Abstract
The colonic mucus gel layer (MGL) is a critical component of the innate immune system acting as a physical barrier to microbes, luminal insults, and toxins. Mucins are the major component of the MGL. Selected microbes have the potential to interact with, bind to, and metabolize mucins. The tolerance of the host to the presence of these microbes is critical to maintaining MGL homeostasis. In disease states such as ulcerative colitis (UC), both the mucosa associated microbes and the constituent MGL mucins have been shown to be altered. Evidence is accumulating that implicates the potential for mucin degrading bacteria to negatively impact the MGL and its stasis. These effects appear more pronounced in UC.
This review is focused on the host-microbiome interactions within the setting of the MGL. Special focus is given to the mucolytic potential of microbes and their interactions in the setting of the colitic colon.
Keywords: colon, mucus gel layer, bacteria, mucins, ulcerative colitis
Introduction
The gastrointestinal tract is a natural reservoir for bacteria as it is a rich source of nutrients that can be used for bacterial metabolism in a temperature environment ideal for bacterial growth. An estimated 1013–1014 bacterial cells reside within the adult intestine, a number that exceeds the total number of cells in the human body by a factor of 10.1 With such favorable conditions for bacterial growth, the human host has had to evolve mechanisms that control bacterial growth, while maintaining the presence of symbiotic bacteria. These symbiotes perform vital roles in energy extraction from foods otherwise indigestible by the host2 while exerting trophic effects on the host epithelium and immune structure and function.3 Although commensal bacteria exist in symbiosis with their host and are typically well tolerated, the potential for deleterious effects on the host still remains, owing to their sheer numbers and large surface area of the intestinal epithelium. The mechanisms by which the bacteria are maintained in homeostasis with the MGL are not fully understood; however, two principles are involved: physical separation of the bacteria from the host epithelium by the MGL and tolerance of the host immune system for the presence of bacteria.4
The physical separation of the bacteria from their host depends on a continuous MGL secreted by host goblet cells, which serves as a barrier between the outer luminal contents and the host epithelium.5 The MGL also contains anti-microbial peptides and IgA, both of which serve to limit the number of bacteria that reach the host epithelium.6 In cases where bacteria breach the MGL, host adaptive immune responses mediated by dendritic cells are employed to eliminate the bacteria.7-9 In health, this process occurs without an overt immunological reaction whereas this response is amplified in UC and other forms of colitis.10 The importance of the adaptive immune system within the colon have been discussed in a number of recent review articles,9,11,12 and therefore will not be discussed here. The focus of this discussion is on the physical separation of the host epithelium and bacterial cells, and the potential for the bacteria to modulate this physical barrier leading to inflammatory consequences. Particular emphasis is placed on the discussion of these properties within the setting of UC.
Properties and Function of the Colonic Mucous Gel Layer
The MGL serves to protect the colonic epithelium from physical, chemical, and biological damage. Its functionality and efficacy are dependent not only on the thickness of the MGL but also on its chemical composition.
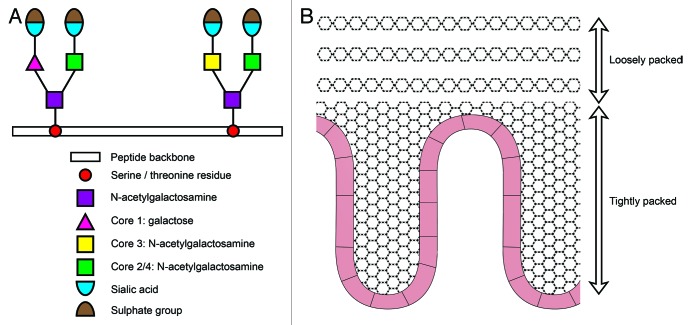
The MGL is produced and secreted by goblet cells resident in intestinal crypts. The number of goblet cells per crypt is greater in the colon than the small bowel reflecting the greater concentration of bacteria within the colonic microbiome.13 The viscoelastic properties of the MGL derive from its glycoprotein constituents (mucins).14 These mucins consist of a peptide backbone containing serine or threonine residues upon which N-acetylgalactosamine residue can be added. This structure is then modified by core transferases, which serve to elongate the oligosaccharide side chains. These side chains are often terminated with sialic acid, which can be post translationally modified to also contain a sulfate group.15 The core structures are formed by the core 1 enzyme β1,3-galactosyltransferase to form the core 1 glycan Galβ1–3GalNAcα-Ser/Thr, the core 3 β1,3 N-acetylglucosaminyltransferase to form a GlcNAcβ1–3GalNAcα-Ser/Thr core, and additional core 2 and core 4 transferases, which serve to act on these precursors with the addition of N-acetylglucosamine.16 (Fig. 1A).
Figure 1. Schematic representation of a mucin structure and organization of the MGL. The four major core types are synthesized by specific glycotransferase enzymes following transfer of N-acetylgalactosamine to serine or theronine. The mucin molecule is then elongated by the addition of galactose or N-acetylgalactosamine and terminated with sialic acid and a sulfate group (A). Schematic representation of the two layered organization of the MGL. Structural organization of mucin molecule (B).
Alterations to the structure or composition of MGL mucins have the potential to affect the protective barrier and play a role in the pathogenesis of inflammation of the underlying epithelium. In UC a number of specific changes have been reported.17-21 During active inflammation the goblet cell population is depleted, and individual goblet cells contain less mucin than those of healthy controls. Additionally the MGL is reduced in thickness.20,21 During periods of disease remission both the number and appearance of goblet cells return to normal.22 In UC changes in O-glycosylation and shortening of the oligosaccharide side chains of MGL mucins have been reported23,24 along with alterations in glycosylation and sulfation17,25,26. In vivo mouse models lacking functional core 2 and 3 enzymes displayed increased susceptibility to colitis in the dextran sulfate sodium colitis model,27,28 while mice lacking core 1 enzymes developed spontaneous colitis.29 These alterations are thought likely to lead to a diminished protective capacity of the MGL.
The presence of sulfate groups is thought to confer a resistance to enzymatic degradation of the mucin;30 thus the reduced content observed in the colitic colon is likely to be disadvantageous. Interestingly bacteria which are capable of cleaving sulfate and utilizing it as a metabolite have been found to be overrepresented in the colitic colon.31,32 Their presence may offer an explanation for the reduced sulfated content of mucin in the colitic colon.
Mucous Gel Layer Organization
Development of methods to measure and characterize the MGL in vivo in animal studies has allowed better understanding of the MGL architecture. The MGL is now known to be organized into a two layer structure; a loosely adherent outer layer and an inner layer which is firmly attached to epithelial cells5 (Fig. 1B). In mouse models, the thickness of this two layered structure is estimated at 150 µm, with the inner layer measuring 50 µm and the outer layer 100 µm.5 The thickness of the MGL in humans remains to be clarified however it is thought to measure between 107 and 155 µm.21 Both layers are composed of MUC2 type mucin, with a well-organized stratified inner layer and a loosely adherent, non-stratified outer layer. A clear boarder exists between the inner and outer layers suggesting a controlled transition of mucus between the layers.5
Analysis of the mucosa associated microbiome within the two layers of the MGL has clearly demonstrated that in health bacteria reside in the outer mucus layer only, leaving the inner layer sterile and devoid of bacteria.33 In a dextran sulfate sodium mouse model of colitis this mucosal homeostasis was absent. Bacteria were found to be resident within crypt structures, and bacteria (mainly Bacteroides) were both in contact with and invading host mucosa.33 This direct contact between bacteria and their host is now known to negatively impact the host cells. Animal models of MUC2 deficient mice, which render the MGL absent and bacteria in direct contact with the colonic epithelium, have resulted in abnormal mucosal morphology,34 increased cellular proliferation of epithelial cells,34,35 decreased apoptosis, development of spontaneous colitis, and increased migration of epithelial cells leading to intestinal tumor formation and subsequent spontaneous progression to invasive carcinoma.35 Given that it is known that the normal colonic microbiome stimulates cell proliferation in colon,36 it has been hypothesized that the increased cell proliferation observed in MUC2 deficient animals could be explained by overt bacterial stimulation due to the diminished MGL.5,34
In addition to the presence of a MGL, the integrity of its mucin components and its stratified organization is also vital to maintenance of mucosal homeostasis. Misfolding of the MUC2 mucin has been shown to lead to endoplasmic reticulum (ER) stress which in the microbial milieu associated with the colon leads to epithelial cell dysfunction and intestinal inflammation.37 Nhe-3 (a sodium hydroxide transporter necessary for normal mucus expansion and organization) deficient mice have shown the MGL to be intact with a two layer organization similar to that of wild type mice. However bacteria were found to be capable of penetrating both the outer and inner MGL resulting in an inflammatory response, suggesting that defects in the MGL have the potential to trigger inflammatory responses. Likewise IL-10 deficient mice were found to have an intact but penetrable MGL which yielded a phagocyte mediated innate inflammatory response, suggesting a link between the MGL integrity, cytokines produced and the immune response.38
Mucosa Associated Microbiome of the Colon
An accurate description of the community composition and structure of mucosa associated bacteria has until recently been difficult to generate. However recent advances in electron microscopy technologies, molecular techniques such as fluorescent in situ hybridization (FISH) and metagenomic sequencing methods have now enabled the mucosal communities to be identified and their distribution mapped without disrupting MGL architecture. The colonic microbiota is unevenly distributed along the longitudinal axis of the colon, with spatial segregation occurring between the luminal and mucosa adjacent regions.33,39 In health this spatial segregation can be further characterized by the existence of bacterial colonization in the looser outer regions of the MGL and a sterile inner region of densely packed mucins which are adjacent to the mucosa.40
The recent advances in molecular techniques and metagenomic studies based on 16S rRNA gene sequences have now made it possible to fully interrogate the resident mucosa associated bacterial communities. A clear differentiation between luminal and mucosa associated communities has been widely reported both in health and diseased states, with Firmicutes and Bacteroidetes reported as the dominant phyla in both locations.26,41-45 In humans the precise spatial differences in bacterial communities remains to be clearly defined. Initial studies suggest a variety of bacteria show a predisposition for colonisation of the human mucosa (Table 1). Included among these is Akkremansia muciniphila,46 which is known to express mucin degrading enzymes. Their presence as part of the healthy mucosa microbiome likely reflects the dualisms that exist between host and microbes, and may have protective or anti-inflammatory roles in health. Murine and rodent models have indicated that the mucosa harbors increased abundances of Lachnospiraceae genera,47 along with Bifidobacterium longum and bifidum species.48 In vitro models have also indicated that Roseburia intestinalis and Eubacterium rectale have a predisposition for colonisation of the mucus layer. It is hypothesized that these bacteria may play a critical role in MGL homeostasis and host epithelium health thorough production of butyrate which serves as an energy source for host colonocytes.49
Table 1. Description of a selection of mucosa associated microbes, their mucin binding proteins, mucin degrading enzymes and their association with health.
| Bacteria | Mucin binding protein | Mucolytic enzyme | Health/Disease association | Reference |
|---|---|---|---|---|
| Akkremansia muciniphila | Unknown | Glycosidase | Deceased in UC | 54, 93 |
| Rumminococcus gnavus | Unknown | α-galactosidases | Increase in active UC | 54, 94 |
|
Rumminococcus torques |
Unknown | α-N-acetylgalactosaminidase | Increase in active UC | 54, 95 |
| Desulfovibrio desulfuricans | Unknown | Sulfatase | Increase in active UC | 31, 32 |
| Bacteroides thetaiotaomicron | Unknown | Sulfatase, neuraminidase, α-fucosidase, β-galactosidase α- N-acetylgalactosaminidase β-N-acetylglucosaminidase |
Decreased mucin sulfation in animal models, | 86, 96 |
| Bacteroides fragilis | Unknown | Neuraminidase, sulfatase, protease, α- N-acetylgalactosaminidase, β-galactosidase, β -N-acetylglucosaminidase, α-fucosidases |
Increase in active UC | 97–99 |
| Bacteroides vulgatus | Unknown | Neuraminidase, α and β-galactosidases, α-fucosidase β-N-acetylglucosaminidase, α and β -N-acetylgalactosaminidase |
Increased in UC | 100 |
| Lactobacillus reuteri | MUC/MucBP | Unknown | Probiotic | 59, 60 |
| Lactobacillus plantarum | Msa | Unknown | Probiotic | 61 |
| Lactobacillus rhamnosus | Spac | Unknown | Probiotic | 62 |
| Lactobacillus johnsoni | GroEL | Unknown | Probiotic | 63 |
| Bifidobacterium breve | Type IV pillus | Unknown | Probiotic | 64 |
| Bifidobacterium longum | Glycoprotein-binding fimbriae protein | Unknown | Probiotic | 65 |
Alterations in Ulcerative Colitis
The MGL of patients with UC is thinner than in health, with alterations to intracellular mucin content, mucin glycosylation, and a reduction in sulfation17,50 coincident with an increase in mucosa associated bacteria and a dysbiosis of the constituent microbes.26,51-53
Studies have revealed an increase in some but not all mucus degrading bacteria (MDB),54 along with decreased levels of butyrate producing bacteria55 (Table 1). Rumminococcus gnavus and torques, both of which possess mucolytic properties, have been shown to be increased in the mucosa of patients with UC. A. muciniphila was found to be decreased in the same cohort of patients, suggesting that A. muciniphila was a constituent of the homeostatic MGL environment. The increased presence of R. gnavus and torques may offer an explanation for the reported increase total mucosa-associated bacteria in UC.54 Additionally the same study showed that these MDB are capable of providing substrates for other non-MDB through degradation of MUC2, providing evidence of the possible syntrophic relationships occurring between colonic bacteria within the MGL.54
MGL-Microbe Interactions
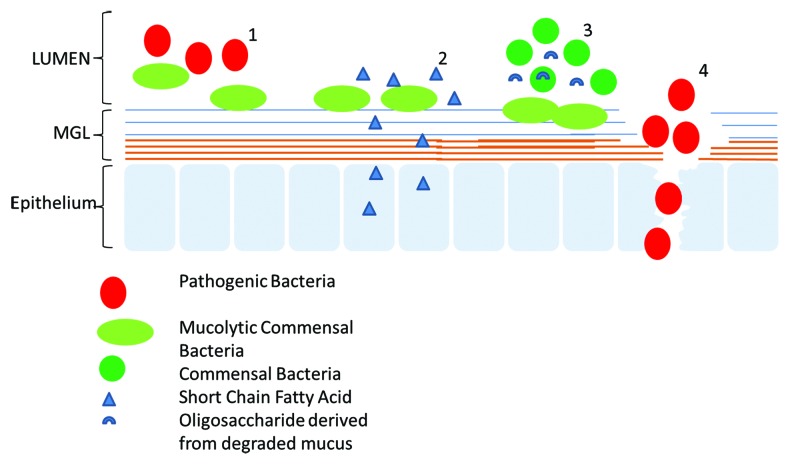
The MGL provides the first line of defense between bacteria in the lumen and the host cells, and, as such, it also acts as the primary site of host-microbe interactions through mucin binding.56 The diverse range of carbohydrate groups that form the peripheral structure of mucins offers a multitude of binding sites for bacteria (both commensal and pathogenic). Bacteria, which are capable of such binding, are likely to gain an advantage over the luminal bacteria through access to additional nutrient sources provided by the MGL itself.57 Indeed in health the MGL provides a dual role, serving to protect the mucosa from potential pathogens while providing a state of mutualism between the host cells and the MGL residential bacteria. In addition to a nutrient source, the MGL provides an initial binding site for selected bacteria upon which they can colonize. Through the provision of such binding sites for commensal bacteria, the MGL can circumvent the interaction of pathogenic bacteria with the host cells and prevent further translocation of pathogens into the mucosa. Additionally the short chain fatty acids produced by fermentation of these commensal bacteria can be utilized as an energy source by the host cells58 (Fig. 2).
Figure 2. Schematic representation of the different association between the MGL and gut microbes. Mucins can act as a barrier to both pathogenic and commensal bacteria. Some commensal bacteria are capable of binding to the MGL and in do so act as antagonists to the binding of pathogen (1). The MGL can provide a source of nutrients for some commensals through mucin degradation; these MDB in turn generate SCFA which serve as an energy source for the host epithelium (2). The MGL can provide a source of nutrients for MDB, which in turn generate nutrients for other commensal bacteria, thereby offering them an ecological advantage (3). Pathogenic bacteria bind to and degrade the MGL, thereby allowing the pathogens access to the host mucosa where they can exert a negative effect on the host cells.
The mechanisms by which commensal bacteria bind to the MGL have been studied in members of the Lactobacillus and Bifidobacterium genera, both of which are common constituents of probiotic formulas. Within the Lactobacillus genera several binding factors have been identified including the mucus-binding protein (MUP) and its homolog MucBP,59,60 the mannose-binding protein Msa,61 a cell wall bound pilli protein SpaC,62 and the cell surface protein GroEL.63 Within the Bifidobacterium genera type IV pilus-type proteins,64 glycoprotein-binding fimbriae protein65 have been identified as potential mucus binding proteins. It should be noted that these proteins have been identified through sequence predictions, and these attributes remain to be validated. An example of the beneficial effects offered by binding of these bacteria is displayed by Lactobacillus johnsonni and its GroEL surface protein. The binding of L. johnsonni to mucins results in aggregation of Helicobacter pylori, decreased H. pylori load, and facilitation of the clearance of the pathogen, thereby suggesting that the binding of such bacteria to mucins may play a role in gastrointestinal homeostasis.63
It is likely that other gut bacteria will present with additional mucus binding proteins, as suggested by Huang et al. in the case of Bacteroides fragilis.66 However, information pertaining to the nature of these binding proteins is absent. Identification of these biologically important motifs will allow recognition bacterial genera and species of biological importance for both health and diseased states.
Mucin Degradation
Certain microbes are known to have the potential to degrade the colonic MGL.67-69 The oligosaccharide side chains of mucins account for 70–80% of the mucin structure and have the potential to act as a significant nutrient source for bacteria that are capable of cleaving these linkages.13 In order to utilize these oligosaccharides as nutrient sources, the bacteria are required to perform extensive degradation of the oligosaccharides to their constituent monosaccharaides. This occurs through enzymatic cleavage with linkage-specific glycosidases.68,70,71 The composition of the mucin sugars in particular glycosylation and terminal linkages are highly variable.15,72 Mucin degradation involves proteolytic cleavage of the non-glycosylated regions from the peptide backbone, degradation of the sugar side chains though a range of glycosidase enzymes tailored to the oligosaccharide constituents, and sialidase and sulfatase enzymes that are capable of degrading the acidic groups on the oligosaccharide chains. Approximately 1% of enteric bacterial species possess the ability to produce the requisite extracellular enzymes,73 and specific enzymes are found in bacteria spanning several genera (Table 1). However, complete degradation of mucin requires the action of a consortium of bacteria as few intestinal bacteria produce all of the requisite glycosidase, sulfatase, and sialidase enzymes.74
Mucin degradation is often thought of as the primary step in bacterial pathogenesis as it disturbs the physical barrier between the lumen and host mucosa. However, this may only apply to excessive degradation as mucin turnover is a normal part of the colonic ecosystem. MGL homeostasis, with mucin degradation through both bacterial enzymatic processes and peristalsis, occurs shortly after birth.55,75 However in chronic inflammation such as that associated with UC, there is an increased presence of the MDB R. gnavus and torques,26,54 offering a possible explanation for increased mucosa associated bacteria found in IBD.
Following binding to, colonization of, and degradation of the MGL, invading microbes have been shown experimentally to have the potential to continue to influence the MGL and exert an effect on the host cells. In a macaque model of the gastric mucosa, invading H. pylori have been shown to signal the host cells to both downregulate expression of mucin genes and to alter the glycosylation profile of the mucins which are expressed.76 While in a Drosophila model a transcriptomic analysis of the host intestinal genes suggested that the mucus barrier was remodeled following infection with Erwinia carotovora.77
Commensal and Pathogen Utilization of Mucin
The ability of enteric bacteria (both commensal and pathogenic) to utilize mucin oligosaccharides as energy sources is now beginning to be elucidated. While only a small proportion of the enteric commensals are predicted to poses the requisite glycosidase enzymes necessary for such processes,73 those which do, appear to be adept to this purpose. Bacteroides thetaiotaomicron and Bifidobacterium bifidum (both common gut commensals) have been extensively studied for their mucin degrading capacity. Transcriptomic analysis has shown both to poses an array of glycosidase genes as well as several carbohydrate transporters which may aid in the import of degraded mucin oligosaccaharides.78,79 B. thetaiotaomicron has also been shown to adaptively direct its glycan foraging to mucin polysaccharides when polysaccharide availability from the diet is reduced thereby contributing to the homeostasis of the gut microbiome.78
In contrast to this it appears that enteric pathogens may be poorly adapted to utilization of mucin and rely on commensal mucin oligosaccharide liberation of metabolites. Both Clostridium difficile and Salomonella typhimurium possess the ability to catabolize mucin sialic acid despite the absence of sialidase enzymes in their genome, suggesting an ability to exploit the sialidase activity of other commensal bacteria.80 Similarly Campylobacter jejuni and both pathogenic and non-pathogenic strains of Escherichia coli have been shown to exploit mucin oligosaccharides liberated by other commensal microbes.81,82 It has been suggested that the absence of mucin degrading capacity of these microbes is due to their transient pathogenic nature.83 Their natural antagonism of the host results in either eradication by the host immune system or a reduction of the host fitness thereby limiting their ability to adapt to changes in the host glycan and/or mucin landscape. Adaption to these cross-feeding events appears to be an important strategy in the survival of enteric pathogens.83
Mucin Degrading Bacteria: Syntrophic and Antagonistic
The microbial diversity that exists within the colonic microbiome which functions as a “microbial organ,” contribute to a multitude of processes and functions.84 Its composition is a result of co-evolution between the microbial communities and the host. It is highly likely that a similar co-evolution has occurred between the mucosa associated bacteria and is key to maintaining mucosal homeostasis. Given the selective pressures under which these microbes are placed, development of syntrophic and antagonistic relationships is not surprising. One such syntrophic relationship exists between the B. thetaiotaomicron and Faecalibacterium prausnitzii. B. thetaiotaomicron possesses a repertoire of genes encoding mucin degrading proteins85,86 and its presence has been shown to result in a decrease in sulfated mucins in rodents.87 However, a co-culture model of both B. thetaiotaomicron and F. prausnitzii results in an increase in the presence of sulfated mucins and maintenance of mucosal homeostasis. These bacteria may be metabolically complementary, with F. prausnitzii metabolizing acetate produced by B. thetaiotaomicron that in turn produces butyrate. This butyrate is then utilized by host cells and stimulates synthesis of mucin serving to maintain mucosal homeostasis.87 F prausnitzii counts are decreased in patients with UC,88 however no information is currently available regarding the co-colonization rates of these bacteria in a UC cohort.
A second aspect of syntrophism is that which exists between MDB and sulfate reducing bacteria (SRB). This relationship has been characterized in an in vitro model, in which a three-stage continuous culture model of the colon was infused with pig gastric mucin. The SRB alone were found to be incapable of directly metabolizing mucin, however when co-cultured with other faecal bacteria an increase in SRB numbers was observed.89 Similarly B. fragilis has been found to release sulfate groups from sulfated mucin in order to utilize the desulfated mucins as an energy source. The released sulfate may then be utilized by D. desulfuricans indicating that SRB may be dependent on other intestinal bacteria to cleave and release sulfate, necessary as a metabolite.74 Within the setting of UC an increased presence of SRB has been identified by several groups,31,32,90 with an association between D. desulfuricans and active inflammation being demonstrated by Rowen et al.32 SRB have also been found to be exclusive to ileal pouches fashioned for UC,91,92 with their presence corresponding to the expression of sulfated mucins.92 Further studies focusing on the co-colonization rates of MDB and SRB during both active and quiescent disease are necessary in order to fully elucidate the role SRB may play in the pathogenesis of UC.
Future Directions of Investigation
Much information relating to host microbe interaction within the MGL remains to be established. To date most studies have focused on single bacterial species within a colonic mucin model or establishing the presence of one or a limited number of mucosa associated bacteria in clinical samples. While helpful in establishing the ability of a given organism to metabolize mucin, such studies do not reflect the complex interactions needed for mucin degradation in the colon. Co-culture experiments with a consortium of bacteria with synergistic profiles will be necessary. Use of metagenomic sequencing to study the microbiome of specific niches will allow co-colonization patterns to be identified and provide the essential background data to allow clinically appropriate studies to be performed. Additionally, metatrascriptomic sequencing will provide information relating to the expression mucolytic enzymes within MDB.
Summary
The MGL is the first line of defense for the mucosa, acting as a physical barrier between microbes and the host epithelium. In health it is in a state of equilibrium allowing resident microbes to colonize and utilize metabolites, while in return obtaining nutrients that were otherwise inaccessible to the host. The diverse nature of the mucin composition has yielded a niche environment for microbes which have co-evolved with the host to survive. The presence of MDB are tolerated by the host, however in the colitic colon this symbiotic relationship is unbalanced. The increased presence of MDB and their symbiotes results in a degradation of the MGL and a reduction in its protective capacities.
Future metagenomic sequencing studies will allow elucidation of the dysbiotic mucosa associated microbiome in UC, particularly when micro-dissection of the MGL is applied prior to sequencing. Moreover to fully address the relationship between host and microbes in health and disease their precise interactions need to be identified. Characterization of mucins and their glycan profiles is needed along with the mucin binding preferences of MDB during periods of both active and quiescent UC.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This manuscript was prepared using funding from Science Foundation Ireland, Grant number 09/IN.1/B2606.
Glossary
Abbreviations:
- MGL
mucus gel layer
- UC
ulcerative colitis
- ER
endoplasmic reticulum
- FISH
fluorescent in situ hybridization
- MDB
mucus degrading bacteria
- MUP
mucus-binding protein
- SRB
sulfate reducing bacteria
References
- 1.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 3.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 4.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 5.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–6. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 8.Beeken W, Northwood I, Beliveau C, Gump D. Phagocytes in cell suspensions of human colon mucosa. Gut. 1987;28:976–80. doi: 10.1136/gut.28.8.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–11. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Campieri M, Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut. 2001;48:132–5. doi: 10.1136/gut.48.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupaul-Chicoine J, Dagenais M, Saleh M. Crosstalk between the intestinal microbiota and the innate immune system in intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2227–37. doi: 10.1097/MIB.0b013e31828dcac7. [DOI] [PubMed] [Google Scholar]
- 13.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–41S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 14.Bansil R, Turner BS. Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci. 2006;11:164–70. doi: 10.1016/j.cocis.2005.11.001. [DOI] [Google Scholar]
- 15.Brockhausen I, Schutzbach J, Kuhns W. Glycoproteins and their relationship to human disease. Acta Anat (Basel) 1998;161:36–78. doi: 10.1159/000046450. [DOI] [PubMed] [Google Scholar]
- 16.Thomsson KA, Holmén-Larsson JM, Angström J, Johansson ME, Xia L, Hansson GC. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology. 2012;22:1128–39. doi: 10.1093/glycob/cws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One. 2011;6:e24447. doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennon G, Balfe A, Bambury N, Lavelle A, Maguire A, Docherty NG, Coffey JC, Winter D, Sheahan K, O Connell PR. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis. 2013 doi: 10.1111/codi.12503. [DOI] [PubMed] [Google Scholar]
- 19.Kaftan SM, Wright NA. Studies on the mechanisms of mucous cell depletion in experimental colitis. J Pathol. 1989;159:75–85. doi: 10.1002/path.1711590115. [DOI] [PubMed] [Google Scholar]
- 20.McCormick DA, Horton LW, Mee AS. Mucin depletion in inflammatory bowel disease. J Clin Pathol. 1990;43:143–6. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–9. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodossi A, Spiegelhalter DJ, Jass J, Firth J, Dixon M, Leader M, Levison DA, Lindley R, Filipe I, Price A, et al. Observer variation and discriminatory value of biopsy features in inflammatory bowel disease. Gut. 1994;35:961–8. doi: 10.1136/gut.35.7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teague RH, Fraser D, Clamp JR. Changes in monosaccharide content of mucous glycoproteins in ulcerative colitis. Br Med J. 1973;2:645–6. doi: 10.1136/bmj.2.5867.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clamp JR, Fraser G, Read AE. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981;61:229–34. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- 25.Morita H, Kettlewell MG, Jewell DP, Kent PW. Glycosylation and sulfation of colonic mucus glycoproteins in patients with ulcerative colitis and in healthy subjects. Gut. 1993;34:926–32. doi: 10.1136/gut.34.7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–U105, e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 27.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–29. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda M, Marth JD. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29:3770–82. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–66. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuw Amerongen AV, Bolscher JGM, Bloemena E, Veerman ECI. Sulfomucins in the human body. Biol Chem. 1998;379:1–18. doi: 10.1515/bchm.1998.379.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Gibson GR, Cummings JH, Macfarlane GT. Growth and Activities of Sulfate-Reducing Bacteria in Gut Contents of Healthy-Subjects and Patients with Ulcerative-Colitis. FEMS Microbiol Ecol. 1991;86:103–11. doi: 10.1111/j.1574-6968.1991.tb04799.x. [DOI] [Google Scholar]
- 32.Rowan F, Docherty NG, Murphy M, Murphy B, Calvin Coffey J, O’Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53:1530–6. doi: 10.1007/DCR.0b013e3181f1e620. [DOI] [PubMed] [Google Scholar]
- 33.Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11:1131–40. doi: 10.3748/wjg.v11.i8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 36.Nowacki MR. Cell proliferation in colonic crypts of germ-free and conventional mice--preliminary report. Folia Histochem Cytobiol. 1993;31:77–81. [PubMed] [Google Scholar]
- 37.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–91. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palestrant D, Holzknecht ZE, Collins BH, Parker W, Miller SE, Bollinger RR. Microbial biofilms in the gut: visualization by electron microscopy and by acridine orange staining. Ultrastruct Pathol. 2004;28:23–7. doi: 10.1080/01913120490275196. [DOI] [PubMed] [Google Scholar]
- 40.Swidsinski A, Sydora BC, Doerffel Y, Loening-Baucke V, Vaneechoutte M, Lupicki M, Scholze J, Lochs H, Dieleman LA. Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflamm Bowel Dis. 2007;13:963–70. doi: 10.1002/ibd.20163. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carroll IM, Ringel-Kulka T, Keku TO, Chang Y-H, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7:1065–75. doi: 10.1002/cbdv.200900322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepage P, Seksik P, Sutren M, de la Cochetière M-F, Jian R, Marteau P, Doré J. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis. 2005;11:473–80. doi: 10.1097/01.MIB.0000159662.62651.06. [DOI] [PubMed] [Google Scholar]
- 45.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5:627–38. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Abbeele P, Gérard P, Rabot S, Bruneau A, El Aidy S, Derrien M, Kleerebezem M, Zoetendal EG, Smidt H, Verstraete W, et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ Microbiol. 2011;13:2667–80. doi: 10.1111/j.1462-2920.2011.02533.x. [DOI] [PubMed] [Google Scholar]
- 49.Rowan FE, Docherty NG, Coffey JC, O’Connell PR. Sulfate-reducing bacteria and hydrogen sulfide in the aetiology of ulcerative colitis. Br J Surg. 2009;96:151–8. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- 50.Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int J Clin Pract. 2008;62:762–9. doi: 10.1111/j.1742-1241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 51.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 53.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–8. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 55.Norin KE, Gustafsson BE, Lindblad BS, Midtvedt T. The establishment of some microflora associated biochemical characteristics in feces from children during the first years of life. Acta Paediatr Scand. 1985;74:207–12. doi: 10.1111/j.1651-2227.1985.tb10951.x. [DOI] [PubMed] [Google Scholar]
- 56.Savage DC. Associations and physiological interactions of indigenous microorganisms and gastrointestinal epithelia. Am J Clin Nutr. 1972;25:1372–9. doi: 10.1093/ajcn/25.12.1372. [DOI] [PubMed] [Google Scholar]
- 57.Probert HM, Gibson GR. Bacterial biofilms in the human gastrointestinal tract. Curr Issues Intest Microbiol. 2002;3:23–7. [PubMed] [Google Scholar]
- 58.Gibson GR, Macfarlane GT, Cummings JH. Sulfate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993;34:437–9. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roos S, Jonsson H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology. 2002;148:433–42. doi: 10.1099/00221287-148-2-433. [DOI] [PubMed] [Google Scholar]
- 60.Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology. 2006;152:273–80. doi: 10.1099/mic.0.28415-0. [DOI] [PubMed] [Google Scholar]
- 61.Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, van der Meer R, Smits MA, Kleerebezem M. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J Bacteriol. 2005;187:6128–36. doi: 10.1128/JB.187.17.6128-6136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc Natl Acad Sci U S A. 2009;106:17193–8. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthésy-Theulaz IE. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:425–34. doi: 10.1128/IAI.74.1.425-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O’Brien F, Flynn K, Casey PG, Munoz JAM, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci U S A. 2011;108:11217–22. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen M-C, Desiere F, Bork P, Delley M, et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci U S A. 2002;99:14422–7. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang JY, Lee SM, Mazmanian SK. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe. 2011;17:137–41. doi: 10.1016/j.anaerobe.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoskins LC, Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968;54:210–7. [PubMed] [Google Scholar]
- 68.Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985;75:944–53. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoskins LC, Boulding ET. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981;67:163–72. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoskins LC, Boulding ET, Gerken TA, Harouny VR, Kriaris MS. Mucin Glycoprotein Degradation by Mucin Oligosaccharide-degrading Strains of Human Faecal Bacteria. Characterisation of Saccharide Cleavage Products and their Potential Role in Nutritional Support of Larger Faecal Bacterial Populations. Microb Ecol Health Dis. 1992;5:193–207. doi: 10.3109/08910609209141586. [DOI] [Google Scholar]
- 71.Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–8. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wesley A, Mantle M, Man D, Qureshi R, Forstner G, Forstner J. Neutral and acidic species of human intestinal mucin. Evidence for different core peptides. J Biol Chem. 1985;260:7955–9. [PubMed] [Google Scholar]
- 73.Miller RS, Hoskins LC. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a “most probable number” method. Gastroenterology. 1981;81:759–65. [PubMed] [Google Scholar]
- 74.Willis CL, Cummings JH, Neale G, Gibson GR. In VitroEffects of Mucin Fermentation on the Growth of Human Colonic Sulfate-Reducing Bacteria. Anaerobe. 1996;2:117–22. doi: 10.1006/anae.1996.0015. [DOI] [Google Scholar]
- 75.Midtvedt AC, Carlstedt-Duke B, Midtvedt T. Establishment of a mucin-degrading intestinal microflora during the first two years of human life. J Pediatr Gastroenterol Nutr. 1994;18:321–6. doi: 10.1097/00005176-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Cooke CL, An HJ, Kim J, Canfield DR, Torres J, Lebrilla CB, Solnick JV. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology. 2009;137:1061–71, e1-8. doi: 10.1053/j.gastro.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 79.Turroni F, Milani C, van Sinderen D, Ventura M. Genetic strategies for mucin metabolism in Bifidobacterium bifidum PRL2010: an example of possible human-microbe co-evolution. Gut Microbes. 2011;2:183–9. doi: 10.4161/gmic.2.3.16105. [DOI] [PubMed] [Google Scholar]
- 80.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–9. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stahl M, Friis LM, Nothaft H, Liu X, Li J, Szymanski CM, Stintzi A. L-fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc Natl Acad Sci U S A. 2011;108:7194–9. doi: 10.1073/pnas.1014125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun. 2008;76:1143–52. doi: 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcobal A, Southwick AM, Earle KA, Sonnenburg JL. A refined palate: bacterial consumption of host glycans in the gut. Glycobiology. 2013;23:1038–46. doi: 10.1093/glycob/cwt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bocci V. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med. 1992;35:251–60. doi: 10.1353/pbm.1992.0004. [DOI] [PubMed] [Google Scholar]
- 85.Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, Henrissat B, Coutinho PM, Minx P, Latreille P, et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5:e156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai HH, Hart CA, Rhodes JM. Production of mucin degrading sulfatase and glycosidases by Bacteroides thetaiotaomicron. Lett Appl Microbiol. 1991;13:97–101. doi: 10.1111/j.1472-765X.1991.tb00580.x. [DOI] [Google Scholar]
- 87.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–9. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 89.Gibson GR, Cummings JH, Macfarlane GT. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–5. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Florin T, Neale G, Gibson GR, Christl SU, Cummings JH. Metabolism of dietary sulfate: absorption and excretion in humans. Gut. 1991;32:766–73. doi: 10.1136/gut.32.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duffy M, O’Mahony L, Coffey JC, Collins JK, Shanahan F, Redmond HP, Kirwan WO. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45:384–8. doi: 10.1007/s10350-004-6187-z. [DOI] [PubMed] [Google Scholar]
- 92.Bambury N, Coffey JC, Burke J, Redmond HP, Kirwan WO. Sulfomucin expression in ileal pouches: emerging differences between ulcerative colitis and familial adenomatous polyposis pouches. Dis Colon Rectum. 2008;51:561–7. doi: 10.1007/s10350-008-9200-0. [DOI] [PubMed] [Google Scholar]
- 93.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 94.Cervera-Tison M, Tailford LE, Fuell C, Bruel L, Sulzenbacher G, Henrissat B, Berrin JG, Fons M, Giardina T, Juge N. Functional analysis of family GH36 α-galactosidases from Ruminococcus gnavus E1: insights into the metabolism of a plant oligosaccharide by a human gut symbiont. Appl Environ Microbiol. 2012;78:7720–32. doi: 10.1128/AEM.01350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoskins LC, Boulding ET, Larson G. Purification and characterization of blood group A-degrading isoforms of alpha-N-acetylgalactosaminidase from Ruminococcus torques strain IX-70. J Biol Chem. 1997;272:7932–9. doi: 10.1074/jbc.272.12.7932. [DOI] [PubMed] [Google Scholar]
- 96.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–6. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 97.Macfarlane GT, Gibson GR. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol Lett. 1991;61:289–93. doi: 10.1111/j.1574-6968.1991.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 98.Macfarlane GT, Macfarlane S, Gibson GR. Synthesis and release of proteases byBacteroides fragilis. Curr Microbiol. 1992;24:55–9. doi: 10.1007/BF01570100. [DOI] [Google Scholar]
- 99.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–4. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Onderdonk AB, Cisneros RL, Bronson RT. Enhancement of experimental ulcerative colitis by immunization with Bacteroides vulgatus. Infect Immun. 1983;42:783–8. doi: 10.1128/iai.42.2.783-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]