Abstract
Galectin-3 is a member of the family of β-galactoside-binding lectins characterized by evolutionarily conserved sequences defined by structural similarities in their carbohydrate-recognition domains. Galectin-3 is a unique, chimeric protein consisting of three distinct structural motifs: (i) a short NH2 terminal domain containing a serine phosphorylation site; (ii) a repetitive proline-rich collagen-α-like sequence cleavable by matrix metalloproteases; and (iii) a globular COOH-terminal domain containing a carbohydrate-binding motif and an NWGR anti-death motif. It is ubiquitously expressed and has diverse biological functions depending on its subcellular localization. Galectin-3 is mainly found in the cytoplasm, also seen in the nucleus and can be secreted by non-classical, secretory pathways. In general, secreted galectin-3 mediates cell migration, cell adhesion and cell–cell interactions through the binding with high affinity to galactose-containing glycoproteins on the cell surface. Cytoplasmic galectin-3 exhibits anti-apoptotic activity and regulates several signal transduction pathways, whereas nuclear galectin-3 has been associated with pre-mRNA splicing and gene expression. Its unique chimeric structure enables it to interact with a plethora of ligands and modulate diverse functions such as cell growth, adhesion, migration, invasion, angiogenesis, immune function, apoptosis and endocytosis emphasizing its significance in the process of tumor progression. In this review, we have focused on the role of galectin-3 in tumor metastasis with special emphasis on angiogenesis.
Keywords: angiogenesis, galectin-3, metastasis
Galectin-3 in tumor metastasis
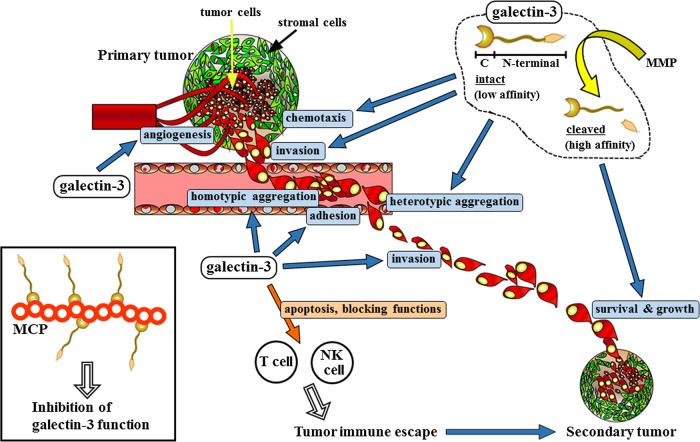
Tumor metastasis is so far the biggest challenge associated with most cancers despite significant improvements in diagnosis, surgical techniques, patient care and adjuvant therapies. It is a complicated biological phenomenon, regulated by multiple interactions between tumor and host cells (Woodhouse et al. 1997). Initiating event in the metastatic cascade is invasion of tumor cells. Proteolytic enzymes (lysosomal hydrolases, collagenases) secreted by tumor cells degrade basement membrane constituents such as type IV collagen, laminin and fibronectin, allowing invading cells access to the underlying connective tissue matrix. The next step is degradation and movement through this matrix, before the cell ultimately invades a vascular endothelial basement membrane or perineurium to enter an adjacent blood or lymphatic vessel. The vascular and lymphatic systems have numerous connections that allow disseminating tumor cells to pass rapidly from one system to the other. Once the tumor cells have made their way into microcirculation, they are carried by the vascular flow to distant organs. The invading tumor cell must survive natural host immune system (macrophages, NK cells and cytotoxic T lymphocytes) in order to enter a distant organ system (secondary invasion). Once the tumor cells have adhered to the microvascular endothelium, including the events of homotypic and heterotypic aggregations, the extravasation of the tumor cells from the blood vessels into the organ begins. After passing the endothelial barrier of the vessel, the tumor cells produce enzymes that break down the components of the basement membrane and underlying connective tissue, thus facilitating their passage into the parenchyma of organ. Finally, tumor cells continue to proliferate in the target organ, which depends on newly established blood vessels (angiogenesis), and form metastatic foci (Folkman 1995). The major obstacle to the treatment of tumor metastasis is the biological heterogeneity of tumor cells in primary and secondary tumors. This heterogeneity is exhibited in a wide range of genetic, biochemical, immunological and biological characteristics including cell morphologies, growth properties and ability to invade. Drug susceptibilities are different between metastatic lesions and their primary tumors, and these differences are believed to result from selective genetic changes (Schnipper 1986). Host microenvironment has been shown to affect the genes that regulate metastasis (Liotta and Kohn 2001). Galectin-3 is reportedly required in many of these steps during tumor metastasis. It has been demonstrated that galectin-3 regulates many biological functions and signaling pathways associated with cell proliferation, adhesion, migration, invasion, angiogenesis and apoptosis (Takenaka et al. 2004;Newlaczyl and Yu 2011). Subcellular localization of galectin-3 seems to be important for tumor cell growth effects, and galectin-3 plays a significant role in cancer progression through the regulation of specific gene expression by transcription factors such as β-catenin (reviewed in Funasaka et al. 2014). Galectin-3 mediates cell–extracellular matrix heterotypic adhesion processes, which may modulate tumor cell detachment from the primary site, and therefore, regulates tumor cell migration and invasion. Galectin-3 was shown to bind to glycoconjugates of extracellular matrix like laminin and fibronectin, as well as hensin, elastin, collagen IV and tenascin-C and -R, both intra- and extracellularly (Sato and Hughes 1992; van den Brule et al. 1995; Kuwabara and Liu 1996; Ochieng et al. 1999; Hikita et al. 2000). In addition, another class of cell adhesion molecules, integrins were shown to be the receptors for galectin-3 (α1β1, α4β7, α6β1, αMβ1, etc.) (Dong and Hughes 1997; Warfield et al. 1997; Ochieng et al. 1999; Matarrese et al. 2000; Saravanan et al. 2009). Moreover, galectin-3 activates focal adhesion kinase (FAK), which is a key regulator of integrin-dependent cell signaling, and Rac1, which is known to play an important role in reorganizing the actin skeleton and the formation of lamellipodial extensions, by N-glycosylation of α3β1 integrin (Saravanan et al. 2009). Dimer or multimer formation of galectin-3 is implicated in aggregation of tumor cells in the circulation during metastasis via bridging with glycoconjugates (Inohara et al. 1996; Ahmad et al. 2004). Galectin-3 mediates both homotypic aggregation of tumor cells invading blood vessels and heterotypic tumor cell adhesion to endothelial cells to keep cells in the blood stream and reach distant organ sites (Inohara et al. 1996; Nangia-Makker et al. 2000; Shekhar et al. 2004). Kim et al. suggested that galectin-3 may be a critical determinant for anchorage-independent cell survival of disseminating cancer cells in the circulation during metastasis (Kim et al. 1999). Galectin-3 is also known to be a chemo-attractant to endothelial cells and to stimulate neovascularization in vitro and in vivo, therefore contributing to tumor angiogenesis (Nangia-Makker et al. 2000). Furthermore, exogenous galectin-3 has been shown to cause apoptosis in activated T cells (Stillman et al. 2006) and galectin-3 suppressed the binding of MHC class I chain-related molecule A to natural killer Group 2, member D which impair the NK cell activation (Tsuboi et al. 2011), from which galectin-3 might be involved in the escape from the host immune system (Figure 1).
Fig. 1.
Multiple functions of galectin-3 in tumor metastasis. Galectin-3 plays a significant role in angiogenesis at the primary tumor site, migration and invasion into the surrounding tissue, emboli formation in the blood or lymph vessels, adhesion to endothelial cells in distant organ and invasion across vessel walls, and tumor cell growth at the secondary tumor. Since it generally occurs via the vascular or lymphangial system to distant organs, metastasis is closely related to the vascular system. MCP is a natural inhibitor of galectin-3 and binds to galectin-3 to prevent its functions (inset). Some other small molecules also inhibit galectin-3 activities. Ocimum gratissimum (OG) inhibits galectin-3 cleavage thus preventing angiogenesis. In addition, galectin-3 induces T-cell apoptosis and blocks NK cell functions, which enables the tumor to escape from the host immune system and to develop. Adapted from Nangia-Makker et al. (2008).
Although the regulatory role of galectin-3 in cancer development and metastasis has been demonstrated using a variety of in vitro and in vivo models, Parco et al. did not find a rate-limiting role for galectin-3 using galectin-3+/+ and galectin-3−/− mouse models of spontaneous colon and mammary carcinomas (Eude-Le Parco et al. 2009). Galectin-3 exhibits moonlighting biological functions. Extracellular galectin-3 mediates several cellular events by interacting with cell surface and extracellular matrix glycoconjugates, and intracellular galectin-3 regulates signaling pathways by interacting with cytoplasmic and nuclear proteins. Moreover, other members of the galectin family show similar interactions through their carbohydrate-binding domain. It is possible that in the knockout mice other compensatory mechanisms come into play.
Galectin-3 in tumor angiogenesis
Angiogenesis is essential for the development and evolution of neoplastic disease, both for tumor growth and metastasis as rapid expansion of a tumor mass requires persistent new blood vessels. For a tumor to progress from a prevascular to a vascular phase, activation of angiogenic switch is required, which reflects the ability of the tumor and inflammatory cells to secrete angiogenic factors in tumor microenvironment (Raica et al. 2009).
A number of proangiogenic growth factors secreted by tumor cells and a plethora of molecules regulate angiogenesis by maintenance and destruction of extracellular matrix and perivascular cells as well as by stimulating endothelial cell division and migration (Holash et al. 1999). The most studied angiogenic factors include vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). VEGF induces vascular permeability, endothelial migration, proliferation and survival of endothelial cells via its activation of the nitric oxide synthase, tyrosine protein kinase CSK, rat sarcoma mitogen-activated protein kinases and phosphatidylinositol 3-kinase-protein kinase B signaling cascades through binding to the VEGF receptor 2 (VEGFR2; Hanahan and Folkman 1996; Bussolati et al. 2011). bFGF is a pleiotropic mitogen produced by macrophages and tumor cells among others and is involved in endothelial cell proliferation, extracellular matrix degradation, endothelial cell migration and modulation of junctional adhesion molecules through FGFR1 receptor on endothelial cells (Hillen and Griffioen 2007). Galectin-3 was first reported to induce angiogenesis by the demonstration that it induced 3D morphogenesis of endothelial cells and an angiogenic response in vivo (Nangia-Makker et al. 2000). A direct binding of galectin-3 to the endothelial cells was demonstrated, which was dependent on its carbohydrate-recognition domain (CRD) as it could specifically be inhibited by a competitive disaccharide, lactose (Nangia-Makker et al. 2000) and a polysaccharide, modified citrus pectin (MCP) (Nangia-Makker et al. 2002). Using a 3D coculture system of in vitro angiogenesis, Shekhar et al. (2004) demonstrated that galectin-3 is important for stabilization of the epithelial and endothelial interactive network, as immuno-neutralization with anti-galectin-3 antibodies abolished these interactions.
A detailed investigation by Markowska et al. suggested that galectin-3 modulates VEGF- and bFGF-mediated angiogenesis. They proposed that galectin-3 CRD binds to GnTV-modified N-glycans on αvβ3 integrin and as a multimer, it cross-links and clusters the integrin and activates FAK-mediated signaling pathways that modulate endothelial cell migration in the angiogenic cascade (Markowska et al. 2010). This group also reported that galectin-3 binds to VEGFR2 and prevents its internalization leading to increased angiogenic response to VEGF-A (Markowska et al. 2011). Using microglia BV2 cells, Wesley et al. (2013) also showed regulation of angiogenic and migratory response in endothelial cells via modulation of integrin-linked kinase signaling. In a recent study, D'Haene et al. (2013) reported that the combined action of galectin-3 and -1 has an enhanced effect on angiogenesis via VEGFR1 activation and reduced receptor endocytosis. The response of EC to galectin-1 and -3 treatment depends on the presence of VEGFR1 or VEGFR2 levels on the cell surface (D'Haene et al. 2013). Increased circulating galectin-3 in cancer patients (Iurisci et al. 2000; Xie et al. 2012) induces secretion of metastasis promoting cytokines such as interleukin-6 (IL-6) and colony-stimulating factor (G-CSF) from the blood vascular endothelium in vitro and in vivo. These cytokines interact with the vascular endothelium in autocrine/paracrine fashion to enhance the expression of endothelial cell surface adhesion molecules resulting in increased association between the cancer cells and endothelial cells leading to increased migration and tubule formation by endothelial cells (Chen et al. 2013). These data were further confirmed by Machado et al., who showed that disruption of galectin-3 in tumor stroma and parenchyma decreased angiogenesis through interfering with the responses of macrophages to the interdependent VEGF and TGFβ-1 signaling pathways (Machado et al. 2014). An interaction of galectin-3 with endothelial cell surface enzyme aminopeptidase N/CD13 has also been reported to regulate endothelial vascularization in the early steps of angiogenesis (Yang et al. 2007). High galectin-3 expression in the tumor induced macrophage infiltration and accelerated angiogenesis (Jia et al. 2013). Stimulatory effect of galectin-3 on the proliferation and angiogenesis of endothelial cells differentiated from bone marrow mesenchymal stem cells was also reported (Wan et al. 2011). Fukushi et al. (2004) showed that NG2, a transmembrane chondroitin sulfate proteoglycan present on the surface of pericytes, induced endothelial cell motility and multicellular network formation in vitro and the stimulation of corneal angiogenesis in vivo mediated by formation of NG2–galectin-3–α3β1 integrin complex suggesting their role for early stages of neovascularization.
Shekhar et al. (2004) had initially reported that proteolytically cleaved galectin-3 displayed ∼20-fold higher affinity for endothelial cells as compared with the full-length protein. Using tumor cells expressing protease resistant galectin-3, we showed a significant inhibition in tumor growth accompanied with reduced tumor angiogenesis (Nangia-Makker et al. 2007), similarly cells expressing an MMP-2 and -9 cleavable variant of galectin-3 showed increased chemotaxis, chemo-invasion and interaction with endothelial cells resulting in increased angiogenesis and 3D morphogenesis compared with non-cleavable form of galectin-3 (Nangia-Makker et al. 2010). Gao et al. (2012) showed that CRD domain of galectin-3 is important for cell surface binding and internalization of galectin-3 by the endothelial cells, which supports the earlier observations made by us and others that the carbohydrate-binding domain showed greater binding to the endothelial cells compared with the full-length protein. Binding of galectin-3 to integrins and VEGFR is also dependent on its carbohydrate-binding property. However, we observed a better migration of endothelial cells when the CRD retained 32 amino acids from the collagen-like domain, which is the result of cleavage by MMP-2 and MMP-9 at an additional site. Whether this interaction is carbohydrate dependent or independent was not established in this study (Nangia-Makker et al. 2010). It is possible that addition of this small domain helps galectin-3 to retain its dimer and multimer forming ability because N-terminal is responsible for dimerization of galectin-3. It is also possible that proliferation, cell interaction or migration of endothelial cells is regulated by different domains of galectin-3 through interactions with different cell surface receptors on endothelial cells via carbohydrate dependent or independent mechanisms.
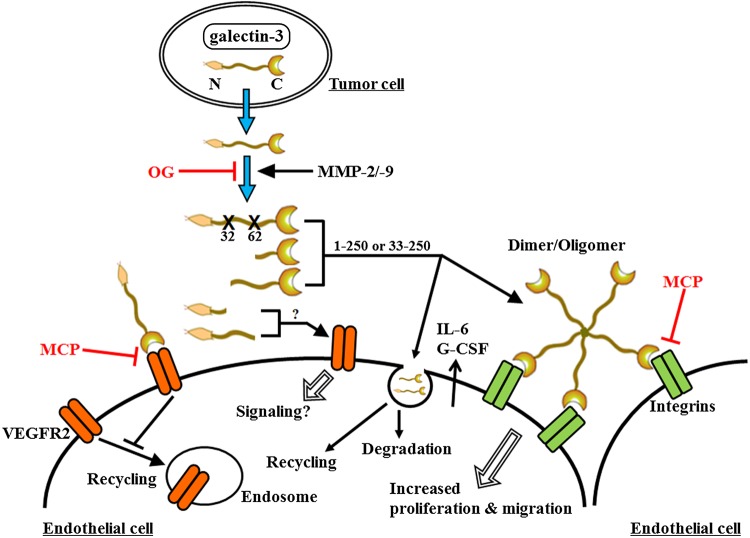
Galectin-3 is not only secreted by tumor cells, there are also reports indicating its expression in vascular endothelial cells. Presence of galectin-3 in the endothelial cells in combination with endothelial hyperplasia was shown to be an independent prognostic factor for immuno-competent primary central nervous system lymphomas (PCNSL) (D'Haene et al. 2008). On the other hand, Thijssen et al. (2008) reported that galectin-3 expression does not increase upon activation of endothelial cells in vitro. Tumor vasculature of various tumors including head and neck cancer (Lotan et al. 1994), hepatocellular carcinoma (Thijssen et al. 2008) and colon carcinoma (Thijssen et al. 2008) showed upregulation of galectin-3. Whether a direct interaction with tumor cells is a prerequisite for increased galectin-3 expression in the activated endothelial cells or its presence in endothelial cells is the result of endocytosis of the galectin-3 secreted by the tumor cells, as suggested by Gao et al. (2012), is difficult to say based on the present information. However, the available data indicate that increased galectin-3 in endothelial cells may be the result of direct endocytosis or via cell surface receptors. In the endothelial cells, galectin-3 follows two pathways, either it is recycled or degraded in the lysosomes. Gao et al. (2012) showed that the presence of N-terminal domain directed galectin-3 towards lysosomes and the presence of CRD directed it towards recycling. Endocytosed galectin-3 also increases the expression and secretion of metastatic proteins IL-6 and G-CSF (Chen et al. 2013). How and whether galectin-3 and its fragments bind to different set of receptors and affect different activities like proliferation or migration of endothelial cells needs to be analyzed in details. Figure 2 summarizes current information on the role of intact and cleaved galectin-3 on angiogenesis.
Fig. 2.
Interactions of galectin-3 with endothelial cells. Galectin-3 is cleaved by MMPs after it is secreted by the tumor cells. The intact galectin-3 and/or its fractions bind to the cell surface receptors on endothelial cells, induce lattice formation by oligomerization, induce secretion of proteins like IL-6 or G-CSF and prevent internalization of VEGFR2. Galectin-3 and its C-terminal containing fractions have also been reported to be endocytosed, either going through recycling or degradation. The N-terminal domain by itself also increased endothelial migration through an as yet unknown signaling mechanism.
Galectin-3 regulated angiogenesis as a therapeutic target
Considering the important role of angiogenesis in tumor cell metastasis, a number of anti-angiogenic agents targeting VEGF, FGF or/and their receptors are currently in clinical use (reviewed by Katoh 2013). The long-term use of these therapies results in drug resistance as a result of expansion of tumor cell clones with up-regulation of other angiogenic factors. Moreover, these inhibitors also produce multiple toxic side effects. In a recent study, Croci et al. (2014) demonstrated that vessels within anti-VEGF-sensitive tumors exhibited high levels of α2-6-linked sialic acid, which prevented galectin-1 binding. In contrast, anti-VEGF refractory tumors secreted increased galectin-1. Although the focus of this review is galectin-3, it has been postulated that after removal of N-terminal, galectin-3 shows galectin-1-like binding properties. Interruption of β1-6GlcNAc branching in endothelial cells or silencing of tumor-derived galectin-1 converted refractory tumors into anti-VEGF-sensitive ones (Croci et al. 2014). Similarly, targeting cleavage of galectin-3 by MMP inhibitors has shown reduced angiogenesis (Nangia-Makker et al. 2013). A few preclinical studies have used galectin-3-binding activity as a target to inhibit angiogenesis and metastasis. When MCP, a high pH and temperature modified hydrolysis product of citrus pectin was fed to nude mice, there was a marked reduction in the metastatic and angiogenic potential of breast cancer cells (Nangia-Makker et al. 2002). It was suggested that MCP interferes with the binding of galectin-3 to its glycoconjugate cell surface receptors. Three novel low-molecular-weight synthetic lactulose amines: N-lactulose-octa-methylenediamine; N,N′-dilactulose-octamethylenedi-amine and N,N′-dilactulose-dodecamethylene diamine exhibiting differential ability to inhibit binding of galectin-3 to the highly glycosylated protein 90K, demonstrated selective regulatory effect in different events linked to endothelial cell morphogenesis and angiogenesis (Rabinovich et al. 2006).
Although the initial studies demonstrating the role of galectin-3 in tumor angiogenesis were reported more than a decade ago, in the recent years an increasing interest in this field confirms its significance. More studies are in order using different models to analyze the significance of galectin-3 on various steps of angiogenesis to get a more comprehensive knowledge of this potentially important and exciting field.
Abbreviations
bFGF, basic fibroblast growth factor; CRDs, carbohydrate-recognition domains; FAK, focal adhesion kinase; G-CSF, colony-stimulating factor; IL-6, interleukin-6; VEGF, vascular endothelial growth factor
Conflict of interest statement
None declared.
Funding
This work was supported by National Institute of Health R37CA46120 (to AR)
References
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Grange C, Camussi G. Tumor exploits alternative strategies to achieve vascularization. FASEB J. 2011;25:2874–2882. doi: 10.1096/fj.10-180323. [DOI] [PubMed] [Google Scholar]
- Chen C, Duckworth CA, Zhao Q, Pritchard DM, Rhodes JM, Yu LG. Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin Cancer Res. 2013;19:1693–1704. doi: 10.1158/1078-0432.CCR-12-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- D'Haene N, Catteau X, Maris C, Martin B, Salmon I, Decaestecker C. Endothelial hyperplasia and endothelial galectin-3 expression are prognostic factors in primary central nervous system lymphomas. Br J Haematol. 2008;140:402–410. doi: 10.1111/j.1365-2141.2007.06929.x. [DOI] [PubMed] [Google Scholar]
- D'Haene N, Sauvage S, Maris C, Adanja I, Le Mercier M, Decaestecker C, Baum L, Salmon I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS ONE. 2013;8:e67029. doi: 10.1371/journal.pone.0067029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj J. 1997;14:267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- Eude-Le Parco I, Gendronneau G, Dang T, Delacour D, Thijssen VL, Edelmann W, Peuchmaur M, Poirier F. Genetic assessment of the importance of galectin-3 in cancer initiation, progression, and dissemination in mice. Glycobiology. 2009;19:68–75. doi: 10.1093/glycob/cwn105. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funasaka T, Raz A, Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol. 2014;27C:30–38. doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liu D, Fan Y, Li X, Xue H, Ma Y, Zhou Y, Tai G. The two endocytic pathways mediated by the carbohydrate recognition domain and regulated by the collagen-like domain of galectin-3 in vascular endothelial cells. PLoS ONE. 2012;7:e52430. doi: 10.1371/journal.pone.0052430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hikita C, Vijayakumar S, Takito J, Erdjument-Bromage H, Tempst P, Al-Awqati Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J Cell Biol. 2000;151:1235–1246. doi: 10.1083/jcb.151.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen F, Griffioen AW. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Inohara H, Akahani S, Koths K, Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996;56:4530–4534. [PubMed] [Google Scholar]
- Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- Jia W, Kidoya H, Yamakawa D, Naito H, Takakura N. Galectin-3 accelerates M2 macrophage infiltration and angiogenesis in tumors. Am J Pathol. 2013;182:1821–1831. doi: 10.1016/j.ajpath.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Katoh M. Therapeutics targeting angiogenesis: Genetics and epigenetics, extracellular miRNAs and signaling networks (review) Int J Mol Med. 2013;32:763–767. doi: 10.3892/ijmm.2013.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939–3944. [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Lotan R, Belloni PN, Tressler RJ, Lotan D, Xu XC, Nicolson GL. Expression of galectins on microvessel endothelial cells and their involvement in tumour cell adhesion. Glycoconj J. 1994;11:462–468. doi: 10.1007/BF00731282. [DOI] [PubMed] [Google Scholar]
- Machado CM, Andrade LN, Teixeira VR, Costa FF, Melo CM, Dos Santos SN, Nonogaki S, Liu FT, Bernardes ES, Camargo AA, et al. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFbeta1-induced macrophages. Cancer Med. 2014;3:201–214. doi: 10.1002/cam4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207:1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, Malorni W, Iacobelli S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85:545–554. [PubMed] [Google Scholar]
- Nangia-Makker P, Balan V, Raz T, et al. Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 2008;1:43–58. doi: 10.1007/s12307-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: A novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Raz T, Tait L, Shekhar MP, Li H, Balan V, Makker H, Fridman R, Maddipati K, Raz A. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol Ther. 2013;14:417–427. doi: 10.4161/cbt.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, Raz A. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlaczyl AU, Yu LG. Galectin-3 – A jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Warfield P, Green-Jarvis B, Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem. 1999;75:505–514. doi: 10.1002/(sici)1097-4644(19991201)75:3<505::aid-jcb14>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Cumashi A, Bianco GA, Ciavardelli D, Iurisci I, D'Egidio M, Piccolo E, Tinari N, Nifantiev N, Iacobelli S. Synthetic lactulose amines: Novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology. 2006;16:210–220. doi: 10.1093/glycob/cwj056. [DOI] [PubMed] [Google Scholar]
- Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45:1924–1934. doi: 10.1016/j.ejca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci. 2009;122:3684–3693. doi: 10.1242/jcs.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992;267:6983–6990. [PubMed] [Google Scholar]
- Schnipper L. Clinical implications of tumor-cell heterogeneity. N Engl J Med. 1986;314:1423–1431. doi: 10.1056/NEJM198605293142206. [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: Functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–1941. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19:543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: Altered expression and localization in activated and tumor endothelial cells. Am J Pathol. 2008;172:545–553. doi: 10.2353/ajpath.2008.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T, et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30:3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule FA, Buicu C, Sobel ME, Liu FT, Castronovo V. Galectin-3, a laminin binding protein, fails to modulate adhesion of human melanoma cells to laminin. Neoplasma. 1995;42:215–219. [PubMed] [Google Scholar]
- Wan SY, Zhang TF, Ding Y. Galectin-3 enhances proliferation and angiogenesis of endothelial cells differentiated from bone marrow mesenchymal stem cells. Transplant Proc. 2011;43:3933–3938. doi: 10.1016/j.transproceed.2011.10.050. [DOI] [PubMed] [Google Scholar]
- Warfield PR, Makker PN, Raz A, Ochieng J. Adhesion of human breast carcinoma to extracellular matrix proteins is modulated by galectin-3. Invasion Metastasis. 1997;17:101–112. [PubMed] [Google Scholar]
- Wesley UV, Vemuganti R, Ayvaci ER, Dempsey RJ. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013;1496:1–9. doi: 10.1016/j.brainres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, He S, Lu CH, Li XY, Jiang F, Ni RZ. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138:1035–1043. doi: 10.1007/s00432-012-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Shim JS, Woo HJ, Kim KW, Kwon HJ. Aminopeptidase N/CD13 induces angiogenesis through interaction with a pro-angiogenic protein, galectin-3. Biochem Biophys Res Commun. 2007;363:336–341. doi: 10.1016/j.bbrc.2007.08.179. [DOI] [PubMed] [Google Scholar]