Abstract
The neonatal gut is rapidly colonized by a newly dominant group of commensal Escherichia coli strains among which a large proportion produces a genotoxin called colibactin. In order to analyze the short- and long-term effects resulting from such evolution, we developed a rat model mimicking the natural transmission of E. coli from mothers to neonates. Genotoxic and non-genotoxic E. coli strains were equally transmitted to the offspring and stably colonized the gut across generations. DNA damage was only detected in neonates colonized with genotoxic E. coli strains. Signs of genotoxic stress such as anaphase bridges, higher occurrence of crypt fission and accelerated renewal of the mature epithelium were detected at adulthood. In addition, we observed alterations of secretory cell populations and gut epithelial barrier. Our findings illustrate how critical is the genotype of E. coli strains acquired at birth for gut homeostasis at adulthood.
Keywords: Escherichia coli, colibactin, genotoxicity, neonate, gut, epithelial proliferation, epithelial differentiation, intestinal barrier
Introduction
Upon birth, the infant gut undergoes a massive microbial colonization which has been suggested to exert marked effects on basic host physiology, immunity and metabolism later in life (for a review see refs. 1 and 2). This initial extra-uterine microbial load originates from the mother and birth environment, and then dynamically evolves toward a complex consortium throughout host development and environmental exposures.3-9 Although diverse among individuals, the composition of the infant fecal microbiota reaches a state of relative equilibrium in humans by the end of the first year of life10 and adopts an adult-like configuration within the 3-y period following birth.9 The host provides a niche in which bacteria ensure their transmission and retention within the gut. In turn, the microbiota, especially in the neonate, contributes to essential host functions such as nutrient supply, energy balance,11-13 metabolic signaling,14 resistance to pathogens colonization,15-17 and has a key role in promoting the postnatal maturation of the intestinal mucosal barrier.18-23
The commensal bacteria Escherichia coli are one of the first colonizer of the mammalian gut at birth.10,24,25 The mother represents the most influential environmental factor for the transmission of E. coli regardless of delivery mode.26 E. coli dominates the gut microbiota within a few days after birth and contributes to the generation of an anaerobic environment favorable for the further establishment of Bacteroides, Bifidobacterium, and Clostridium species. E. coli, among other Enterobacteriaceae, becomes the predominant facultative anaerobic bacteria within the mature gut microbiota in which it establishes a stable lifelong interaction with the host.10,27,28 Although E. coli exhibits a very high genomic plasticity, its core genome representing less that 40% of the genes present in each E. coli strains, its genetic structure is predominantly clonal.29 Phylogenetic analyses of E. coli populations revealed at least five main phylogenetic groups: A, B1, B2, D, and E.30 Over the past 30 y, epidemiologic studies have established a shift from the phylogenetic group A to the phylogenetic group B2 strains in the fecal microbiota of populations living in industrialized countries,28,29,31,32 including infants.33-35 Enriched dietary habits or increased level of hygiene are presumably the main factors accounting for this major modification in the phylogenetic group distribution of fecal E. coli population. Moreover, up to 30% of commensal B2 strains have acquired a genomic island, called pks, by horizontal transfer.36 Fecal carriage of commensal B2 pks+ E. coli strains in infants has been estimated in Western countries and revealed that up to 18% of Swedish infants are colonized three days after birth, in a study performed 10 y ago.37 Carriage of the pks island was also associated with long-term persistence of such neonatal acquired commensal B2 strains.37
The pks genomic island encodes for a nonribosomal peptide synthetase (NRPS) and polyketide synthetase (PKS) machinery that allows the synthesis of a peptide-polyketide hybrid genotoxin, named colibactin.36 A short and direct contact of cultured human cells or mouse enterocytes, in colon loop experiments, with pks+ E. coli strains induced transient DNA double strand breaks, characterized by the formation of phosphorylated histone H2AX (γH2AX) foci. This was followed by incomplete DNA repair, leading to anaphase bridges, chromosomal abnormalities and senescence in dividing cells.38,39 In IL10−/−, azoxymethane (AOM)-treated, pks+ E. coli monocolonized mice, a significant increase of γH2AX foci was observed in colonocytes suggesting that inflammation creates an environment potentiating the genotoxicity of E. coli strain.40 In addition, the presence of pks+ E. coli accelerated the progression from dysplasia to invasive carcinoma in mono-associated AOM/IL10−/− mice whereas pks+ E. coli mono-associated AOM/WT mice did not develop any dysplasia/tumors.40 Taken together, these studies suggest that pks+ E. coli could promote colorectal carcinogenesis in a pro inflammatory environment.
We hypothesized that the postnatal period is a critical period where direct interaction between pks+ E. coli and immature intestinal barrier is susceptible to modulate intestinal homeostasis. Persistence of such neonatal gut residents is actually not known and impact of early exposure to these genotoxic strains on the developing intestine has never been explored. Therefore, we developed a novel animal model that mimics the natural transmission of E. coli from mothers to newborns in order to investigate whether colibactin can alter the development and function of the intestinal epithelial barrier. We observed that early colonization with pks+ E. coli triggers genotoxicity in the gut epithelium of neonates whereas γH2AX foci were no longer detected in adults. Nevertheless, adult rats early exposed to genotoxic E. coli strains exhibited an increased cell turnover in both small intestine and colon. Further examination of the gut epithelium revealed that occurrences of anaphase bridges and crypt fissions were abnormally high, whereas pattern of differentiated epithelial cells was reshaped in adult rats early exposed to pks+ E. coli. In addition, these phenomenons were transmissible across generations. The analyze of the intestinal epithelium permeability revealed that the integrity of the gut barrier was altered in animals early colonized with pks+ E. coli. In conclusion, we characterized an example of long lasting regulation of the host intestinal barrier function by genotoxic E. coli strains acquired at birth and demonstrated a transferability of host intestinal phenotypes across generations according to the phenotype of the pioneer gut bacteria.
Results
Prevalence of B2 E. coli carrying the pks island in neonates
We analyzed a collection of stools from 184 healthy neonates collected between October and November 2010 at the University Hospital of Limoges in France. At three days of age, 56.5% of infants were colonized with E. coli (104 neonates) and 47.1% of them carried at least one B2 E. coli strain (49 neonates), corresponding to 26.6% of all infants. Over the 52 B2 E. coli isolates collected and further characterized (Table S1), 30 carried the pks island. The rate of pks+ E. coli colonization at three days of life was finally 26.9% in infants colonized with E. coli or 15.2% (28 neonates) in the entire infant population.
These pks+ E. coli isolates were further analyzed by multilocus sequence typing (MLST)41 and serotyping. Subgroup VII (STc14) isolates were the most prevalent, all of them except two, being from serogroup O75 (Table S1).
Generation of non-genotoxic isogenic mutant in a commensal B2 E. coli strain carrying the pks island
M1/5 pks+ E. coli strain (E. coli WT), further used in this study, was isolated from the stool of a healthy adult (Table S2). This pks+ E. coli strain of serogroup O75, which belongs to the subgroup VII of the phylogenetic group B2 and for which shotgun sequence data was available, induced the formation of nuclear γH2AX foci in infected rat intestinal epithelial cells (IEC-6), as previously observed with other pks+ E. coli strains.36,42 The clbA gene coding for a 4'-phosphopantetheinyl transferase (Pptase), which is essential for the synthesis of colibactin, has been deleted to generate an isogenic E. coli ∆clbA mutant unable to induce the generation of γH2AX foci. Chromosomal complementation of the ∆clbA mutant (E. coli ΔclbA::clbA) fully restored this genotoxic activity (Fig. S1A and B).
Genotoxic and non-genotoxic commensal E. coli strains are transmitted from mother to the offspring and equally colonized the gut from birth to adulthood in an asymptomatic manner
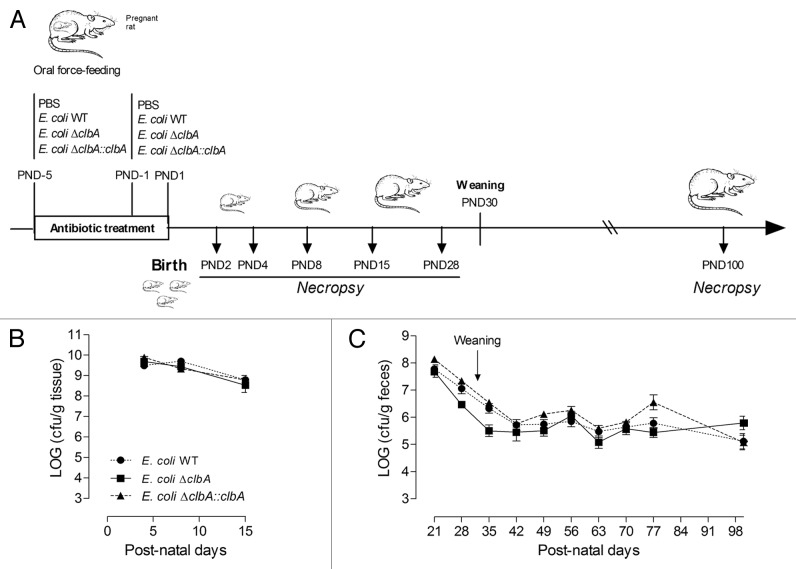
We developed an experimental animal model mimicking the early colonization of neonates by E. coli from the mother microbiota (Fig. 1A). Pregnant Wistar rats were fed with E. coli WT, E. coli ΔclbA, E. coli ΔclbA::clbA or PBS. These B2 E. coli strains were the dominant Enterobacteriaceae species isolated in mother fecal microbiota during the perinatal period (Fig. S2A and B). The natural transmission of E. coli strains to the offspring was detected as soon as post-natal day 2 (PND2). All neonates were then equally colonized and gut E. coli population reached the highest density between PND4 and PND8 (Fig. 1B). Fecal bacteria counts gradually decreased from PND21 to PND35, then stabilized and the adult fecal E. coli strain levels remained around 105,6 CFU per gram of feces (Fig. 1C). This decline of E. coli population was associated with the weaning transition and may result, as previously described in the literature, from the increased diversity of the gut microbiota composition and redox modifications of the gut microenvironment.43 E. coli originating from the mothers was always the predominant bacterial specie among Enterobacteriaceae present in the offspring gut microbiota (Fig. S2C). During the four-month period of our experiment, the animals were healthy and we did not observe any alteration of body weight or overall health status in early colonized animals with commensal E. coli strains (Fig. S3). In conclusion, using this novel rodent model, we observed that both kinetic and dynamic of gut colonization with commensal B2 E. coli strains reflected a similar pattern than the one previously described in humans, in a completely asymptomatic manner.
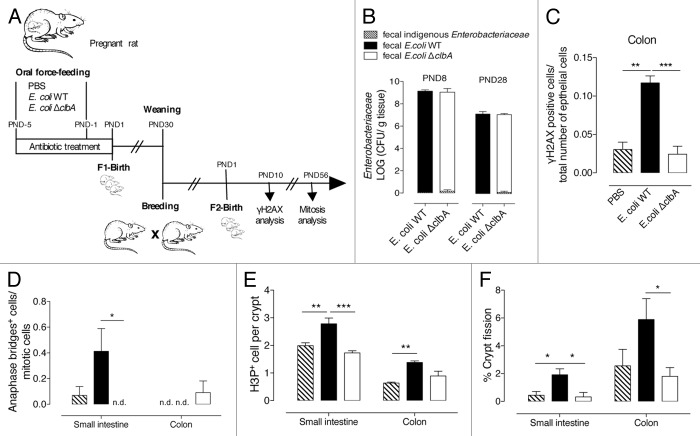
Figure 1. Commensal E. coli strains are transmitted from mother to the offspring and persistently colonized the gut at adulthood. (A) Experimental design of a rat model mimicking long-lasting colonization of the gut by commensal E. coli strains from birth to adulthood. Pregnant rats were treated with streptomycin (5 g/L) in drinking water and were inoculated twice with 109 CFU by intragastric gavage before parturition with E. coli WT, ΔclbA or ΔclbA::clbA strains or with PBS. Newborns were sacrificed at Post-Natal Days (PND) PND2, PND4, PND8, PND15, PND28 and PND100. (B) Evaluation of gut colonization in cecum/colon homogenate of neonates at early time-points (PND4, PND8 and PND15). (C) Evaluation of gut colonization in feces homogenate at later time-points (PND21, PND28, PND35 to PND100). Weaning is highlighted with a black arrow. Groups of 3–4 rats (PND4), 6–7 (PND8), 6–8 (PND15) and 15–20 (PND21-PND100) were used. Mean values ± SEM are shown.
Maternally acquired commensal genotoxic E. coli strains exacerbate DNA damage in the gut epithelial cells of neonates
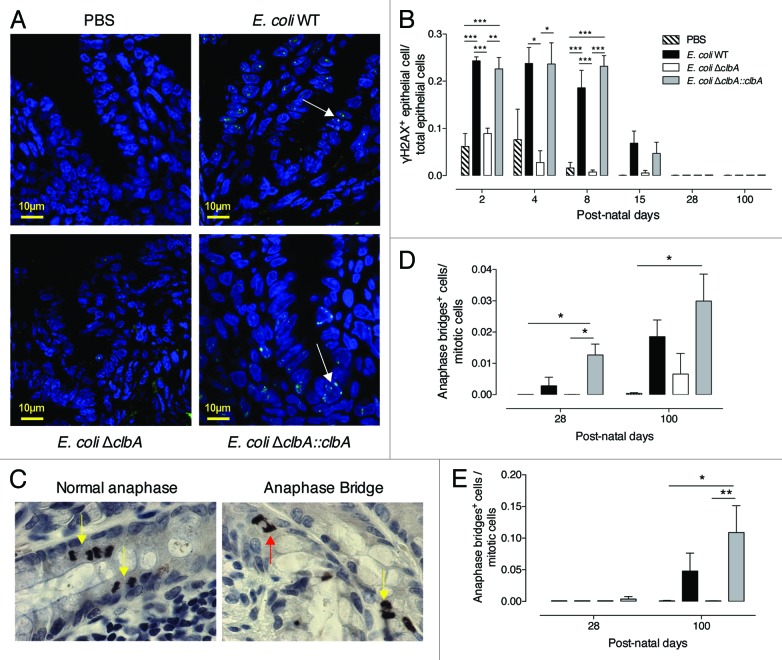
We asked whether maternally acquired commensal B2 E. coli strains were genotoxic to the neonatal epithelium. From PND2 to PND8, we observed a significant increased number of γH2AX+ cells along the entire length of the intestinal mucosa in pups originating from clbA+ (WT or ΔclbA::clbA) E. coli treated mother as compared with pups originating from E. coli clbA- (ΔclbA or PBS) treated mother (Fig. 2A and B). During this first week of life, the intensity of γH2AX signature observed in the intestinal epithelium of neonates colonized with clbA+ E. coli strains was similar to that observed with 0.5-Gy-whole body γ irradiation (Fig. S4A and B). Once pups reached PND8, γH2AX signal progressively declined, and genotoxicity was no longer detectable in rats older than PND28, when the number of genotoxic bacteria was estimated to be below 107 CFU per gram of feces (Fig. 1C).
Figure 2. Gut colonization with commensal E. coli strains producing colibactin exacerbates DNA double-strand breaks in gut epithelial cells of neonates and induces signs of increased chromosomal instability at adulthood. Immunofluorescence analysis of intestinal and colonic epithelium of neonates and adults (PND2-PND100) colonized since birth by commensal E. coli WT, E. coli ΔclbA or E. coli ΔclbA::clbA strains or treated with PBS. (A) Representative colon frozen sections at PND4. DNA was stained in blue and γH2AX foci in green. Scaled bars = 10 µm. White arrows show γH2AX foci in nucleus. (B) Quantification of γH2AX-positive epithelial cells. Groups of 3–6 rats were analyzed. Mean value ± SEM are shown. Two-way ANOVA with Bonferroni Multiple Comparison test, * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001. Immunohistochemestry analysis of intestinal and colonic epithelium of rats at PND28 and PND100. (C) Representative colon sections at PND100. Phospho-H3 was stained in black and allows detection of normal anaphases (left, yellow arrows) and abnormal mitosis with anaphase bridge (right, red arrow). Magnification used for photomicrographs = x20. Yellow arrows show normal anaphase figures and red arrow shows anormal mitotic cell with anaphase bridge. (D-E) Quantification of mitotic cells with anaphase bridge in small intestine (D) and colon (E). Groups of 5–10 rats were analyzed. Mean ± SEM are shown. Two-way ANOVA with Bonferroni Multiple Comparison test, * P ≤ 0.05 and ** P ≤ 0.01.
Adult gut stably colonized since birth with a commensal genotoxic E. coli displays epithelial cells with signs of increased chromosomal instability
Misrepaired double-strand breaks (DSBs) can induce chromosome fusions resulting in anaphase bridging.44,45 We previously observed such chromosomal instability biomarker within cultured epithelial cells shortly exposed to pks+ E. coli.38 Thus, we quantified the anaphase bridge index within different segments of the adult gut epithelium in rats early colonized with genotoxic or non-genotoxic E. coli strains (Fig. 2C). Interestingly, a significant increased number of anaphase bridges was detected in the small intestine of animals early colonized with clbA+ E. coli strains as compared with clbA- E. coli at PND28 and PND100 (Fig. 2D). A similar pattern was also observed in the colon (Fig. 2E), suggesting that DNA repair that follows DSBs triggered by clbA+ E. coli strains during the neonatal period was likely effective but DNA exhibited scars of misrepaired DSBs that will lead to genomic instability at adulthood.
Adult gut stably colonized since birth with a commensal genotoxic E. coli displays increased rate of epithelial cell apoptosis
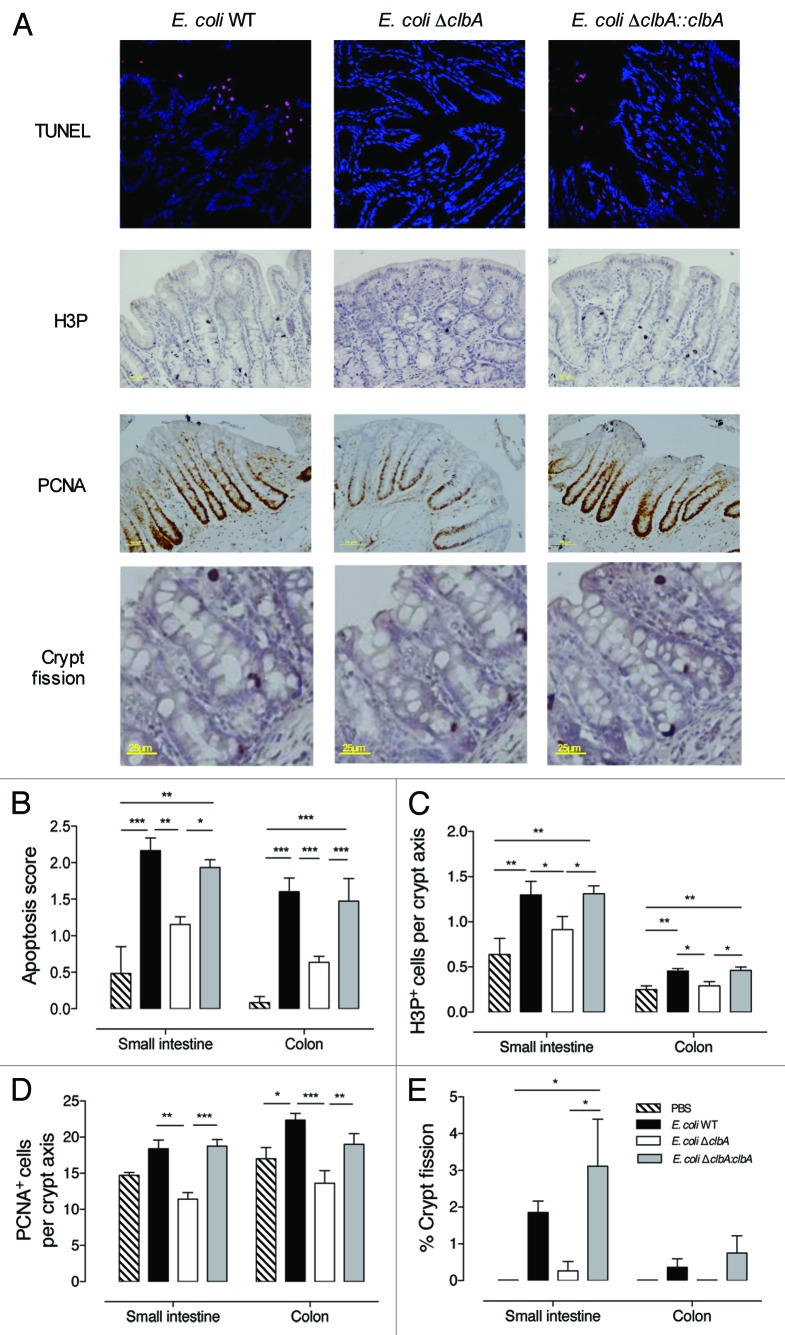
Intestinal epithelial cells continuously undergo spontaneous apoptosis and shedding as a result of constant renewal of the epithelial monolayer throughout postnatal life. Given that colibactin-induced DNA damage, following in vitro infection with pks+ E. coli, leads to cell death by apoptosis38 and that intestinal epithelial cells displayed DNA damage in rats colonized with clbA+ E. coli (Fig. 2B), we quantified apoptosis in epithelial cells along the gut at PND100. Regardless of the gut compartment, a significant increased number of apoptotic cells was observed in animals early colonized with clbA+ E. coli strains as compared with animals early colonized with the clbA- strain or controls (Fig. 3A and B). This phenomenon may occur as soon as PND28 (Fig. S5A and B). However, in offspring, gross histological architecture of the crypt-villus units did not reveal apparent differences according to mother treatment (Fig. S6A–D).
Figure 3. Early gut colonization with commensal E. coli strains producing colibactin increases the intestinal epithelial cells apoptosis, proliferation and crypt fission at adulthood. Histological and immunofluorescence analysis of the intestinal and colonic epithelium of adult animals early colonized by commensal E. coli WT, ΔclbA or ΔclbA::clbA strains or treated with PBS. (A) Representative colon frozen sections at PND100. DNA was stained in blue and apoptotic cells were stained in red with anti-TdT antibody (Tunel). Scale bars = 10 µm. Representative colon sections at PND100. Phospho-H3 was stained in black. PCNA was stained in black. Representative colon sections at PND100 and stained with hematoxylin-eosin. (B) Quantification of intestinal apoptotic score (see Methods section). (C) Quantification of H3P+ cells per crypt. (D) Quantification of PCNA+ cells per crypt. (E) Quantification of crypt fission. Black arrows show the fission of crypt in the colon. Magnification used for photomicrographs = x20. Groups of 5–10 rats were used, Mean values ± SEM are shown. One-way ANOVA, Bonferroni Multiple Comparison test, * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001.
Adult gut stably colonized since birth with a commensal genotoxic E. coli displays increased rate of epithelial cell proliferation
A higher rate of apoptotic cells without any change in the gut epithelium morphology, including villus and crypt depth, prompted us to examine the proliferative cell compartment within intestinal crypts at adulthood. Immunohistochemical staining of phosphorylated histone H3, a marker of mitotic cells, revealed that increased apoptosis rates previously observed in the mature gut epithelium of rats early exposed to clbA+ E. coli, was associated with a marked increase of mitotic cells number (Fig. 3A and C; Fig. S5C and D). Consistently, staining of these adult gut sections with a marker of the replicative S-phase (Proliferative Cell Nuclear Antigen, PCNA) revealed that the expansion of the proliferative compartment was restricted within the crypts of adult rats early exposed to clbA+ E. coli (Fig. 3A and D; Fig. S5E and F).
Early gut colonization with a commensal genotoxic E. coli hastened intestinal epithelial cells migration at adulthood
In order to investigate whether the increased proliferation and apoptosis in the crypt compartment of adult rats early colonized with genotoxic E. coli strains have an impact on cell turnover, we monitored epithelial cell migration along the crypt-villus axis, by performing a 12–24 h BrdU chase (Fig. S7A). We observed that the migration front of BrdU-positive cells was significantly higher in rats early colonized with clbA+ E. coli strains as compared with rats early colonized with clbA- E. coli or controls (Fig. S7B and C). Moreover, 48 h after BrdU injection (Fig. S7D), intestinal epithelial cells in rats from clbA+ E. coli treated mother progressed more rapidly along the crypt-villus axis as compared with those from clbA- E. coli treated mother or controls. This functional assay indicated that early acquired commensal genotoxic E. coli modulates intestinal epithelium renewal in adult rats.
Adult gut stably colonized since birth with a commensal genotoxic E. coli displays an abnormal crypt fission rate
Crypt proliferation is a critical process driving the postnatal growth of the gut in rodents and humans. When crypts divide, two flask-shaped based joining in a single unit are observed at the top of the crypt. This feature is characteristic of crypt fission. The number of crypt fission increases in the gut throughout the three first postnatal weeks in rats and then decreases significantly with age.46-48 Using our model, we observed that the percentage of crypt fission segregated according to the E. coli strain acquired at birth (Fig. 3A and E; Fig. S5G and H). Indeed, it remained abnormally elevated in 100 d-old rats colonized with clbA+ E. coli strains as compared with control rats or rats colonized with clbA- E. coli. Increased number of crypt fissions is considered as a dominant mechanism of early adenoma development.49,50
Maternally acquired commensal genotoxic E. coli strains influence secretory intestinal cell lineage at adulthood
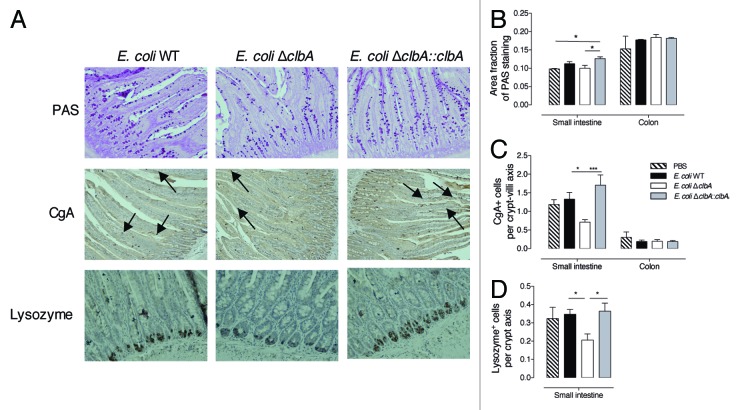
The proliferative cells spend approximately two days in the crypt, in which they divided 4–5 times before terminally differentiated into absorptive cells, mucous-secreting goblet cells, hormone-secreting enteroendocrine cells or bactericidal defensin peptides and lyzozyme-secreting Paneth cells.51 Based on our previous results showing a significant increase of proliferative cells in the intestine of adult rats from clbA+ E. coli treated mother, we next assessed cell lineage selection using secretory cell differentiation markers. We did not detect any modification of colonocyte differentiation in the offspring at PND100 (Fig. 4B and C). However, staining of small intestinal sections with Periodic-Acid Shiff revealed an increase of mucous-secreting goblet cells in rats early colonized with the genotoxic E. coli (Fig. 4A and B). In addition, we noticed a significant reduction of enteroendocrine cells, as determined by the immunohistochemistry analysis of chromogranin A, in clbA- E. coli-colonized rats as compared with clbA+ E. coli-colonized rats (Fig. 4A and C). Finally, we observed an increased number of Paneth cells, stained with anti-lyzozyme antibody, in adult rats colonized at birth with a genotoxic E. coli strain (Fig. 4A and D).
Figure 4. Early gut colonization with commensal E. coli strains producing colibactin modulates small intestine epithelial cell differentiation at adulthood (PND100). Histological analysis of the intestinal epithelium of adult animals early colonized by commensal E. coli WT, ΔclbA or ΔclbA::clbA strains or treated with PBS. (A) Representative small intestine sections stained with Periodic-Acid Shiff (PAS) method showing goblet cells in magenta. Enteroendocrine cells (black arrows) were stained in brown with anti-chromogranin A (CgA) antibody. Paneth cells were stained in brown with anti-Lysozyme (Lyz) antibody. (B) Quantification of PAS staining area on total epithelial surface. (C) Quantification of CgA+ cells along the crypt-villus axis. (D) Quantification of Lyz+ cells along the crypt-villus axis in the small intestine. Magnification used for photomicrographs = x20. Groups of 5–10 rats were used. Mean values ± SEM are shown. One-way ANOVA with Bonferroni Multiple Comparison test, * P ≤ 0.05, ** P ≤ 0.01.
Commensal genotoxic E. coli strains maternally acquired since birth increase intestinal epithelial permeability at adulthood
We have established that the mature gut epithelium of rats early colonized with commensal genotoxic E. coli displays altered patterns of apoptosis, proliferation, and differentiation. We next assessed whether these alterations may alter epithelial barrier function. We analyzed the transcellular permeability to intact HRP in small intestine using Ussing chambers (Fig. 5A). No significant changes were observed; suggesting that transcytosis of high molecular weight macromolecules in intact form is not affected by commensal B2 E. coli colonization. We next examined intestinal paracellular permeability to 4kDa dextran-FITC and electrical resistance (R) (Fig. 5B and C). Rats early colonized with clbA+ E. coli strains exhibited, at both PND28 and PND100, a significant increase in apical to serosal cumulative permeation of 4kDa dextran-FITC as compared with control rats or rats early colonized with the clbA- E. coli strain. In accordance with this increased 4kDa dextran-FITC permeability, a significant decrease of the electrical resistance (R) was observed in rats early colonized with the clbA+ E. coli strains, suggesting that adult rats early colonized with genotoxic E. coli have persistent epithelial barrier dysfunction.
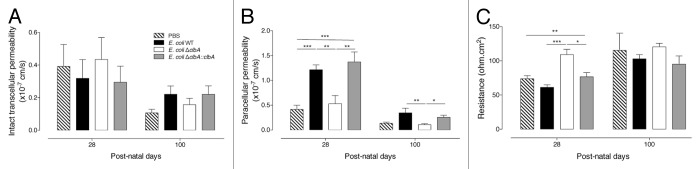
Figure 5. Early gut colonization with commensal E. coli strains producing colibactin alters intestinal permeability at adulthood (PND28 and PND100). Duodenal permeability was analyzed in adult animals early colonized by commensal E. coli WT, ΔclbA or ΔclbA::clbA strains or treated with PBS. (A) Transcellular permeability was assessed measuring mucosal to serosal flux of HRP. (B) Paracellular permeability was assessed measuring mucosal to serosal flux of FITC-dextran. (C) Electric transepithelial resistance was recorder during the permeability analysis. Groups of at least 10 rats were used. Mean values ± SEM are shown. One-way ANOVA with Bonferroni Multiple Comparison test, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Alterations observed in the gut epithelia of rats colonized with a maternally acquired genotoxic commensal E. coli strain are transmissible across generations
We extended this study by examining transgenerational impact of the natural transmission of a maternally acquired genotoxic E. coli. To this end, adult rats colonized since birth with E. coli WT or E. coli ΔclbA strain as well as control rats were bred (Fig. 6A). Fecal bacteria analysis of the second generation revealed that both genotoxic and non-genotoxic E. coli strains were naturally transmitted to the offspring with comparable levels than in the first generation (Fig. 6B). As expected, at PND10, was significantly increased within the gut epithelium of neonates colonized with genotoxic E. coli (Fig. 6C) and associated, at PND56 with an increased occurrence of anaphase-bridges (Fig. 6D), mitotic cells (Fig. 6E), and crypt fission (Fig. 6F). Collectively, these findings demonstrated that a specific E. coli strain present at a physiological level of colonization in the gut microflora of the mother can be transmitted across generations. The genotoxic potential of these early colonizing E. coli strains elicits short- and long-term physiological alterations of the gut physiology.
Figure 6. Alterations in intestinal physiology, induced by early gut colonization with commensal E. coli producing colibactin, are transmitted across generations. (A) Experimental design of a rat model mimicking transgenerational colonization of the gut with commensal E. coli strains. Adult rats, early gut colonized with commensal E. coli WT, ΔclbA strains or treated with PBS (F1), are breed and give rise to a second generation (F2) that is subsequently analyzed. (B) Evaluation of gut colonization in cecum/colon homogenate of neonates at PND8 and feces homogenate of adults at PND28. (C) Quantification of colonic γH2AX-positive epithelial cells. (D) Quantification of mitotic cells with anaphase bridge in small intestine and colon. (E) Quantification of H3P+ cells per crypt in small intestine and colon. (F) Quantification of crypt fission in small intestine and colon. Groups of 6 rats were used. Mean values ± SEM are shown. One-way ANOVA with Bonferroni Multiple Comparison test, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Discussion
Bacterial colonization of the gastrointestinal tract occurs during the first year of life. After this period, the microbiota composition is rather stable throughout adulthood.52 Adult human intestine harbored approximately 1014 bacterial cells, comprising more than 500 different bacterial species.53 This dense microbial environment affects metabolic, neurologic, inflammatory and immunologic host functions.18,54 Nonetheless, the neonatal period remains largely unexplored while likely having a tremendous impact on metabolic and cellular programming of the host.56 Perturbations in this fragile ecosystem, during this highly vulnerable period may have consequences in the later life.
E. coli belongs to the pioneer microbiota that colonized the mammalian gut within few days after birth.29 As reported by our group,36 B2 E. coli strains harbored a specific genomic island called pks, which encodes the genotoxin colibactin. These colibactin-producing E. coli induced DNA double strand breaks, chromosomal abnormalities and genomic instability in mammalian cells.38 Therefore, we did a survey for the presence of pks+E. coli in early life. In this study, we showed that pks+E. coli strains belong to the early microbiota of around 15% of three-days-old French neonates. This is in accordance with data generated from a 10 y old Swedish cohort in which 18% of three-days-old neonates was colonized by pks+E. coli. Thus, the investigation of the impact of an early exposure with these genotoxic strains on the developing intestine was of particular interested. In this context, we developed an innovative and realistic animal model of natural colonization by vertical transmission. This model reproduced the natural sequence of events that precedes human gut colonization, with levels reaching the same order of magnitude than those observed in neonates7,26,57 and adults.29,58 In addition, this model eliminated pups manipulation and thus circumvented the neonatal stress that is known to impact the gut mucosal barrier.59-61 The use of germfree or gnotobiotic animals gave insights into the causative role of microbiota in shaping host gut responses but lacked of accuracy as the result of large intestinal developmental defects or an artificial monocolonized environment. Thus, this vertical model allowed us to investigate in a global and natural way the role of pioneer genotoxic commensal E. coli strain on the intestinal homeostasis.
E. coli harboring the pks island have been shown to be more frequently detected in colorectal tissue from inflammatory bowel disease (IBD) and colorectal colon cancer (CRC) patients as compared with non-IBD/non CRC controls,40,62 and may directly impact carcinogenesis. However, it is not clear whether dysbiosis promoting the emergence of E. coli is a cause or a consequence of CRC. We hypothesized that E. coli, which is the major pioneer bacteria colonizing the gut of neonates, may be involved in the initiation steps of colon carcinogenesis. Using our model allowing maternal transmission of E. coli, we demonstrated that early acquired colibactin-producing B2 E. coli establishes a lifelong niche in the gut ecosystem behaving as an autochthonous microbe. As a consequence, B2 colibactin-producing E. coli altered intestinal epithelial cells integrity during the neonatal period, by activating the DNA damage response, and at adulthood, by increasing signs of genotoxic damage including anaphase bridges, crypt fission, intestinal proliferation and cellular renewal. These data strongly suggested that early colonizing colibactin-producing E. coli strains could participate in intestinal tumorigenesis. Several studies have reported that the use of genotoxic therapies in young children exacerbates the risk to develop cancer at adulthood.63-65 Our data revealed that the intestinal genotoxicity intensity observed in animals early colonized by colibactin-producing E. coli was similar to that observed with 0.5Gy whole-body γ-irradiation. Beside, epidemiological follow-up of atomic-bomb survivors indicated that low-dose (~1Gy) increased the frequency of mutation associated with colorectal cancer.66 Elaboration of longitudinal birth cohort in order to further explore a potential link between carriage of colibacin-producing B2 E. coli strain at birth and predisposition to colorectal cancer is required.
Moreover, the present study revealed a role of colibactin-producing B2 E. coli in lineage-specific maturation of intestinal epithelial cells. While in the small intestine of animals early colonized by colibactin-producing E. coli, amplified differentiation of Paneth, goblet and enteroendocrine cell lineages might reflect an increase in proliferative dynamics, the crypt hyperplasia observed in the colon was not related to any modification of epithelial cell differentiation. This selective cell lineage commitment in the small intestine suggest that rather than a passive effect of deregulated cell proliferation, early intestinal colonization with colibactin-producing E. coli might affect intestinal cell programming. The renewal of the intestinal epithelium from stem cells to functionally mature cells is tightly regulated by multiple factors and pathways involving epithelial-epithelial and epithelial-mesenchymal interactions.67 In this study, we showed that it may also respond to environmental conditions and especially to the composition of the neonatal gut microbiota.
A concomitant increase of Paneth and goblet cell numbers has been reported after a systemic treatment with the DNA-damaging agent doxorubicin and may be related to regeneration processes.68 Indeed, it has been demonstrated that Paneth cells may influence the stem cells micro-environment and function.70,71 In addition Paneth cells synthesize and secrete numerous factors that are known to modulate proliferation and migration of enterocytes.72 It is likely that intestinal DNA damage induced during the neonatal period, where the intestinal mucosal barrier is largely immature, by colibactin-producing E. coli may be exemplified by an increased numbers of Paneth and goblet cells at adulthood, probably due to the recruitment of cells from a pre-existing pool of secretory intermediate progenitor cells which are known to be induced after DNA damage.73 This specific point was not examined in the present study and will be the focus of future experiments.
Intestinal epithelial cells are key players of the intestinal barrier by forming a physical barrier allowing the transport of nutrients to the lamina propria while keeping larger molecule and bacteria in the lumen. Intestinal permeability controls interplay between host and external environment. Mechanisms regulating the integrity of the gut epithelial barrier, and thus the maintenance of tolerance between the host and the gut environment, critically involve the gut microbiota.54 Our experiments revealed that the presence of genotoxic E. coli dampened paracellular permeability. However, we did not observe any difference in transcellular permeability but we did not analyze the transcytosis of macromolecules in degraded form. Indeed, luminal antigens crossing the epithelium can reach the serosal compartment in intact (10%) or degraded form (90%).74 Our results exclude an effect of E. coli colonization on intact transcellular permeability but did not exclude an effect on the lysosomal pathway involved in degraded antigens transcytosis. There are mounting evidences suggesting that disturbance of intestinal barrier function and especially enhanced gut permeability may be involved in several gut dysfunctions, including irritable bowel syndrome (IBS)75 and IBD.76 In addition, enhanced translocation of luminal components could alter tolerance between the host and its environment leading to the development of allergic or auto-immune manifestations. Indeed, recent epidemiological and clinical studies indicate an association between the composition of intestinal microbial communities and the increased incidence of allergic asthma, eczema or rhinitis.77-81 Taken together with the results of our study, these data lead us to hypothesize that early gut colonization with genotoxic B2 E. coli might be a risk factor in the incidence of such diseases. In addition, we established that the early life is a critical period for the host and that the composition of the intestinal microbiota and its interaction with the intestinal epithelium is likely to give important information about the development of diseases that manifest both during infancy and adult life.
The gut microbiome is approximately 150 times larger than the human genome, with an estimated 3.3 million microbial genes. In this study, we have shown that the disruption of a single gene in the genome of B2 E. coli strains acquired at birth is critical for gut homeostasis of the host. As suggested in an assay wrote in 2009 by Blaser and Falkow,82 it is predictable that social and medical progress that affects the composition of the microbiota will have consequences for our physiology and health. Such evolution is observed not only at the level of the microbiota but also at the level of phylogenetic groups, bacterial species and genes. There is a concomitant increase in population of industrialized countries of immune-mediated diseases and colorectal cancer along with a shift in E. coli population from the declining phylogenetic group A to the newly dominant phylogenetic group B2.29 A large proportion of these B2 E. coli strains are both genotoxic and pioneer bacteria colonizing the gut of neonates. Thus, our data lead us to hypothesize that early gut colonization with the newly dominant B2 E. coli group associated with genotoxic activity might be a risk factor in the incidence of immune-mediated diseases and colorectal cancer.
Material and Methods
Analysis of feces in neonates
Healthy neonates were defined as asymptomatic neonates, which did not receive any antibiotic after birth. Sex, gestational age, mode of delivery as well as per-partum antibiotic prophylaxis during labor were recorded anonymously with approval of local ethics committee. Characterization of E. coli isolates is described in Supplemental Materials and Table S1.
Bacterial strains, mutagenesis procedures and growth Conditions
Bacterial strains used in this study are listed in Table S2. The commensal Escherichia coli strain M1/5, kindly provided by Pr. Ulrich Dobrindt, was isolated from the stool of an asymptomatic human and maintained with minimal genetic manipulation. Pks chromosomal isogenic mutant and complemented strain were generated in the laboratory. Procedures, primers and templates used for gene mutagenesis and resultant mutants are presented in the Supplemental Materials and Table S2 respectively. Before oral administrations, all E. coli strains were grown 6 h in LB broth supplemented with antibiotics at 37 °C with shaking. These cultures were diluted 1:100 in LB broth without antibiotics and cultured overnight at 37 °C with shaking. Bacterial pellets from these overnight cultures were suspended in sterile PBS to the concentration of 109 colony forming units (CFU)/ml.
Experimental animal model of long-lasting gut bacterial colonization
Primiparous timed-pregnant WISTAR female rats were obtained from Janvier on gestational day 15 and housed separately under specific-pathogen-free conditions and had access to food and water supplemented with streptomycin (5g/L) ad libitum. Pregnant females were inoculated twice with 109 bacteria by intragastric gavage at PND-5 and PND-1. Toxalim animal facility (INRA, UMR 1331, Toulouse) is licensed by the French Ministry of Agriculture (agreement n° B31.555.13). All animal experiments complied with the European Union regulation, reviewed by the regional ethics committee (CNREEA n°1; MP/03/62/11/11). Bacterial colonization, body weight, gut development and maturation of offspring were specifically monitored from birth to weaning (PND2 to PND28) or at adulthood (PND56 and PND100) when gut maturation was completed (Fig. 1A).
Colonic bacterial load
Before PND15, cecum and colon homogenates were prepared in 0.6 mL of isotonic saline solution using Precellys tissue homogenizer (Bertin Technologies). After PND15, feces homogenates were prepared. 10-fold serial dilution of homogenates was plated on MacConkey agar plates (Biovalley) supplemented or not with appropriate antibiotics. Plates were incubated at 37 °C and the numbers of CFU were enumerated after 24 h. Colonies found growing on MacConkey’s agar plates without antibiotic were considered to be enterobacteria-like bacteria belonging to the family Enterobacteriaceae.
Immunofluorescence analysis
Small intestine and colon samples were placed immediately in optimum cutting temperature (OCT, Sakura) compound and snap-frozen in liquid nitrogen. Sections (5µm) were fixed in 4% formaldehyde, permeabilized with PBS-0,25% Triton X100, blocked with PBS-0.1% Tween20–5% NGS and stained with mouse anti-phospho H2AX antibody (Millipore, clone JBW301) followed by goat anti-mouse-FITC antibodies (Zymed). DNA was stained for 1 min with TO-PRO-3 (Invitrogen) and mounted in VectaShield containing DAPI (Vector Laboratories).
Cell death was quantified by terminal deoxynucleotidyl transferase-mediated tetramethylrhodamine-dUTP (TMR-dUTP) nickened labeling (TUNEL kit, Roche). Frozen sections of small intestine and colon were fixed with 4% paraformaldehyde and permeabilized with PBS-0.1% Triton X100–0.1% Sodium citrate. DNA was labeled with TO-PRO-3 (Invitrogen). Scoring (0–4) was based on the number of TUNEL-positive cells in intestinal epithelium. 0 = no TUNEL-positive cells; 1 = < 5 TUNEL-positive cells; 2 = 5 to 10 TUNEL-positive cells; 3 = 11 to 40 TUNEL-positive cells; 4 = > 41 TUNEL-positive cells. Images were acquired with an Olympus IX70 laser scanning confocal microscope equipped with the Fluoview software FV500. Confocal aperture was set to achieve a z optical thickness of ~0.5 μm for foci quantification and ~0.26 μm for γH2AX foci localization analysis.
Histology, immunohistochemistry, and cell lineage analysis
Small intestine and colon samples were collected, fixed with 10% formalin for 24 h, dehydrated and embedded in paraffin according to standard histological procedures. Sections (5 µm) were mounted on SuperFrost® Plus Slides then dewaxed in a xylene bath for 10 min and rehydrated in graded alcohol baths. Slides were stained with hematoxylin and eosin (H&E) and analyzed for intestinal morphometry (crypt depth, villi length, and crypt fission). For immunohistochemistry, endogenous peroxidase activity was quenched using 5% H2O2 in PBS (or methanol). Antigen retrieval was performed by heating section in 10mM sodium citrate (pH 6.0) for 10 min. Non specific binding was blocked, incubating with 2.5% normal goat serum for 1 h at room temperature (RT). Sections were incubated with primary antibody anti-phospho-Histone3 (H3P, 1/50e, Cell Signaling Technology, overnight at 4 °C), anti-chromogranin A (CgA, 1/500e, Immunostar, 1 h at RT) or anti-lysozyme (Lyz, 1/100e, Invitrogen, 1 h at RT) antibodies. Detection was performed by incubating with biotin secondary antibody followed by ABC complex (Vectastain, Vector Laboratories).
Sections were incubated with anti-PCNA (Proliferating Cellular Nuclear Antigen, 1/1000e, Santa Cruz) 1 h at RT. Detection was performed using IMPRESS reagent (Kit anti-mouse IMPRESS reagent, Vector laboratory). Staining was developed with DAB substrate (Vector Laboratories) and sections were counterstained with Hematoxylin QS (Vector Laboratories). After dehydratation, sections were mounted in Vectamount (Vector Laboratories) and images were acquired with a Leica DMRB fluorescence microscope or Nicon Eclipse 90i and analyzed with NIS Elements AR program. For goblet cells observation, slides were stained using periodic-acid Schiff method (PAS): incubation with 0.5% of periodic acid for 7 min at RT followed by Schiff reagent for 15 min at 4 °C.
Cell proliferation was analyzed by assessing the number of mitotic cells (H3P+ cells), proliferative cells (PCNA+ cells) per crypt axis (25 to 100 crypts were analyzed per sections). Goblet cells number was evaluated by measuring the mean area of PAS staining on 5 photomicrographs per sample. Enteroendocrine cells (CgA+ cells) and Paneth cells (Lyz+ cells) were enumerated per crypt-villus axis (25 to 100 crypts were analyzed per sections). 1–3 sections were analyzed per rat. Crypt depth and villus length were obtained from at least 10 well-oriented crypt-villus axes per animal. The number of fissioning crypts was obtained by observing at least 100 crypts per animal.48 All slides were analyzed by two investigators who were blinded for the treatment. Images were acquired with a Leica DMRB or Nikon Eclipse 90i microscope. Analyses were performed using Image J software (http://rsb.info.nih.gov/ij/).
Intestinal permeability
Two specimens of duodenum fragments were mounted in Ussing chambers (Easy Mount, Physiologic Instruments) exposing a surface area measuring 0.1 cm2. They were bathed on each side with 1 ml of oxygenated thermostated Kreb’s solution (Sigma). Electrical parameters, including potential difference, short-circuit current (Isc) and total electrical resistance (R), were recorded at regular intervals during the 2-h period of experimentation. A change in electrical resistance was considered an index of altered paracellular permeability. Horseradish peroxidase (HRP) transport was measured as an index of macromolecular permeability, and FITC-dextran 4kDa epithelial passage was measured as a marker of paracellular permeability to small molecules. After equilibration of electrical parameters, HRP was added to the mucosal compartment at a final concentration of 0.4 mg/ml, and FITC-dextran 4kDa at a final concentration of 2.2 mg/ml. The 2 markers were applied simultaneously in the mucosal compartment. Epithelial permeability to intact HRP was determined by an enzymatic assay measuring HRP activity using an automatic Infinite M200 microplate reader (Tecan). Epithelial permeability to FITC-Dextran 4kDa was determined by measuring the fluorescence intensity (FI) 485nm/525nm using an automatic Infinite M200 microplate reader (Tecan). By measuring the specific activity of the markers expressed in FI/µg or HRP activity/µg and the FI or HRP activity values found in the serosal compartment, intestinal fluxes (µg/2 h·cm2) were calculated. Permeability was calculated as the ratio of flux/concentration, as previously described.
Statistical analysis
Statistical evaluation of differences between the experimental groups was determined by using one-way or two-way analysis of variance (ANOVA) followed by a Bonferroni post-test (which allows comparison of all pair of groups). All tests were performed with GraphPad Prism 5.03 (GraphPad Software Inc.). All data are presented as mean ± standard error of the mean (SEM). A P value < 0.05 was considered significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Pr Ulrich Dobrindt for providing the M1/5 E. coli strain and shotgun sequence data (Münster University, Germany). The authors wish to thank the staff at the animal service facility of Toxalim for animal care. We also thank members of the Joost Van Meerwijk lab (Centre de Physiopathologie de Toulouse-Purpan, CPTP) for obtaining irradiated rats, the staff of the histopathology platform of UMS US 006 and imagery platform of CPTP for excellent technical help. This work was supported by grants from the French National Research Agency (ANR-09-MIEN-005–01) and the Association pour la Recherche sur le Cancer (ARC DOC20110602919).
Glossary
Abbreviations:
- AOM
azoxymethane
- BrdU
bromodeoxyuridin
- CFU
colony forming unit
- CRC
colorectal cancer
- DAPI
4’,6’ diamidino 2 phenylindol
- DSB
double strand break
- E. coli
Escherichia coli
- FITC
fluorescein isothiocyanate
- H&E
Hematoxylin and eosin
- H3P
histone 3 phosphorylated
- HRP
horseradish peroxidase
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- IEC
intestinal epithelial cell
- LB
Luria-bertani
- NGS
normal goat serum
- NRPS
nonribosomal peptides synthetase
- OCT
optimum cutting temperature
- PKS
polyketide synthetase
- PCNA
proliferating cell nuclear antigen
- Pptase
phosphopantetheinyl transferase
- PND
post natal day
- RT
room temperature
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick and labeling
- WT
wild type
References
- 1.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–76. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 2.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 3.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 4.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 8.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–57. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003;100:10452–9. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–93. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heczko U, Abe A, Finlay BB. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J Infect Dis. 2000;181:1027–33. doi: 10.1086/315348. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 20.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–5. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 24.Park HK, Shim SS, Kim SY, Park JH, Park SE, Kim HJ, Kang BC, Kim CM. Molecular analysis of colonized bacteria in a human newborn infant gut. J Microbiol. 2005;43:345–53. [PubMed] [Google Scholar]
- 25.Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–45S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–21. [PubMed] [Google Scholar]
- 28.Nowrouzian FL, Adlerberth I, Wold AE. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 2006;8:834–40. doi: 10.1016/j.micinf.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–17. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 30.Gordon DM, Clermont O, Tolley H, Denamur E. Assigning Escherichia coli strains to phylogenetic groups: multi-locus sequence typing versus the PCR triplex method. Environ Microbiol. 2008;10:2484–96. doi: 10.1111/j.1462-2920.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- 31.Smati M, Clermont O, Le Gal F, Schichmanoff O, Jauréguy F, Eddi A, Denamur E, Picard B, Coliville Group Real-time PCR for quantitative analysis of human commensal Escherichia coli populations reveals a high frequency of subdominant phylogroups. Appl Environ Microbiol. 2013;79:5005–12. doi: 10.1128/AEM.01423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escobar-Páramo P, Grenet K, Le Menac’h A, Rode L, Salgado E, Amorin C, Gouriou S, Picard B, Rahimy MC, Andremont A, et al. Large-scale population structure of human commensal Escherichia coli isolates. Appl Environ Microbiol. 2004;70:5698–700. doi: 10.1128/AEM.70.9.5698-5700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon DM, Stern SE, Collignon PJ. Influence of the age and sex of human hosts on the distribution of Escherichia coli ECOR groups and virulence traits. Microbiology. 2005;151:15–23. doi: 10.1099/mic.0.27425-0. [DOI] [PubMed] [Google Scholar]
- 34.Nowrouzian F, Hesselmar B, Saalman R, Strannegard IL, Aberg N, Wold AE, Adlerberth I. Escherichia coli in infants’ intestinal microflora: colonization rate, strain turnover, and virulence gene carriage. Pediatr Res. 2003;54:8–14. doi: 10.1203/01.PDR.0000069843.20655.EE. [DOI] [PubMed] [Google Scholar]
- 35.Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J Infect Dis. 2005;191:1078–83. doi: 10.1086/427996. [DOI] [PubMed] [Google Scholar]
- 36.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–51. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 37.Nowrouzian FL, Oswald E. Escherichia coli strains with the capacity for long-term persistence in the bowel microbiota carry the potentially genotoxic pks island. Microb Pathog. 2012;53:180–2. doi: 10.1016/j.micpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107:11537–42. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Secher T, Samba-Louaka A, Oswald E, Nougayrède JP. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One. 2013;8:e77157. doi: 10.1371/journal.pone.0077157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Gall T, Clermont O, Gouriou S, Picard B, Nassif X, Denamur E, Tenaillon O. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol Biol Evol. 2007;24:2373–84. doi: 10.1093/molbev/msm172. [DOI] [PubMed] [Google Scholar]
- 42.Olier M, Marcq I, Salvador-Cartier C, Secher T, Dobrindt U, Boury M, Bacquié V, Pénary M, Gaultier E, Nougayrède JP, et al. Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut Microbes. 2012;3:501–9. doi: 10.4161/gmic.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholtens PA, Oozeer R, Martin R, Amor KB, Knol J. The early settlers: intestinal microbiology in early life. Annu Rev Food Sci Technol. 2012;3:425–47. doi: 10.1146/annurev-food-022811-101120. [DOI] [PubMed] [Google Scholar]
- 44.McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941;26:234–82. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmond DA, Tucker JD, Thomas P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–32. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 46.Cummins AG, Jones BJ, Thompson FM. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Sci. 2006;51:718–23. doi: 10.1007/s10620-006-3197-9. [DOI] [PubMed] [Google Scholar]
- 47.St Clair WH, Osborne JW. Crypt fission and crypt number in the small and large bowel of postnatal rats. Cell Tissue Kinet. 1985;18:255–62. doi: 10.1111/j.1365-2184.1985.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 48.Dehmer JJ, Garrison AP, Speck KE, Dekaney CM, Van Landeghem L, Sun X, Henning SJ, Helmrath MA. Expansion of intestinal epithelial stem cells during murine development. PLoS One. 2011;6:e27070. doi: 10.1371/journal.pone.0027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasan HS, Park HS, Liu KC, Mandir NK, Winnett A, Sasieni P, Bodmer WF, Goodlad RA, Wright NA. APC in the regulation of intestinal crypt fission. J Pathol. 1998;185:246–55. doi: 10.1002/(SICI)1096-9896(199807)185:3<246::AID-PATH90>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Preston SL, Wong WM, Chan AO, Poulsom R, Jeffery R, Goodlad RA, Mandir N, Elia G, Novelli M, Bodmer WF, et al. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 2003;63:3819–25. [PubMed] [Google Scholar]
- 51.Drozdowski L, Thomson AB. Intestinal hormones and growth factors: effects on the small intestine. World J Gastroenterol. 2009;15:385–406. doi: 10.3748/wjg.15.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol. 2006;21:517–23. doi: 10.1016/j.tree.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 55.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neu J. Perinatal and neonatal manipulation of the intestinal microbiome: a note of caution. Nutr Rev. 2007;65:282–5. doi: 10.1111/j.1753-4887.2007.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 57.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 58.Bailey JK, Pinyon JL, Anantham S, Hall RM. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010;59:1331–9. doi: 10.1099/jmm.0.022475-0. [DOI] [PubMed] [Google Scholar]
- 59.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–6. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res. 2007;62:240–5. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- 61.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–90. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 62.Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut. 2014;63:761–70. doi: 10.1136/gutjnl-2013-304739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brassesco MS, Xavier DJ, Camparoto ML, Montaldi AP, de Godoy PR, Scrideli CA, Tone LG, Sakamoto-Hojo ET. Cytogenetic instability in childhood acute lymphoblastic leukemia survivors. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/230481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenney ME, Levitt GA. The quality of survival after childhood cancer. Eur J Cancer. 2002;38:1241–50, discussion 1251-3. doi: 10.1016/S0959-8049(02)00091-6. [DOI] [PubMed] [Google Scholar]
- 65.Rice SC, Vacek P, Homans AH, Messier T, Rivers J, Kendall H, Finette BA. Genotoxicity of therapeutic intervention in children with acute lymphocytic leukemia. Cancer Res. 2004;64:4464–71. doi: 10.1158/0008-5472.CAN-03-3940. [DOI] [PubMed] [Google Scholar]
- 66.Nakachi K, Hayashi T, Hamatani K, Eguchi H, Kusunoki Y. Sixty years of follow-up of Hiroshima and Nagasaki survivors: current progress in molecular epidemiology studies. Mutat Res. 2008;659:109–17. doi: 10.1016/j.mrrev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 67.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 68.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G461–70. doi: 10.1152/ajpgi.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–6. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 70.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 71.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–43. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 73.King SL, Mohiuddin JJ, Dekaney CM. Paneth cells expand from newly created and preexisting cells during repair after doxorubicin-induced damage. Am J Physiol Gastrointest Liver Physiol. 2013;305:G151–62. doi: 10.1152/ajpgi.00441.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heyman M, Desjeux JF. Significance of intestinal food protein transport. J Pediatr Gastroenterol Nutr. 1992;15:48–57. doi: 10.1097/00005176-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–81. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 77.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–7. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hong PY, Lee BW, Aw M, Shek LP, Yap GC, Chua KY, Liu WT. Comparative analysis of fecal microbiota in infants with and without eczema. PLoS One. 2010;5:e9964. doi: 10.1371/journal.pone.0009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97:1173–8. doi: 10.1016/S0091-6749(96)70181-1. [DOI] [PubMed] [Google Scholar]
- 81.Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89:227–9. doi: 10.1136/adc.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.