and neck squamous cell carcinoma
Conflict of interest statement
None declared.
Funding
This work was supported by grant from the National Institutes of Health (R01 CA161585-02A1).
References
- Amano M, Suzuki M, Andoh S, Monzen H, Terai K, Williams B, Song CW, Mayo KH, Hasegawa T, Dings RP, et al. Antiangiogenesis therapy using a novel angiogenesis inhibitor, anginex, following radiation causes tumor growth delay. Int J Clin Oncol. 2007;12:42–47. doi: 10.1007/s10147-006-0625-y. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiation Oncol Biol Phys. 1997;38 doi: 10.1016/s0360-3016(97)00101-6. 285–189. [DOI] [PubMed] [Google Scholar]
- Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H, Kaltner H, Hadari Y, Ruchoux MM, Brotchi J, et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001;11:12–26. doi: 10.1111/j.1750-3639.2001.tb00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- Cindolo L, Benvenuto G, Salvatore P, Pero R, Salvatore G, Mirone V, Prezioso D, Altieri V, Bruni CB, Chiariotti L. Galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int J Cancer. 1999;84:39–43. doi: 10.1002/(sici)1097-0215(19990219)84:1<39::aid-ijc8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma. J Exp Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- Deschner EE, Gray LH. Influence of oxygen tension on x-ray-induced chromosomal damage in Ehrlich ascites tumor cells irradiated in vitro and in vivo. Radiation Res. 1959;11:115–146. [PubMed] [Google Scholar]
- Dings RP, Williams BW, Song CW, Griffioen AW, Mayo KH, Griffin RJ. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J Cancer. 2005;115:312–319. doi: 10.1002/ijc.20850. [DOI] [PubMed] [Google Scholar]
- Ferreira MB, De Souza JA, Cohen EE. Role of molecular markers in the management of head and neck cancers. Curr Opin Oncol. 2011;23:259–264. doi: 10.1097/CCO.0b013e328344f53a. [DOI] [PubMed] [Google Scholar]
- Gandhi MK, Moll G, Smith C, Dua U, Lambley E, Ramuz O, Gill D, Marlton P, Seymour JF, Khanna R. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood. 2007;110:1326–1329. doi: 10.1182/blood-2007-01-066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Koonce NA, Dings RP, Siegel E, Moros EG, Brauer-Krisch E, Corry PM. Microbeam radiation therapy alters vascular architecture and tumor oxygenation and is enhanced by a galectin-1 targeted anti-angiogenic peptide. Radiation Res. 2012;177:804–812. doi: 10.1667/rr2784.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen AW, van der Schaft DW, Barendsz-Janson AF, Cox A, Struijker Boudier HA, Hillen HF, Mayo KH. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354:233–242. doi: 10.1042/0264-6021:3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EY, Chanchien CC, Lin H, Wang CC, Wang CJ, Huang CC. Galectin-1 is an independent prognostic factor for local recurrence and survival after definitive radiation therapy for patients with squamous cell carcinoma of the uterine cervix. Int J Radiation Oncol Biol Phys. 2013;87:975–982. doi: 10.1016/j.ijrobp.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Huang EY, Chen YF, Chen YM, Lin IH, Wang CC, Su WH, Chuang PC, Yang KD. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis. 2012;3:e251. doi: 10.1038/cddis.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Koonce NA, Halakatti R, Li X, Yaccoby S, Swain FL, Suva LJ, Hennings L, Berridge MS, Apana SM, et al. Repression of multiple myeloma growth and preservation of bone with combined radiotherapy and anti-angiogenic agent. Radiation Res. 2010;173:809–817. doi: 10.1667/RR1734.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, Al Nakouzi N, Sacca P, Casas G, Mazza O, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- Le QT, Kong C, Lavori PW, O'Byrne K, Erler JT, Huang X, Chen Y, Cao H, Tibshirani R, Denko N, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiation Oncol Biol Phys. 2007;69:167–175. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O'Byrne KJ, et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko SA, Drocourt JL, Yang SU. Deoxyribonucleic acid-protein and deoxyribonucleic acid interstrand cross-links induced in isolated chromatin by hydrogen peroxide and ferrous ethylenediaminetetraacetate chelates. Biochemistry. 1982;21:5010–5015. doi: 10.1021/bi00263a026. [DOI] [PubMed] [Google Scholar]
- Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- Massie HR, Samis HV, Baird MB. The kinetics of degradation of DNA and RNA by H 2 O 2. Biochim Biophys Acta. 1972;272:539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- Mathieu V, de Lassalle EM, Toelen J, Mohr T, Bellahcene A, Van Goietsenoven G, Verschuere T, Bouzin C, Debyser Z, De Vleeschouwer S, et al. Galectin-1 in melanoma biology and related neo-angiogenesis processes. J Invest Dermatol. 2012;132:2245–2254. doi: 10.1038/jid.2012.142. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Prise KM, Davies S, Michael BD. Cell killing and DNA damage in Chinese hamster V79 cells treated with hydrogen peroxide. Int J Radiation Biol. 1989;55:583–592. doi: 10.1080/09553008914550631. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Ramhorst RE, Rubinstein N, Corigliano A, Daroqui MC, Kier-Joffe EB, Fainboim L. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002;9:661–670. doi: 10.1038/sj.cdd.4401009. [DOI] [PubMed] [Google Scholar]
- Rajendran JG, Schwartz DL, O'Sullivan J, Peterson LM, Ng P, Scharnhorst J, Grierson JR, Krohn KA. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorive S, Belot N, Decaestecker C, Lefranc F, Gordower L, Micik S, Maurage CA, Kaltner H, Ruchoux MM, Danguy A, et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia. 2001;33:241–255. doi: 10.1002/1098-1136(200103)33:3<241::aid-glia1023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- Saussez S, Camby I, Toubeau G, Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck. 2007;29:874–884. doi: 10.1002/hed.20559. [DOI] [PubMed] [Google Scholar]
- Sotomayor CE, Rabinovich GA. “Galectin-1 induces central and peripheral cell death: Implications in T-cell physiopathology”. Dev Immunol. 2000;7:117–129. doi: 10.1155/2000/36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik HM, Schmidt K, Lingor P, Tonges L, Kugler W, Nitsche M, Rabinovich GA, Bahr M. Galectin-1 expression in human glioma cells: Modulation by ionizing radiation and effects on tumor cell proliferation and migration. Oncol Rep. 2007;18:483–488. [PubMed] [Google Scholar]
- Szoke T, Kayser K, Baumhakel JD, Trojan I, Furak J, Tiszlavicz L, Horvath A, Szluha K, Gabius HJ, Andre S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology. 2005;69:167–174. doi: 10.1159/000087841. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- Upreti M, Jamshidi-Parsian A, Apana S, Berridge M, Fologea DA, Koonce NA, Henry RL, Griffin RJ. Radiation-induced galectin-1 by endothelial cells: A promising molecular target for preferential drug delivery to the tumor vasculature. J Mol Med (Berl) 2013;91:497–506. doi: 10.1007/s00109-012-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brule FA, Fernandez PL, Buicu C, Liu FT, Jackers P, Lambotte R, Castronovo V. Differential expression of galectin-1 and galectin-3 during first trimester human embryogenesis. Dev Dyn. 1997;209:399–405. doi: 10.1002/(SICI)1097-0177(199708)209:4<399::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol. 2001;193:80–87. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH730>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue F, Jiang Y, Chen GQ, Zhao KW. Hypoxia inducible factor-1 mediates expression of galectin-1: The potential role in migration/invasion of colorectal cancer cells. Carcinogenesis. 2010;31:1367–1375. doi: 10.1093/carcin/bgq116. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Zhao KW, Jiang Y, Zhao M, Chen GQ. Synergistic induction of galectin-1 by CCAAT/enhancer binding protein alpha and hypoxia-inducible factor 1alpha and its role in differentiation of acute myeloid leukemic cells. J Biol Chem. 2011;286:36808–36819. doi: 10.1074/jbc.M111.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zips D, Zophel K, Abolmaali N, Perrin R, Abramyuk A, Haase R, Appold S, Steinbach J, Kotzerke J, Baumann M. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105:21–28. doi: 10.1016/j.radonc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Zuniga E, Gruppi A, Hirabayashi J, Kasai KI, Rabinovich GA. Regulated expression and effect of galectin-1 on Trypanosoma cruzi-infected macrophages: Modulation of microbicidal activity and survival. Infect Immun. 2001;69:6804–6812. doi: 10.1128/IAI.69.11.6804-6812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]