Abstract
Lactobacillus gasseri ATCC 33323 is a member of the acidophilus-complex group, microbes of human origin with significant potential for impacting human health based on niche-specific traits. In order to facilitate functional analysis of this important species, a upp-based counterselective chromosomal integration system was established and employed for targeting the lipoteichoic acid (LTA) synthesis gene, ltaS, in L. gasseri ATCC 33323. The ltaS gene encodes a phosphoglycerol transferase responsible for building the glycerol chain of LTA. No isogenic mutant bearing the deletion genotype was recovered, but an integration knockout mutant was generated with insertion inactivation at the ltaS locus. The ltaS deficient derivative exhibited an altered cellular morphology and significantly reduced ability to adhere to Caco-2 intestinal cell monolayers, relative to the wild-type parent strain.
Keywords: integration, deletion, mutation, probiotic, commensal, lipoteichoic acid, adhesion
Introduction
Lactobacillus gasseri ATCC 33323 is a commensal bacterium that is commonly found in the human microbiome.1 It is an autochthonous microorganism that colonizes the human mucosa, including the oral cavity, vagina, and gastrointestinal tract (GIT) in healthy individuals.2-7L. gasseri is also among the predominant microorganisms in the initial colonization of the neonatal GIT following childbirth.8 Niche-related phenotypes have been defined that likely confer its capacity to colonize the mucosa and potentially contribute to the ability of L. gasseri to positively impact health. Specifically, L. gasseri exhibits bile resistance, adhesion to Caco-2 intestinal epithelial cells, antimicrobial activity, the ability to degrade oxalate, and immunomodulatory properties.1,9-11 Consumption of L. gasseri has been associated with health benefits substantiated by randomized human clinical trials, such as the capacity to reduce severity and duration of symptoms associated with acute diarrhea and upper respiratory viral infections alike, as well as suppression of Helicobacter pylori infection and maintenance of vaginal homeostasis.12-14 Given the considerable potential of L. gasseri for probiotic applications, this species warrants genetic efforts to correlate genotypes with phenotypic traits potentially impacting health and well-being.
The published genome sequence of L. gasseri ATCC 33323 facilitates identification and subsequent functional analysis of relevant genes involved in survival and activity in the GIT, such as genes encoding mucus-binding proteins, putative adhesion factors, and immunomodulatory components.1 This study established a deletion and/or knockout strategy for genes in L. gasseri using a upp (uracil phosphoribosyltransferase) based markerless gene replacement system.15 The system was used to investigate the role of the cell surface component lipoteichoic acid (LTA), a conserved Gram-positive structural macroamphiphile, in microbe-host interaction. Previously, elimination of LTA from the surface of Lactobacillus acidophilus was correlated with a shift to anti-inflammatory cytokine profiles from dendritic cells (DCs) and mitigation of both colitis and colon cancer outcomes in mouse models.16,17 An insertion knockout of ltaS, encoding a phosphoglycerol transferase involved in LTA synthesis, in L. gasseri was created in order to elucidate how LTA potentially contributes to gastrointestinal homeostasis by conferring the capacity to adhere to intestinal epithelial cells.
Results
Construction of L. gasseri NCK2253 ∆upp background host, 5-FluorouracilR
In microbes with a functional uracil ribonucleotide salvage pathway, the toxic uracil analog 5-fluorouracil (5-FU) is imported and incorporated into the pathway, resulting in the formation of 5-fluoro-deoxy-uridine-monophosphate, lethally acting as a thymidylate synthase inhibitor. L. gasseri ATCC 33323 was first evaluated for sensitivity to 5-FU by plating approximately 108 cfu of a 16 h stationary phase culture onto glucose semi-defined medium (GSDM) supplemented with a final concentration of 100 µg/mL of 5-FU. Approximately 10 colonies appeared, indicating that spontaneous mutation rates to a 5-FUR phenotype occurred at a frequency of 10−7. The typical excision rate of integrated pORI-based plasmids from lactobacilli has been observed to be roughly 0.2% to 1%.15 Therefore, due to the quantity of cells plated to select for excision recombinants, 5-FUR colonies arising from spontaneous mutation would occur at an incidence of <0.01 clones per plate, making the number of spontaneous mutants relative to the excision recombinant clones negligible.
The 5-FUS of the wild-type strain indicated that L. gasseri ATCC 33323 contains an active uracil ribonucleotide salvage pathway. Analysis of the genome confirmed that L. gasseri ATCC 33323 lacked a complete pathway for synthesis of uracil de novo, but encoded a putative Upp which enzymatically catalyzes the phosphorylation of imported uracil.1 In L. gasseri ATCC 33323, Upp is encoded by LGAS_1245, with 78% nucleotide sequence identity and 82% predicted amino acid identity to the confirmed upp gene in L. acidophilus NCFM. The upp gene was targeted for deletion in L. gasseri ATCC 33323 harboring pTRK669 (NCK2092) through allelic replacement. For this, the integration plasmid pTRK1065, containing genomic regions upstream and downstream of the gene locus but deficient in 571 bp of the coding sequence for upp, was used. Integration of pTRK1065 was mediated by site-specific homologous recombination of the flanking sequences and was induced by preventing plasmid replication through loss of the helper plasmid, pTRK669. PCR using primers flanking the deletion junction were used to screen for excision recombinants, which yielded a putative upp deletion genotype in an EmS clone. The potential positive clone was sequenced at the deletion region, which confirmed fidelity of the flanking regions and loss of the 571 bp sequence (Fig. 1). The deletion clone was designated NCK2253 and was plated on GSDM supplemented with 5-FU to confirm that deletion of LGAS_1245 conferred 5-FU resistance phenotype. NCK2253 was electroporated with the temperature sensitive helper plasmid pTRK669 to provide RepA in trans for the pORI-based non-replicative broad host range integration vector pTRK935, thus facilitating further gene deletion experiments in L. gasseri. A clone of NCK2253 exhibiting the CmR phenotype was confirmed to contain pTRK669 by PCR screening of the plasmid specific repA gene, and designated NCK2254 (Table 1).
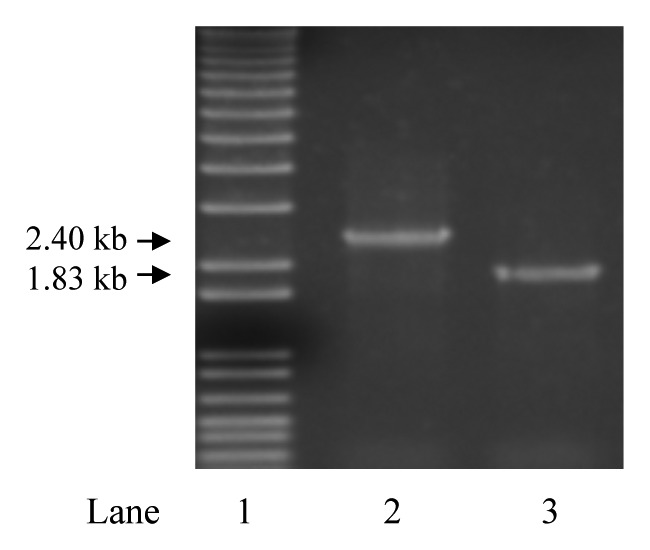
Figure 1. PCR confirmation of upp deletion. Agarose gel electrophoresis image of PCR amplicons from genomic DNA (gDNA) with primers flanking upp in L. gasseri ATCC 33323 strains. Lane 1 is a 1 kb ladder (Invitrogen). Lane 2 is the amplicon from gDNA of the wild-type control and lane 3 is the amplicon from gDNA of the putative ∆upp clone. The loss of 571 bp in the deletion clone (lane 3) was confirmed by DNA sequencing.
Table 1. Bacterial strains and plasmids used in this study.
| Strain designation | Description | Original Reference |
|---|---|---|
| E. coli EC1000 | Host for pORI plasmids, chromosomal repA+ (pWVO1), KnR | 41 |
| NCK1911 | Host for pTRK935, EmR | 15 |
| L. gasseri ATCC 33323 | Human intestinal isolate, neotype strain | 42 |
| NCK2092 | L. gasseri ATCC 33323 with pTRK669 | This study |
| NCK2253 | L. gasseri ATCC 33323 ∆upp, 5-FUR | This study |
| NCK2254 | NCK2253 with pTRK669 | This study |
| NCK2270 | NCK2253 with integrated pTRK1070, LTA- | This study |
| Plasmids | ||
| pORI28 | Broad host range non-replicative vector, EmR | 41 |
| pTRK669 | Temperature sensitive-helper plasmid, repA+, CmR | 37 |
| pTRK935 | pORI-based counterselective integration vector, P-upp, EmR | 15 |
| pTRK1065 | pORI28::∆upp, EmR | This study |
| pTRK1070 | pTRK935::∆ltaS, EmR | This study |
In L. acidophilus NCFM deficient in upp, counterselection was accomplished through plasmid-based complementation with the native P-upp cassette from the parent strain, facilitating 5-FU selection of clones devoid of the plasmid.15 Since the upp genes in L. gasseri and L. acidophilus exhibit high sequence identity, it was hypothesized that pTRK935, containing the P-upp cassette from L. acidophilus, would similarly complement the upp deletion in L. gasseri and thus confer a 5-FUS phenotype. To investigate the utility of pTRK935 for gene replacement in L. gasseri, NCK2254 was subsequently electroporated with pTRK935 and assessed for complementation by plating on GSDM supplemented with 5-FU. The pTRK935 containing clones of NCK2254 were sensitive to 5-FU and therefore, the L. acidophilus P-upp construct in pTRK935 successfully complemented, in trans, the upp deletion in L. gasseri. Complementation of the deletion and restoration of the 5-FUR phenotype following curing of the pTRK935 plasmid confirmed the utility of the integration vector by facilitating positive selection for plasmid-free excision recombinants in L. gasseri ATCC 33323.
Construction of L. gasseri NCK2270 ∆upp ltaS-, 5-FUR, EmR
In order to abolish LTA biosynthesis in L. gasseri NCK2253 for phenotypic analysis, the pTRK935 integration vector was employed to delete ltaS. LTA in lactobacilli structurally consists of a long hydrophobic tail, a carbohydrate moiety and a polymeric glycerol phosphate (poly-Gro-P) chain that is primarily substituted with d-alanine and glycosyl residues.18 The biosynthesis of LTA begins on the cytoplasmic side of the cell membrane, where glycosyltransferases attach various saccharides to diacylglycerol, dependent on the species.19 The glycolipid is then flipped to the extracellular surface of the cell membrane by the putative transmembrane LtaA flippase, where LtaS catalyzes the polymerization of the poly-Gro-P chain. On a separate locus in the chromosome, the dlt operon encodes enzymes that substitute d-alanine on the completed poly-Gro-P backbone to yield canonical LTA in lactobacilli. In L. acidophilus NCFM and L. gasseri ATCC 33323 the genes encoding the LTA biosynthetic enzymes are organized within a putative operon flanked by two putative rho-independent terminators. The putative operons in L. gasseri and L. acidophilus share conserved structural synteny (73% nucleotide sequence homology), containing two putative glycosyltransferases, a putative ltaA-encoded transmembrane “flippase” and an ItaS-encoded phosphoglycerol transferase. Notably, a gene encoding SecG is present in the LTA operon in L. acidophilus but was absent in L. gasseri. However, L. gasseri encodes SecG, albeit at an unrelated locus where it is flanked by rho-independent terminators. It is unknown whether SecG is involved in the regulation, synthesis, or export of LTA components. LGAS_1586 encodes for a putative phosphoglycerol transferase, which shared 78% predicted amino acid homology to LtaS in L. acidophilus NCFM and was targeted for deletion by allelic replacement to create an LTA deficient L. gasseri ATCC 33323 mutant.
The integration plasmid pTRK1070 was constructed by cloning a splicing by overlap extension (SOE)-PCR construct of 5′ and 3′ regions within the LGAS_1586 gene. The deletion construct contained two segments of sequences homologous to the coding sequence of ltaS at 631 bp and 640 bp, respectively, excluding internal 786 bp of the gene. Consequently, any homologous recombination of pTRK1070 in the chromosome resulted in an integration knockout of the putative phosphoglycerol transferase gene by disruption of the coding sequence with the potential for causing a frameshift or introducing a nonsense mutation. Gene replacement by excision of the plasmid was expected to result in an in-frame 786 bp deletion. Plasmid integrants were successfully recovered and PCR amplification of the integration junctions confirmed the insertion knockout of ltaS (LGAS_1586). Extensive screening for 5-FUR excision recombinants for allelic replacement failed to recover an ΔltaS deletion mutant. Interestingly, clones screened were 5-FUR, plasmid cured and Ems, but all contained a wild-type ltaS allele. This suggests that although knockout of ltaS by integration is not lethal, the selective pressure to maintain the wild-type genotype results in heavy skewing of the double recombinants to maintain the wild-type allele. Subsequently, a single clone with pTRK1070 integrated into ltaS was selected for phenotypic characterization and designated NCK2270. The NCK2270 mutant did not exhibit an aberrant growth phenotype when using standard propagation techniques, but appeared to have an elongated cellular morphology consistent with that observed from L. acidophilus NCFM ΔltaS (Fig. 2).17

Figure 2. Phase-contrast light microscopy images of L. gasseri ATCC 33323 Δupp (NCK2253) (left) and L. gasseri ATCC 33323 Δupp ltaS- (NCK2270) (right). NCK2253 displayed a cellular morphology similar to the wild-type, but NCK2270 appeared to be relatively elongated. Images were generated at 400× amplification.
Relative adhesion of NCK2270 to Caco-2 cell monolayers
The ltaS knockout mutant NCK2270 was phenotypically analyzed for adhesion to Caco-2 cell line monolayers to determine whether the ltaS knockout would alter the capacity of L. gasseri ATCC 33323 to adhere to intestinal epithelial cells in vitro. Approximately 1 × 108 cfu stationary phase bacterial cells were incubated with Caco-2 monolayers for 1 h at 37 °C and 5% CO2. The ltaS knockout mutant exhibited a 41% decrease in adhesion to the Caco-2 cell monolayer (Fig. 3). The decrease in adhesion observed in the NCK2270 ltaS knockout indicates that LTA plays a role in adhesion of wild-type L. gasseri ATCC 33323 to intestinal epithelial cells, in vitro.
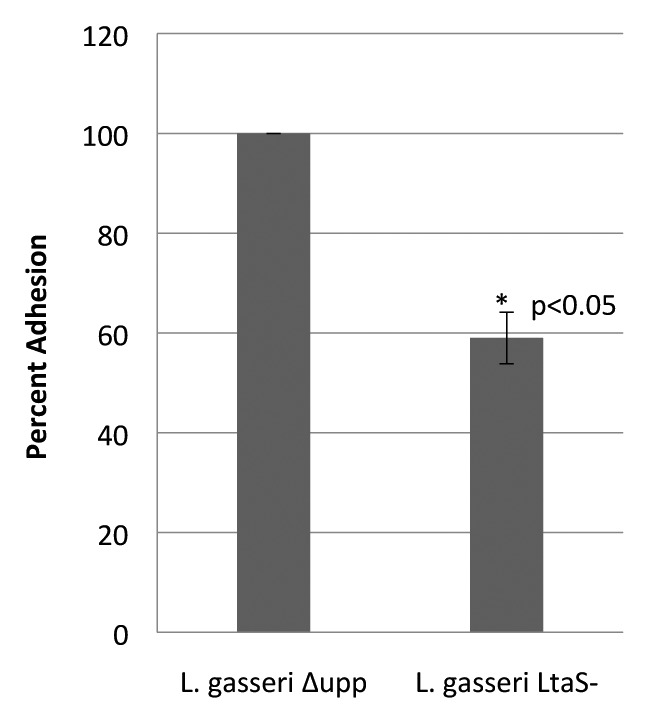
Figure 3. Adhesion of ltaS deficient L. gasseri to Caco-2 cell line. Adherence capacity of L. gasseri ∆upp ltaS- to the Caco-2 intestinal epithelial cell monolayer in vitro. Results are presented as percentage of adhesion when compared with the parent strain, NCK2253. The mean percentage of adhesion was calculated based on three replicates from three independent experiments. Student's t test was used to determine statistical significance (α = 0.05).
Discussion
L. gasseri is an autochthonous microbe inhabiting the human mucosa which likely exerts benefits to health as a member of the commensal microbiota. As a member of the acidophilus-complex, this Lactobacillus species has been used extensively in a number of industrial applications as both a dairy starter culture and a probiotic. Comparative genome analysis of L. gasseri ATCC 33323 offers insights into the genotypes that potentially contribute to colonization of the human GIT and serves to guide targeted functional genomic analysis for investigating genes of interest.
In this study, a deletion within the upp gene (LGAS_1245) of L. gasseri was accomplished in order to develop a genetic platform for allelic replacement in this important commensal species. Previously, the validity of using an upp-deficient parent strain for phenotypic analysis was thoroughly investigated in L. acidophilus NCFM.15 The upp deficient L. acidophilus NCFM derivative was not phenotypically disparate from the parent wild-type in growth, cell morphology, or resistance to stress. Furthermore, microarray expression analysis confirmed that the ∆upp mutant did not exhibit any significant differential expression relative to the parent strain. Genes successfully deleted using this gene replacement strategy in L. acidophilus NCFM include surface-layer (S-layer) protein X, (LBA0512), aggregation promoting factor (LBA0493), myosin cross-reactive antigen (LBA0649), and lactose permease (LBA1463).15,20-22 Establishing this system in L. gasseri provides numerous advantages, notably the ability to positively select for excision recombinants yielding gene deletions or replacements. In concurrent efforts, this system was used to successfully and efficiently delete the sortase A gene (srtA) from L. gasseri (Call et al., submitted). Markerless allelic replacement facilitated by the upp-based counterselective system also allows for stable chromosomal expression of recombinant proteins, with potential applications for food grade expression of biotherapeutics or subunit vaccines.23 Site-directed homologous recombination of gene cassettes downstream of specific promoters allows design for constitutive or inducible expression of the encoded protein at various levels.24 This method offers advantages over plasmid-based expression of recombinant proteins, which requires antibiotic selection and yields unstable expression in vivo.
Knockout of LTA synthase, a phosphoglycerol transferase enzyme catalyzing the polymerization of the Gro-P backbone, abolishes the activity of LTA, resulting in pleiotropic effects on bacterial physiology and bacterial-mammalian cell interactions.17 Specifically, deficiency in biosynthesis of LTA contributes to an elongated cellular morphology in rod-shaped Gram-positive bacteria due to aberrant septa formation. LTA localizes at the septa during cell division and regulates autolysins via direct and indirect mechanisms.25 In this study a knockout insertion mutant was created in ltaS in order to investigate phenotypic outcomes of this mutation in L. gasseri. Insertion mutagenesis has been broadly and historically employed as a tool for efficient generation of gene knockouts for functional analysis. Integration of pTRK1070 was predicted to result in disruption of the coding sequence by the linearized vector, with the likely possibility of causing either frameshift or nonsense mutations. However, extensive efforts to delete the ltaS gene were unsuccessful, for unknown reasons. The study was continued using the insertion knockout of ltaS for functional and phenotypic analysis.
The L. gasseri ltaS knockout mutant displayed an altered cellular morphology, but growth was not deficient under standard propagation techniques. The mutant also exhibited a decrease in adhesion to Caco-2 monolayers relative to the parent strain. This is in accordance with previous studies indicating the role of LTA in adhesion of lactobacilli to mammalian cells.26 LTA may contribute to adhesion to epithelial cells by lactobacilli through a few proposed mechanisms. Electrostatic interaction as a general mechanism of adhesion is supported by the decrease of adhesion observed with increased anionic charge of dlt mutants deficient in d-alanine substitution.27 Inactivation of the dlt operon abrogated d-alanine substitution in Lactobacillus plantarum NCIMB8826, which is compensated for by increases in glycosyl residue substitution on the poly-Gro-P backbone, thus decreasing the presence of cationic charges.27 Moreover, pathogenic dlt knockout derivatives exhibited decreased adhesion to mammalian cells which further substantiates the role of d-alanine substitution in adherence capacity.28,29 However, deletion of the phosphoglycerol transferase gene, which potentially abolishes the poly-Gro-P backbone would eliminate both the cationic and anionic charges associated therewith. Thus, the inhibited adhesion phenotype observed in ltaS knockout mutants could be attributed to the lowered cation content on the cell surface or be suitably explained by the general removal of charge from the cell surface. Interestingly, the random distribution of d-alanine observed in other lactobacilli marginally precludes the notion of localized areas of net cationic or anionic nature that would facilitate electrostatic attraction.30,31 This is in contrast with other organisms that display a gradient of substitution based on chain length that may exhibit localized regions of net negative or net positive charges.32-34 Considerable work in elucidating the relationship of cell surface molecules and the structure of LTA as they relate to adhesion is required to adequately explain the mechanisms responsible for the altered adherence phenotype in organisms expressing modified LTA.
The upp counterselection system has been rigorously applied for gene replacement in L. acidophilus NCFM as a platform for efficient generation of deletions in key genetic determinants conferring probiotic activity. Thus the same method was established in the closely related but phenotypically distinct L. gasseri by deleting the native upp gene to create a suitable host for the counterselectable integration vector, pTRK935. The system was successfully employed to efficiently delete the srtA gene in L. gasseri (Call et al., submitted). In this study, the system was used successfully to achieve an integration knockout of ltaS, but attempts to recover a mutant with a complete deletion in ltaS were unsuccessful. The ltaS integration mutant exhibited an elongated cellular morphology and decreased adhesion to Caco-2 intestinal epithelial cells. Collectively, the results establish an upp-counterselection gene replacement system in L. gasseri, and also highlight important physiological roles of LTA in this microbe and its potential implications for human interactions as a commensal bacterium.
Materials and Methods
Bacterial strains
All bacterial strains and plasmids are listed in Table 1. Bacterial cultures were cryopreserved in their respective media with a 15% (vol/vol) glycerol concentration and stored at -80 °C. L. gasseri was propagated in de Man, Rogosa and Sharpe (MRS) (Difco Laboratories, Inc.) broth under statically aerobic conditions at 37 °C, or on MRS agar (1.5% wt/vol agar, Difco) incubated anaerobically at 37 °C for 48 h. Concentrations of 2 µg/mL of erythromycin (Em) (Sigma-Aldrich) and 5 µg/mL of chloramphenicol (Cm) (Sigma) were used for plasmid selection in L. gasseri ATCC 33323, when appropriate. Selection for 5-FU resistant L. gasseri was performed by supplementing GSDM35 agar with a final concentration of 100 µg/mL of 5-FU (Sigma). Escherichia coli EC1000 was propagated aerobically in Luria-Bertani (Difco) broth at 37 °C with shaking, or on brain-heart infusion (Difco) solid medium supplemented with 1.5% agar. Antibiotic selection of E. coli was maintained with 40 µg/mL of kanamycin (Kn) and 150 µg/mL of Em for recombinant E. coli, when appropriate. L. gasseri deficient in LTA and the parent strain were propagated overnight in MRS broth at 37 °C, subcultured at a 1% (vol/vol) inoculum in fresh medium and incubated for 16 h. The cell pellet was vortexed to resuspend the cells and morphology was visualized using a Nikon Eclipse E600 phase contrast microscope with a Q-Imaging Micropublisher Camera attachment.
DNA isolation, manipulation, and transformation
All kits, enzymes, and reagents were used according to the manufacturers' instructions. DNA purification and cloning were performed as previously described.15 Purification of genomic DNA from L. gasseri employed a ZR Fungal/Bacterial MiniPrep kit (Zymo Research Corp). Plasmid DNA was isolated from E. coli using Qiagen Spin miniprep kit (Qiagen Inc). High fidelity PCR amplification of DNA was performed with PfuUltra II Fusion HS DNA polymerase (Stratagene Corp). Routine PCRs were conducted with Choice-Taq Blue polymerase (Denville Scientific Inc). Primers for PCR amplification were purchased from Integrated DNA Technologies and are listed in Table S1. DNA amplicons were separated using 0.8% agarose gel electrophoresis and stained with ethidium bromide for visualization. DNA extraction from agarose gels was performed with a Zymoclean Gel DNA Recovery kit (Zymo Research Corp). Restriction endonucleases BamHI and SacI and corresponding buffers were acquired from Roche Molecular Biochemicals. Ligations were performed with New England Biolabs Quick Ligation kit. Sequencing was performed by Davis Sequencing Inc. Rubidium chloride competent E. coli cells were prepared as previously described and frozen at -80 °C.36 Newly constructed integration plasmids were sequenced across the multiple cloning site containing the insert to ensure fidelity and subsequently electroporated into competent L. gasseri containing the temperature-sensitive helper plasmid, pTRK669, according to methods described previously.37,38 Penicillin G at a concentration of 10 µg/mL was employed in the preparation of the competent cells to promote electroporation efficiency.39
Construction of upp deletion in L. gasseri
Primers were designed for amplification of genomic regions flanking the putative upp gene in L. gasseri ATCC 33323. The upstream and downstream regions were 579 bp and 580 bp, respectively, resulting in a 571 bp in-frame deletion. The flanking PCR amplicons were purified and subsequently combined using splicing overlap extension (SOE)-PCR,40 resulting in two homologous sequences to regions flanking the upp gene in the host chromosome. Restriction endonuclease sites were incorporated into the 5′ ends of the primers to enable forced directional cloning into pORI28, resulting in the integration vector pTRK1065. The pTRK1065 plasmid was electroporated into competent NCK2092, an ATCC 33323 derivative harboring the temperature-sensitive helper plasmid pTRK669 with repA, provided in trans for replication of the pORI-based integration plasmid. Transformants were selected on MRS agar with 2 µg/mL Em and 5 µg/mL Cm and confirmed by PCR with plasmid specific primers. Confirmed transformants were inoculated into MRS broth with the appropriate antibiotic selection and then subcultured three times at a 1% inoculum into MRS with Em selection for the integration plasmid, but without Cm selection for pTRK669, at 42 °C. Loss of the helper plasmid occurred upon propagation at the non-permissive plasmid replication temperature of 42 °C. The culture was subsequently diluted and plated for isolation on MRS with 2 µg/mL Em, after which colonies were replica plated on MRS supplemented with 2 µg/mL Em and MRS with 5 µg/mL Cm. Clones devoid of the helper plasmid which exhibited a EmR, CmS phenotype, were subsequently screened by PCR for the absence of the repA amplicon from pTRK669. Colonies exhibiting a EmR, CmS phenotype and no repA amplicon were inoculated into broth culture containing Em and their genomic DNA were purified. Chromosomal integration of pTRK1065 was confirmed by PCR amplification of the integration junctions using primers rooted in the genome and the Erm gene on the plasmid. Confirmed integrants were subsequently subcultured into MRS without Em selection to allow resolution and excision of pTRK1065. Clones were replica plated onto MRS and MRS with Em to screen for EmS colonies, which were then screened by PCR with primers flanking the deletion junction to confirm deletion of the upp gene. Positive clones were sequenced at the deletion junction to ensure fidelity.
Knockout mutation of ltaS in L. gasseri
Construction of the integration plasmid for deletion of ltaS was performed essentially as described above, with minor exceptions. Specifically, the homologous regions in the integration plasmid were amplified from within the ltaS gene, rather than flanking it, such that any recombination would result in an insertional knockout. The two regions within the gene that were amplified were 631 bp on the 5′ and 640 bp on the 3′ end, respectively, resulting in an in-frame deletion of 786 bp. The construct was cloned into the previously created pTRK93515 to create pTRK1070, which was electroporated into the Δupp deletion background host (see above) harboring pTRK669, NCK2254 (Table 1). Following integration of the plasmid into the host chromosome by site-directed recombination, the EmR culture was propagated without antibiotic selection, and subsequently plated on GSDM supplemented with 5-FU to select for excision recombinants.
Adhesion to Caco-2 epithelial cell line
All cell culture media and reagents were obtained from Gibco (Gibco-Invitrogen Corp). Caco-2 cell line ATCC HTB-37 was purchased from the American Type Culture Collection and maintained in minimal essential medium (MEM) supplemented with 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, 100 µg/mL penicillin, and 0.25 µg/mL amphotericin. Cells were cultivated in 5% CO2 at 37 °C and monolayers were prepared by seeding approximately 6.5 × 104 cells per well with 2 mL of MEM in 12 well plates.15 The MEM was replaced every two days and the monolayers were used for assays 21 d post-seeding. Cells were used between passage 23 and 40. Prior to the addition of bacterial cells, the monolayers were washed twice with 1 mL of 37 °C phosphate-buffered saline (PBS) (pH 7.4) after which 1 mL of warm MEM with no antibiotic supplementation was added to each well. The monolayers were incubated at 37 °C and 5% CO2 prior to co-incubation with bacterial cells. For preparation of bacterial cells, lactobacilli were cultivated in MRS broth from a frozen stock and subcultured once overnight at a 1% inoculum, subsequently subcultured, and incubated for 16 h. Stationary phase cells were harvested by centrifugation at 10 000 x g for 2 min, the supernatant discarded and washed once in room temperature PBS. Cultures were centrifuged again and resuspended in PBS. The optical density of the cell suspension was measured at 600 nm and diluted in PBS to achieve approximately 1 × 108 cfu/mL for each strain. One mL of the bacterial suspension was then added to the monolayers in triplicate and co-incubated for 1 h at 37 °C and 5% CO2. The monolayers were then washed five times with 37 °C PBS. Finally, 1 mL of 0.05% (vol/vol) Triton X-100 was added to each well and incubated for 10 min with agitation. Monolayers were disrupted by pipetting, removed, and vortexed. The cell suspension was plated on MRS using a Whitley automatic spiral plater (Don Whitley Scientific Ltd). Following incubation, colonies were enumerated using a ProtoCOL (Synoptics Ltd) colony counter to determine the number of adhered cells.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This research was supported by the North Carolina Agricultural Foundation and Danisco USA/Dupont Nutrition and Health. Kurt Selle was partially supported by the 2013–2014 Dannon Probiotics Fellow Program (The Dannon Company, Inc) during the later stages of this work. We gratefully acknowledge Akinobu Kajikawa, Emma Call, Rosemary Sanozky-Dawes, Evelyn Durmaz, and Brant Johnson for their counsel, technical support and scientific discussions.
References
- 1.Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O’Flaherty S, Buck BL, Dobson A, Duong T, et al. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl Environ Microbiol. 2008;74:4610–25. doi: 10.1128/AEM.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Bello F, Hertel C. Oral cavity as natural reservoir for intestinal lactobacilli. Syst Appl Microbiol. 2006;29:69–76. doi: 10.1016/j.syapm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, Temmerman M, Vaneechoutte M. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol. 2007;7:115. doi: 10.1186/1471-2180-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado S, Suárez A, Mayo B. Dominant cultivable Lactobacillus species from the feces of healthy adults in northern Spain. Int Microbiol. 2007;10:141–5. [PubMed] [Google Scholar]
- 5.Hernández-Rodríguez C, Romero-González R, Albani-Campanario M, Figueroa-Damián R, Meraz-Cruz N, Hernández-Guerrero C. Vaginal microbiota of healthy pregnant Mexican women is constituted by four Lactobacillus species and several vaginosis-associated bacteria. Infect Dis Obstet Gynecol. 2011;2011:851485. doi: 10.1155/2011/851485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hojo K, Mizoguchi C, Taketomo N, Ohshima T, Gomi K, Arai T, Maeda N. Distribution of salivary Lactobacillus and Bifidobacterium species in periodontal health and disease. Biosci Biotechnol Biochem. 2007;71:152–7. doi: 10.1271/bbb.60420. [DOI] [PubMed] [Google Scholar]
- 7.Kiss H, Kögler B, Petricevic L, Sauerzapf I, Klayraung S, Domig K, Viernstein H, Kneifel W. Vaginal Lactobacillus microbiota of healthy women in the late first trimester of pregnancy. BJOG. 2007;114:1402–7. doi: 10.1111/j.1471-0528.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 8.Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol. 2001;2:43–53. [PubMed] [Google Scholar]
- 9.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A. 2005;102:2880–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh T, Fujimoto Y, Kawai Y, Toba T, Saito T. Inhibition of food-borne pathogenic bacteria by bacteriocins from Lactobacillus gasseri. Lett Appl Microbiol. 1995;21:137–41. doi: 10.1111/j.1472-765X.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 11.Stoeker L, Nordone S, Gunderson S, Zhang L, Kajikawa A, LaVoy A, Miller M, Klaenhammer TR, Dean GA. Assessment of Lactobacillus gasseri as a candidate oral vaccine vector. Clin Vaccine Immunol. 2011;18:1834–44. doi: 10.1128/CVI.05277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonyaritichaikij S, Kuwabara K, Nagano J, Kobayashi K, Koga Y. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter. 2009;14:202–7. doi: 10.1111/j.1523-5378.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- 13.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24:481–91. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Margreiter M, Ludl K, Phleps W, Kaehler ST. Therapeutic value of a Lactobacillus gasseri and Bifidobacterium longum fixed bacterium combination in acute diarrhea: a randomized, double-blind, controlled clinical trial. Int J Clin Pharmacol Ther. 2006;44:207–15. doi: 10.5414/CPP44207. [DOI] [PubMed] [Google Scholar]
- 15.Goh YJ, Azcárate-Peril MA, O’Flaherty S, Durmaz E, Valence F, Jardin J, Lortal S, Klaenhammer TR. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2009;75:3093–105. doi: 10.1128/AEM.02502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, Blatner NR, Owen JL, Klaenhammer TR, Mohamadzadeh M. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2012;109:10462–7. doi: 10.1073/pnas.1207230109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4623–30. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebeer S, Claes IJJ, Vanderleyden J. Anti-inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol. 2012;20:5–10. doi: 10.1016/j.tim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Rahman O, Dover LG, Sutcliffe IC. Lipoteichoic acid biosynthesis: two steps forwards, one step sideways? Trends Microbiol. 2009;17:219–25. doi: 10.1016/j.tim.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JM, Barrangou R, Abou Hachem M, Lahtinen S, Goh YJ, Svensson B, Klaenhammer TR. Transcriptional and functional analysis of galactooligosaccharide uptake by lacS in Lactobacillus acidophilus. Proc Natl Acad Sci U S A. 2011;108:17785–90. doi: 10.1073/pnas.1114152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh YJ, Klaenhammer TR. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2010;76:5005–12. doi: 10.1128/AEM.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Flaherty SJ, Klaenhammer TR. Functional and phenotypic characterization of a protein from Lactobacillus acidophilus involved in cell morphology, stress tolerance and adherence to intestinal cells. Microbiology. 2010;156:3360–7. doi: 10.1099/mic.0.043158-0. [DOI] [PubMed] [Google Scholar]
- 23.Douglas GL, Goh YJ, Klaenhammer TR. Integrative food grade expression system for lactic acid bacteria. Methods Mol Biol. 2011;765:373–87. doi: 10.1007/978-1-61779-197-0_22. [DOI] [PubMed] [Google Scholar]
- 24.Douglas GL, Klaenhammer TR. Directed chromosomal integration and expression of the reporter gene gusA3 in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2011;77:7365–71. doi: 10.1128/AEM.06028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichmann NT, Gründling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granato D, Perotti F, Masserey I, Rouvet M, Golliard M, Servin A, Brassart D. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl Environ Microbiol. 1999;65:1071–7. doi: 10.1128/aem.65.3.1071-1077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102:10321–6. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, Trieu-Cuot P. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol Microbiol. 2002;43:1–14. doi: 10.1046/j.1365-2958.2002.02723.x. [DOI] [PubMed] [Google Scholar]
- 29.Fittipaldi N, Sekizaki T, Takamatsu D, Harel J, Domínguez-Punaro MdeL, Von Aulock S, Draing C, Marois C, Kobisch M, Gottschalk M. D-alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis. Infect Immun. 2008;76:3587–94. doi: 10.1128/IAI.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batley M, Redmond JW, Wicken AJ. Nuclear magnetic resonance spectra of lipoteichoic acid. Biochim Biophys Acta. 1987;901:127–37. doi: 10.1016/0005-2736(87)90264-1. [DOI] [PubMed] [Google Scholar]
- 31.Childs WC, 3rd, Taron DJ, Neuhaus FC. Biosynthesis of D-alanyl-lipoteichoic acid by Lactobacillus casei: interchain transacylation of D-alanyl ester residues. J Bacteriol. 1985;162:1191–5. doi: 10.1128/jb.162.3.1191-1195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer W. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography, exemplified with lipoteichoic acids. Anal Biochem. 1993;208:49–56. doi: 10.1006/abio.1993.1007. [DOI] [PubMed] [Google Scholar]
- 33.Fischer W. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography. J Microbiol Methods. 1996;25:129–44. doi: 10.1016/0167-7012(96)00009-7. [DOI] [Google Scholar]
- 34.Leopold K, Fischer W. Heterogeneity of lipoteichoic acid detected by anion exchange chromatography. Arch Microbiol. 1992;157:446–50. doi: 10.1007/BF00249103. [DOI] [PubMed] [Google Scholar]
- 35.Kimmel SA, Roberts RF. Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii ssp. bulgaricus RR. Int J Food Microbiol. 1998;40:87–92. doi: 10.1016/S0168-1605(98)00023-3. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D. 1985. Techniques for transformation of E. coli, p. 109-135.In D. M. Glover (ed.), DNA cloning: a practical approach, vol. 1. IRL Press Ltd., Oxford, England. [Google Scholar]
- 37.Russell WM, Klaenhammer TR. Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl Environ Microbiol. 2001;67:4361–4. doi: 10.1128/AEM.67.9.4361-4364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker DC, Aoyama K, Klaenhammer TR. Electrotransformation of Lactobacillus acidophilus group A1. FEMS Microbiol Lett. 1996;138:233–7. doi: 10.1111/j.1574-6968.1996.tb08163.x. [DOI] [PubMed] [Google Scholar]
- 39.Wei M-Q, Rush CM, Norman JM, Hafner LM, Epping RJ, Timms P. An improved method for the transformation of Lactobacillus strains using electroporation. J Microbiol Methods. 1995;21:97–109. doi: 10.1016/0167-7012(94)00038-9. [DOI] [Google Scholar]
- 40.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 41.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer E, , Kandler O. Lactobacillus gasseri sp. nov., a new species of the subgenus Thermobacterium. . . . . . Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Orig. C. 1980;;1:75–78. doi: 10.1016/0378-1119(89)90359-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


