Abstract
Objective: An increasing number of studies that are using high-throughput molecular methods are rapidly extending our knowledge of gut microbial colonization in preterm infants whose immaturity and requirement for extensive treatment may result in altered colonization process. We aimed to describe the profile of gut microbiota in 50 extremely low birth weight (<1200 g) critically ill infants at three different time points during the first two months of life by using 16S rRNA gene specific sequencing.
Patients and Methods: Stool samples were collected at the age of one week, one month and two months. Bacterial community profiling was done using universal amplification of 16S rRNA gene and 454 pyrosequencing.
Results: The diversity of gut microbiota in preterm neonates in the first week of life was low but increased significantly over two months. The gut microbiota was dominated by facultative anaerobic bacteria (Staphylococcus spp. and Enterobacteriaceae) and lacked colonization with bacteria known to provide resistance against pathogens (Bacteroides, Bifidobacterium, and Lactobacillus) throughout the study. Colonization of Escherichia coli and uncultured Veillionella was positively correlated with maturity. Infants born to mothers with chorioamnionitis had significantly higher bacterial diversity than those without.
Conclusions: High prevalence and abundance of potentially pathogenic Enterobacteriaceae and Staphylococcaceae with low prevalence and abundance of colonization resistance providing taxa bifidobacteria, Bacteroides and lactobacilli may lead to high infection risk via microbial translocation from the gut. Additionally, our data suggest that maternal chorioamnionitis may have an effect on the diversity of infants’ gut microbiota; however, the mechanisms involved remain to be elucidated.
Keywords: gut microbiota, microbiome profiling, preterm neonates, 16S rRNA gene sequencing, extremely low birth weight
Introduction
Normal gut colonization begins straight after birth with maternal vaginal and faecal microbiota being the key source of colonizing microbes.1 In term neonates during the first days of life the most abundant colonizers are staphylococci, γ-proteobacteria (e.g., Enterobacteriaceae), and bifidobacteria,2-4 but the composition changes over time and gradually becomes more adult-like with the dominance of Bacteroidetes and Firmicutes.3
Within the past two decades, the survival rate of preterm infants has greatly increased due to improved standards of neonatal intensive care. For this group of infants, many parameters of normal development of gut bacterial colonization must be redefined as, in addition to immaturity, preterm infants often require intensive care and antibiotic treatment, all having a considerable effect on the development of the gut microbiota.5 Previous studies have shown that the gut microbiota in preterm infants is dominated by members of family Enterobacteriaceae and has decreased numbers of lactobacilli and bifidobacteria.6,8 Also, during the first days of life preterm infants are colonized with only a few microbial species.6-8 Increasing number of studies using 16S rRNA gene specific sequencing are in general confirming but on the other hand expanding the previous knowledge.9-14 We aimed to broaden the understanding of the composition of gut microbiota in preterm infants by describing the profile of gut microbiota in 50 extremely low birth weight (ELBW; birth weight [BW] <1200 g) neonates, all requiring treatment in 3rd level neonatal intensive care unit (NICU) and receiving antibiotic treatment, with the focus of composition dynamics between three time points collected during the first two months of life by using 16S rRNA gene specific sequencing.
Results
Sequencing data
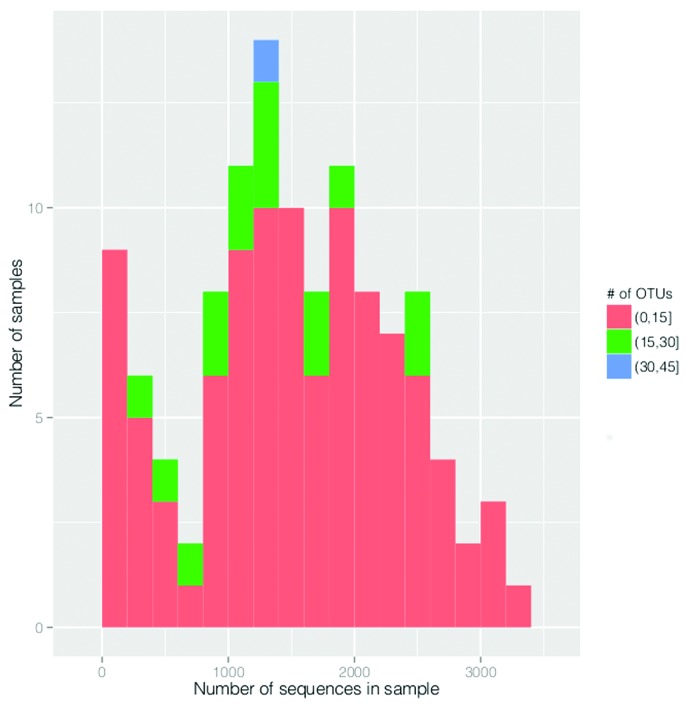
We retrieved 188 222 high-quality sequence reads with an average length of 280 bp. The number of trimmed sequences was higher than 500 in 101 out of 118 samples (30 samples collected at the age of one week, 42 at one month, and 29 at two months). The cut-off was set to 500 sequences per sample because this was the level whereby the rarefaction curves for 95% of the samples reached a 1% plateau. This means that the increasing number of sequences per sample does not increase the number of OTUs obtained in 95% of the samples (Fig. 1). Eighty-five operational taxonomic units (OTUs) met the criteria of having at least 5 sequences assigned to them and thus were included into further analysis (Accession numbers for representative sequences of OTUs in GenBank are KJ527501-KJ527585). The mean (SD) taxonomic richness per sample was 8.15 (4.19). This did not change between the age of one week and one month being 7.13 (5.15) and 7.83 (3.77), respectively, but it increased significantly by the age of two months (9.64 [3.34]; P = 0.04). The mean (SD) Shannon diversity index was 0.67 (0.51) and it increased over time being 0.38 (0.4) at one week, 0.71(0.54) at one month, and 0.92 (0.44) at two months of age (all P < 0.01).
Figure 1. The distribution of 118 samples according to number of sequences and number of OTUs. Round and square brackets in the figure indicate to the exclusion and inclusion of adjacent value, respectively.
Prevalence and mean relative abundance of bacterial phyla, families, and genera
Retrieved sequences were distributed between seven bacterial phyla, 24 families and 35 genera. Major phyla (prevalence > 10% and relative abundance > 0.01) were Proteobacteria (prevalence 94%; mean relative abundance [SD] 0.51 [0.42]), whose abundance increased significantly between the age of one week and one month (from 0.28 [0.44] to 0.66 [0.39]; P < 0.01); Firmicutes (100%; 0.45 [0.41]), whose abundance decreased significantly between the age of one week and one month (from 0.65 [0.45] to 0.32 [0.37]; P < 0.01); and Bacteroidetes (13%; 0.02 [0.14]). Minor phyla were Actinobacteria (13%; 0.002 [0.007]) and Verrucomicrobia, which was present in one sample with relative abundance of 0.14.
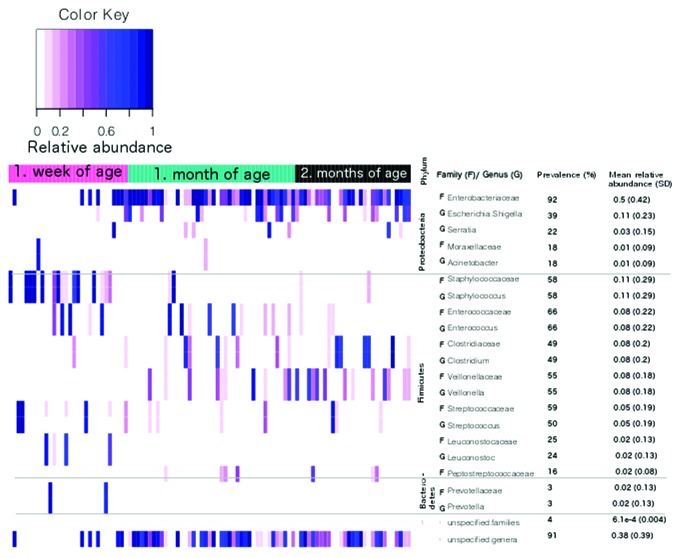
Ten most abundant families and genera are presented in Figure 2. Among these families, the prevalence of Enterobacteriaceae and Enterococcaceae increased significantly between one week and one month of age (from 77% to 98% and from 53% to 81%; P < 0.01 and P = 0.02, respectively). This increase was also observed for mean relative abundance values of Enterobacteriaceae and Veillionellaceae that increased from 0.25 (0.42) to 0.66 (0.38) (P < 0.001) and from 0.003 (0.009) to 0.09 (0.19) (P < 0.01), respectively. At the same time the prevalence of Prevotellaceae and the abundance of Staphylococcaceae decreased significantly from 13% to 0 (P = 0.03) and 0.33 (0.43) to 5.1e-4 (0.15) (P < 0.001), respectively. The only change observed between the age of one month and two months was the decrease in abundance of Enterococcaceae from 0.1 (0.25) to 0.02 (0.03) (P = 0.03). The distribution of 10 most abundant families in individual samples can be seen in Figure S1.
Figure 2. The distribution of 10 most abundant families (f) and genera (g) between the ages of one week, one month, and two months.
The majority of these changes were also observed in approximately the same proportions among the most abundant genera belonging to aforementioned families. These genera included Staphylococcus, Enterococcus, Veillonella, and Prevotella (Fig. 2). In family Enterobacteriaceae, most abundant genus was Escherichia/Shigella, which similarly to family Enterobacteriaceae, increased significantly between the ages of one week and one month with the values of 17% vs. 41% for prevalence and 0.02 (0.1) vs. 0.16 (0.3) for mean (SD) relative abundance (P = 0.04 and P < 0.01, respectively). It has to be taken into consideration, that significant proportion of sequences (prevalence 91% and mean relative abundance [SD] 0.38 [0.39]) was unspecified on genus level (Fig. 2). The majority of these sequences belonged to family Enterobacteriaceae (88%, 0.35 [0.39]), in particular to OTU named Enterobacteriaceae.100.293 (86%, 0.29 [0.38]). Based on additional identification against NCBI nt database this OTU matched to genus Klebsiella.
Prevalence and relative abundance of bacterial OTUs
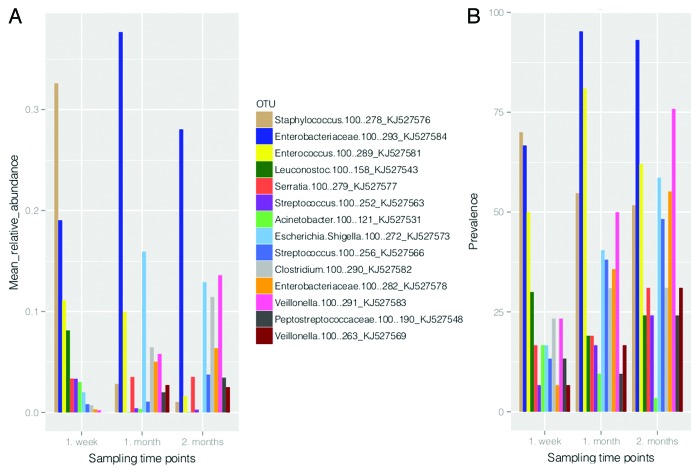
Fourteen OTUs out of 85 dominated the gut microbiota of ELBW infants (prevalence and mean relative abundance higher than 10% and 0.01, respectively). The distribution of these OTUs in studied time points is shown in Figure 3. At the age of one week, three of the most prevalent and abundant OTUs in gut microbiota of ELBW infants were Staphylococcus.100.278 (GenBank accession number KJ527576), Enterobacteriaceae.100.293 (Klebsiella sp.; KJ527584), and Enterococcus.100.289 (KJ527581). By the age of one month, OTUs corresponding to Escherichia/Shigella.100.272 (KJ527573) together with Enterobacteriaceae.100.293 and Enterococcus.100.289 predominated. At the age of two months the most prevalent and abundant OTUs were Enterobacteriaceae.100.293 (Klebsiella sp.), Veillonella.100.291 (KJ527583), Enterococcus.100.289, and Escherichia/Shigella.100.272 (Fig. 3).
Figure 3. The distribution of the most dominant OTUs (prevalence > 10% and mean relative abundance > 0.01) between the ages of one week, one month and two months based on their mean relative abundance (A) and prevalence (B).
None of the OTUs assigned to genus Bifidobacterium crossed the detection limit. Also, only one OTU was assigned to genus Bacteroides and two to Lactobacillus. These three OTUs had prevalence lower than 4%, although relative abundance was high in one or two samples (>0.01, reaching up to 0.6).
Correlation between gut microbiota and clinical parameters
We observed positive correlation between Shannon diversity index and corrected gestational age (GA+ postnatal age; P < 0.001) and negative correlation between Shannon diversity index and the starting day of total enteral feeding (P < 0.01). Also, Shannon diversity index was significantly higher among ELBW infants born to mothers with chorioamnionitis than in infants born to mothers without (mean values [SD] 0.82 [0.59] vs. 0.59 [0.45]; P < 0.01).
The prevalence and relative abundance of dominating OTUs correlated only with corrected GA: relative abundance of Staphylococcus.100.272 decreased (P = 0.02) and the prevalence of Escherichia/Shigella.100.272 and Veillonella.100.291 increased with corrected GA (P < 0.01 and P = 0.03, respectively).
Discussion
Current study is a thorough addition to several recent 16S rRNA gene sequencing based studies analyzing the composition of gut microbiota in preterm infants. We observed significant changes in the gut microbiota of ELBW infants during the first weeks of life when extensive interventions (NICU stay, artificial lung ventilation, total parenteral nutrition [TPN], and empiric and broad-spectrum antibiotic treatment) were applied. By the age of one month, however, the composition of gut microbiota had shifted toward more stable complex with the changes occurring between the age of one and two months being not as extensive as in the first weeks of life regardless of significant increase in diversity. Nevertheless, key colonization resistance providing bacteria (Bifidobacterium, Bacteroides, and Lactobacillus) were lacking during the whole study period. These results are generally confirmatory to previous knowledge on this subject. Interestingly, ELBW infants born to mothers with chorioamnionitis had significantly higher bacterial diversity in their gut. This raises the question if diversity may be affected by more extensive exposure to microbes, inflammatory reactions and antibiotic treatment in case of chorioamnionitis or is the presence of chorioamnionitis correlated with some unknown factor affecting the diversity in these infants. In clinical data available for our study, chorioamnionitis did not correlate with any factor.
Similarly to previous studies assessing the composition of gut microbiota in preterm infants, we observed gradual increase of diversity in ELBW infants throughout the study.7,15 As the gradual increase of diversity has also been reported in vaginally born fullterm infants,16 it seems to be a normal process of diversification of gut microbiota in early age regardless of maturity. Nevertheless, Jacquot et al.17 have shown that bacterial diversity increases more slowly in extremely preterm (GA < 28 wk) than in moderately preterm infants (GA ≥ 28 wk),17 supported by the increase of Shannon diversity index with increasing corrected GA seen in our study. Additionally, many studies have concluded that preterm infants have generally low bacterial diversity,8,10,15 which can be seen by us in ELBW infants as well.
Shannon diversity index was higher among ELBW infants who started to receive enteral feeds earlier. This is most probably due to a shorter period of nutrition deficiency in the gut resulting from parenteral feeding. We also found that Shannon diversity index was significantly higher among ELBW infants born to mothers with chorioamnionitis (frequent causative factor for preterm birth) as opposed to those without, which is contradicting the results of Madan et al.18 Unfortunately, we did not have the additional information about the type or duration of antibiotics used to treat these women. Thus, we can only hypothesize that the β-lactams in amnionic fluid, exposure to inflammation and potential colonizers (chorionamnionitis is a polymicrobial infection; over 65% of positive amniotic fluid cultures involve two or more organisms19) may, among other factors, contribute to more diverse gut microbiota in these infants.
Members of family Enterobacteriaceae from phylum Proteobacteria were the dominating organisms of the gut microbiota of ELBW infants except immediately after birth when the slight predominance of Staphylococcaceae from phylum Firmicutes was observed. Increased levels of Proteobacteria have been reported extensively by studies assessing the composition of gut microbiota of preterm infants14,20-22 and interestingly it has lately been linked to necrotizing enterocolitis.14,22,23
The dominance of Staphylococcaceae in the gut microbiota of newborns has been hypothesized to be the result of improved hygienic condition during delivery.24 High abundance of staphylococci may increase the risk for acquiring coagulase-negative staphylococcal infections, for which preterm neonates are especially predisposed to.25 A correlation between higher abundance of most dominant OTU from genus Staphylococcus and smaller corrected GA of ELBW infants indicate that the risk of acquiring these infections through staphylococcal colonization is at its highest during the first week of life.
A representative from genus Escherichia/Shigella was one of the dominating OTUs, which higher prevalence was correlated with increasing corrected GA. The most widely reported member of genus Escherichia in gut microbiota of preterm infants has historically been Escherichia coli,6,8,17 with the prevalence of this microbe rising during the first month of life.8 If we consider our results confirmatory to this knowledge, we verify that E. coli is a habitant of more mature gut microbita. Nevertheless, it has to be noted that although it is very likely, we cannot confirm with full certainty if this OTU is corresponding to E. coli or not due to the limited capacity of resolving different species based on short lengths of sequences.
Similarly to OTU corresponding to genus Escherichia/Shigella, more mature ELBW infants were more likely to harbor OTU corresponding to genus Veillonella in their gut microbiota. The increase of Veillonella in gut microbiota over time has also been described in healthy infants.2
Although similar overall dynamics of bacterial families has been observed in fullterm infants3 there is a much wider inter-individual variability of the composition of gut microbiota in fullterm than in preterm infants.7 Also, high abundance of bifidobacteria and Bacteroides in fullterm1,2,26 but low abundance or absence in preterm infants has been frequently observed.6,8-10,26,27 Moreover, Butel et al.28 have shown that when the GA at birth is less than 33 wk the gut colonization by bifidobacteria is decreased.28 This may be an explanation why bifidobacteria did not cross the detection limit in our study in which GA for all ELBW infants was less than 32 wk. In addition to lack of bifidobacteria, low prevalence and abundance of Bacteroides and Lactobacillus are all indicators of impaired colonization process. It has been widely hypothesized that gut colonization with bifidobacteria and lactic acid bacteria such as lactobacilli, is supported by breast-feeding although there are increasing evidence that this may not be the case.29 Nevertheless, our results may seem to favor this hypothesis as most of the ELBW infants participating in current study did not receive breast-feeding and the levels of lactic acid bacteria were very low to statistically argue against it, but based on other similar studies it is unlikely that feeding regimen is affecting this colonization in preterm infants. For instance, Butel et al.28 failed to see a statistical correlation between breast-feeding and bifidobacterial colonization in preterm infants.28 Also, studies using 16S rRNA gene based sequencing are supporting these findings by reporting low levels of lactobacilli, bifidobacteria, and Bacteroides despite of studying mainly breast-fed preterm infants.9,10
One of the limitations in our study is a possible primer bias especially toward bifidobacteria as 27F primer has been shown to have low affinity for this group of bacteria.30 Nevertheless, by analyzing the levels of bifidobacteria with quantitative Real-Time PCR and genus Bifidobacterium specific primers, we were able to validate that the prevalence and levels of bifidobacteria are indeed low in ELBW infants. We also ruled out primer bias against genera Bacteroides and Lactobacillus. Thus, in the context of current study the primer bias does not have a considerable effect on the results.
In conclusion, the detailed description of gut microbiota in critically ill ELBW infants during the first two months of life confirmed low prevalence and abundance of bifidobacteria, Bacteroides, and lactobacilli throughout the study period, suggesting prevailing impaired colonization resistance, and showed the shift in dominance from Staphylococcaceae to Enterobacteriaceae during first weeks of life. Our data suggest that maternal chorioamnionitis may have an effect on the diversity of ELBW infants’ gut microbiota; however, the mechanisms involved remain to be elucidated.
Patients and Methods
Participants and sample collection
A total of 118 stool samples collected from 50 preterm infants met the inclusion criteria. Briefly, approximately 200 mg of stool was available from infants with BW <1200 g participating in a cluster-randomized study comparing the efficacy of ampicillin and penicillin, both combined with gentamicin, in risk-factor based empiric treatment of suspected early onset neonatal sepsis. The details of the study are described elsewhere.31 Thus, the patients were all receiving antibiotic treatment. Stool samples were collected in a clean screw-top container at the age of one week (n = 38), one month (n = 45), and two months (n = 35). Samples were initially stored at 4 °C for a maximum of 4 h and then transferred to -80 °C until analyzed.
Additionally, extensive demographic and clinical data were obtained for the participants (Table 1). The study was conducted in the NICUs of Tallinn Children’s Hospital and Tartu University Hospital. Altogether 50 mothers (median age [min;max] was 30.5 [16;44] years) participated in this study. Twelve mothers received antenatal antibiotics and 23 received antibiotics during delivery. Eighteen mothers were suffering from chorioamnionitis and 12 had premature rupture of membranes >18 h (PROM). The diagnosis of chorioamnionitis was confirmed based on clinical criteria: fever and/or high C reactive protein levels, odorous amniotic fluid, and placental inflammation.
Table 1. Demographic and clinical factors for participating ELBW infants (n = 50).
| Factor | Value |
|---|---|
| Duration of NICU days; median (IQR) | 25.7 (12.5–41.8) |
| Number of patients/ Duration of artificial lung ventilation (days); median (IQR) | 48/ 5.8 (3.4–17.5) |
| Number of patients in hospital ward A | 28 |
| Number of patients born with caesarean section | 22 |
| GA (weeks); mean (SD) | 26.64 (0.32) |
| BW (g) mean (SD) | 886.64 (28.27) |
| Number of male patients | 26 |
| Number of patients receiving empiric antibiotic treatment with penicillin | 25 |
| Duration of empiric antibiotic treatment in days; median (IQR) | 3 (0.5–14.5) |
| Number of patients receiving broad-spectrum antibiotic treatment: | 37 |
| β-lactam + betalactamase inhibitors | 23 |
| III ja IV generation cephalosporines | 10 |
| carbapenems | 6 |
| Starting day of broad-spectrum antibiotic treatment; median (IQR) | 7 (0–25) |
| Duration of broad-spectrum antibiotic treatment in days; median (IQR) | 11.5 (0–38.8) |
| Number of patients on following feeding regimen at day 7*: | |
| TPN | 12 |
| Breast milk containing regimen | 17 |
| Formula | 21 |
| Mean % (range) of breast milk in breast milk containing regimen | 81.4 (25–100) |
| Starting day of enteral feeding regimen; median (IQR) | 3 (1–7) |
| Starting day of total enteral feeding; median (IQR) | 17 (14–22) |
| Number of participants suffering from sepsis: | 24 |
| Early onset sepsis | 2 |
| Late onset sepsis | 22 |
| Number of NEC II, III patients | 7 |
| Number of patients who died in hospital | 2 |
Feeding regimen of ELBW infants was documented on Day 7 and categorized into three groups based on the route and character of the feeds as follows: (1) TPN, only parenteral feeding or including enteral feeds providing calories less than 10% of daily total; (2) breast milk containing regimen, breast milk constituting at least 11% of enteral feeds (the actual proportion of breast milk ranged from 12 to 100%); and (3) formula feeding, formula constituting more than 89% of enteral feeds. In case of breast milk containing regimen only biological mother’s fresh or frozen breast milk was used; pasteurization or donor milk were not available. For formula feeding a ready-made liquid preterm formula Nenatal by Nutricia providing 82 Kcal/100 ml was used. Fortification was started only when enteral volume of 100 ml/kg was reached. TPN was started with glucose (4–6 g/kg/d) and amino acids (1 g/kg/d) within the first hours of life and increased by 2 g/kg/d for glucose and 1 g/kg/d for aminoacids as tolerated. Lipids (0.5–1 g/kg/d) were started on the second day of life and advanced by 0.5–1 g/kg/d as tolerated.
Of note, two outbreaks of bloodstream infections were observed during the study. In hospital ward B an outbreak of methicillin-resistant Staphylococcus aureus infection involving three patients and in hospital ward A five patients had a blood stream infection caused by K. pneumoniae.4
454 pyrosequencing of 16S rRNA gene
DNA was extracted from stool samples using QiaAmp Stool DNA Mini kit (Qiagen) according to manufacturer’s instructions and stored at -20 °C. The amplification of 16S rRNA V1-V4 hypervariable region was performed with primers that included 454 specific adaptor sequences at 5′ end following the 8-bp barcode marked as Ns (unique sequence tag to barcode each sample) and universal 27F and 685R primers.32,33 Full primer sequences were as follows: 27F with B adaptor 5′CCTATCCCCT GTGTGCCTTG GCAGTCTCAG NNNNNNNNAG AGTTTGATCC TGGCTCAG3′ and 685R with A adaptor 5′CCATCTCATC CCTGCGTGTC TCCGACTCAG NNNNNNNNTC TACGCATTTC ACCGCTAC3′.
Cycling parameters were 3 min at 95 °C, followed by 5 cycles of 30 s at 95 °C, 30 s at 47 °C and 60 s at 72 °C, then 30 cycles of 30 s at 95 °C, 30 s at 71 °C and 60 s at 72 °C with a final extension at 72 °C for 10 min. PCR reactions were performed in total volume of 25 μL including 3 μL of DNA template and primers at concentration 0.2 μM. PCR products were purified using Agencourt AMPure XP (Beckman Coulter) and sequenced with 454 FLX+ systems at GATC Biotech AG.
Additional PCR and qPCR were performed for genera Lactobacillus, Bacteroides, and Bifidobacterium to analyze primer 27F bias toward these genera in current study (Fig. S2).
Data analysis
The initial pre-trimmed data set was denoised using PyroNoise and UChime (“chimera.uchime” task in de novo mode) implemented in MOTHUR software 1.27.0. Only sequences longer than 150 bp were included for further processing. OTUs were generated with the average neighbor hierarchical clustering algorithm with identity threshold of 97%. For additional denoising, OTUs with less than 5 sequences were removed. Reference sequences of aligned 16S rDNA were obtained from the SILVA rRNA database34 against what the taxonomic assignments were performed using Naive Bayesian classifier with a confidence cutoff of 90%.35 OTU naming was based on the lowest taxonomic level identified for OTUs based on SILVA database match followed by the value of this match (100 equals 100% match) and serial number in the OTU list generated for this study (the list was generated in random order). Additional taxonomic assignment for identifying species to which OTUs potentially corresponded to, was performed using BLASTN against the NCBI nt database (last accessed in February, 2014). The relative abundance values and sequence counts were all normalized.
Statistics
Statistical analysis was performed using R 2.13.2 software. For analyzing general bacterial diversity Shannon diversity index was calculated.36 Categorical values were compared with Fisher exact test and the continuous variables by Welch Two sample T-test. Mixed effect models were used to analyze the correlation of clinical parameters with microbial characteristics (Shannon diversity index; prevalence and relative abundance of five most dominating OTUs). These models included the following fixed effects: corrected GA (GA+ postnatal age in weeks); PROM; mode of delivery; duration of artificial lung ventilation; duration of empiric antibiotic treatment; the starting day and duration of broad spectrum antibiotic treatment (carbapenems, third or fourth generation cephalosporins, and β-lactamase resistant penicillins); feeding regimen; presence of late onset sepsis; maternal usage of antibiotics prior to delivery and during delivery; and presence of maternal chorioamnionitis. Feeding regimen included three separate variables: the volume of received breast milk documented on Day 7; the starting day of enteral feeding, which consisted of breast milk containing regimen (at least 11% of breast milk in enteral feeds) and formula feeding (more than 89% of formula in enteral feeds); and the starting day of total enteral feeding (marks the end of TPN). The random effects in mixed effect models were hospital ward (A and B) and the type of empiric antibiotic treatment used (penicillin or ampicillin plus gentamicin).
All analyses were performed with Holm-Bonferroni correction and the overall level of a significant difference was set at 5%.
Ethics
The study was approved by the Ethics Committee of University of Tartu and informed consent was signed by parents or guardians.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This study was supported by grants of Estonian Ministry of Education and Research (target financing No SF0180132s08) and the Estonian Science Foundation (grant No 6984 and grant No 9059).
Glossary
Abbreviations:
- ELBW
Extremely low birth weight
- BW
birth weight
- NICU
Neonatal intensive care unit
- OTU
Operational taxonomic unit
- GA
Gestational age
- LOS
Late onset sepsis
- NEC
Necrotizing enterocolitis
- qPCR
Quantitative Real-Time PCR
- PROM
Premature rupture of membranes
- TPN
Total parenteral nutrition
References
- 1.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggesbø M, Moen B, Peddada S, Baird D, Rugtveit J, Midtvedt T, Bushel PR, Sekelja M, Rudi K. Development of gut microbiota in infants not exposed to medical interventions. APMIS. 2011;119:17–35. doi: 10.1111/j.1600-0463.2010.02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parm Ü, Metsvaht T, Sepp E, Ilmoja M-L, Pisarev H, Pauskar M, Lutsar I. Mucosal surveillance cultures in predicting Gram-negative late-onset sepsis in neonatal intensive care units. J Hosp Infect. 2011;78:327–32. doi: 10.1016/j.jhin.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Mshvildadze M, Neu J, Mai V. Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutr Rev. 2008;66:658–63. doi: 10.1111/j.1753-4887.2008.00119.x. [DOI] [PubMed] [Google Scholar]
- 6.Magne F, Abély M, Boyer F, Morville P, Pochart P, Suau A. Low species diversity and high interindividual variability in faeces of preterm infants as revealed by sequences of 16S rRNA genes and PCR-temporal temperature gradient gel electrophoresis profiles. FEMS Microbiol Ecol. 2006;57:128–38. doi: 10.1111/j.1574-6941.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwiertz A, Gruhl B, Löbnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res. 2003;54:393–9. doi: 10.1203/01.PDR.0000078274.74607.7A. [DOI] [PubMed] [Google Scholar]
- 8.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–73. doi: 10.1136/fn.80.3.F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156:20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaTuga MS, Ellis JC, Cotton CM, Goldberg RN, Wynn JL, Jackson RB, Seed PC. Beyond bacteria: a study of the enteric microbial consortium in extremely low birth weight infants. PLoS One. 2011;6:e27858. doi: 10.1371/journal.pone.0027858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, Shuster J, Sharma R, Hudak ML, Neu J. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8:e52876. doi: 10.1371/journal.pone.0052876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart CJ, Marrs EC, Nelson A, Lanyon C, Perry JD, Embleton ND, Cummings SP, Berrington JE. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS One. 2013;8:e73465. doi: 10.1371/journal.pone.0073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torrazza RM, Ukhanova M, Wang X, Sharma R, Hudak ML, Neu J, Mai V. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS One. 2013;8:e83304. doi: 10.1371/journal.pone.0083304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claud EC, Keegan KP, Brulc JM, Lu L, Bartels D, Glass E, Chang EB, Meyer F, Antonopoulos DA. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1:20. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart CJ, Marrs ECL, Magorrian S, Nelson A, Lanyon C, Perry JD, Embleton ND, Cummings SP, Berrington JE. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012;101:1121–7. doi: 10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 16.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquot A, Neveu D, Aujoulat F, Mercier G, Marchandin H, Jumas-Bilak E, Picaud J-C. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158:390–6. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, Sogin ML, Foster JA, Edwards WH, Palumbo P, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F456–62. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tita ATN, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett E, Kerr C, Murphy K, O’Sullivan O, Ryan CA, Dempsey EM, Murphy BP, O’Toole PW, Cotter PD, Fitzgerald GF, et al. The individual-specific and diverse nature of the preterm infant microbiota. Arch Dis Child Fetal Neonatal Ed. 2013;98:F334–40. doi: 10.1136/archdischild-2012-303035. [DOI] [PubMed] [Google Scholar]
- 21.Chang JY, Shin SM, Chun J, Lee J-H, Seo J-K. Pyrosequencing-based molecular monitoring of the intestinal bacterial colonization in preterm infants. J Pediatr Gastroenterol Nutr. 2011;53:512–9. doi: 10.1097/MPG.0b013e318227e518. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegård I-L, Wold AE. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006;59:96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/S0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 26.Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de Los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol. 2012;79:763–72. doi: 10.1111/j.1574-6941.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, Altaye M, Wagner M, Gevers D, Ward DV, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1:13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butel M-J, Suau A, Campeotto F, Magne F, Aires J, Ferraris L, Kalach N, Leroux B, Dupont C. Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J Pediatr Gastroenterol Nutr. 2007;44:577–82. doi: 10.1097/MPG.0b013e3180406b20. [DOI] [PubMed] [Google Scholar]
- 29.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 30.Sim K, Cox MJ, Wopereis H, Martin R, Knol J, Li M-S, Cookson WO, Moffatt MF, Kroll JS. Improved detection of bifidobacteria with optimised 16S rRNA-gene based pyrosequencing. PLoS One. 2012;7:e32543. doi: 10.1371/journal.pone.0032543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metsvaht T, Ilmoja M-L, Parm U, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs. penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatr. 2010;99:665–72. doi: 10.1111/j.1651-2227.2010.01687.x. [DOI] [PubMed] [Google Scholar]
- 32.Keinänen-Toivola MM, Revetta RP, Santo Domingo JW. Identification of active bacterial communities in a model drinking water biofilm system using 16S rRNA-based clone libraries. FEMS Microbiol Lett. 2006;257:182–8. doi: 10.1111/j.1574-6968.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- 33.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5:235–7. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–96. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magurran AE. Chapter four: An index of diversity... In: Measuring biological diversity. Malden, MA: Blackwell Publishing; 2004: 100-130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.