Abstract
Helicobacter pylori chronically persists in 50% of the human population and causes serious gastric and duodenal pathologies in 15% of infected people. Research on the immune response to the infection has mainly focused on the induction of CD4+ T cell responses. Human studies emphasize the potential clinical relevance of CD8+ cytotoxic T lymphocytes, however this cell type has barely been reported in studies employing mouse or gerbil models. Traditionally characterized as an extracellular bacterium, H. pylori has been identified inside epithelial and immune cells. Similarly to other intracellular bacteria, H. pylori infection of macrophages can alter autophagy and phagosome processing. A novel animal model of H. pylori infection demonstrates for the first time the induction of cytotoxic CD8+ T cell responses in pigs and localization of intracellular H. pylori within lymphoid aggregates. Here, we discuss novel mechanisms of host-H. pylori interactions that could lead to the induction of cytotoxic responses.
Keywords: Helicobacter pylori, CD8 T cells, intracellular bacteria, pig models, autophagy, immunohistochemistry, gastritis, Tbet, gastric epithelial cells, macrophages
Introduction
Helicobacter pylori is a gram negative, spiral-shaped bacterium with a unique capacity to colonize and survive in the human gastric mucosa. Approximately 50% of the human population carries this bacterium, however there are substantial differences regarding its geographic distribution. In Asia and Africa, the prevalence can reach 90%. In contrast, only 20% of the population in North America and Western Europe are colonized by H. pylori, which is the result of changes in hygiene, lifestyle habits, and the generalized use of antibiotics.1 Most H. pylori carriers develop chronic superficial gastritis that does not lead to severe disease, but 15% of infected individuals will develop serious gastric and duodenal pathologies. The main diseases caused by H. pylori are peptic and duodenal ulcers, gastric adenocarcinoma and gastric lymphoma. In fact, H. pylori comprises the main risk factor in 60–80% of stomach cancers, which is the fourth most common form of cancer worldwide.2,3 Conversely, there is increasing evidence that the presence of H. pylori protects against esophageal and cardial pathologies,4-7 childhood asthma,8,9 childhood allergies,10 and inflammatory bowel disease.11
Whether H. pylori exerts protective effects in the context of a dysregulated immune response or whether it contributes to cell damage and malignant transformation is dependent on various host and pathogen-related factors, including the host’s genetic background, age, and immune status, and the bacterium’s ability for antigenic variation, molecular mimicry, intracellular persistence, and expression of pathogenicity factors.12 Two of the most important pathogenicity factors of H. pylori are the effector protein CagA, which is part of the cag (cytotoxin-associated gene) pathogenicity island (PAI) and the secreted toxin vacuolating cytotoxin A (VacA). Infection with strains bearing the cag PAI has been associated with the development of peptic ulcer disease, gastric lymphoma, and gastric adenocarcinoma.13 VacA can be endocytosed into activated human primary T cells14 where it inhibits cell proliferation and thus the clonal expansion of H. pylori antigen specific T-cells15,16 contributing to immune evasion. Furthermore, it exists in different isoforms, which differ in their cytotoxin activity and the associated risk for developing gastroduodenal disease.16
Immune Response to Helicobacter pylori
The hallmark of the immune response to H. pylori in humans is the infiltration of the gastric mucosa by T helper (Th) 1, regulatory T cells (Treg), and neutrophils.17 This inflammatory response is unsuccessful in clearing the bacteria from the stomach and can lead to more severe immunopathology.18 Adaptive immune responses to H. pylori have been extensively studied in mouse and gerbil models of infection. Similar to humans, the animal models of H. pylori infection are characterized by the induction of mixed CD4+ T cell responses mediated by Th1, Th17, and regulatory T cell (Treg) subsets, which also fail to eradicate the bacterium.19 In recent years data from human clinical studies emphasize the potential relevance of another T cell subset, the CD8+ cytotoxic T lymphocytes, in the context of the immune responses to H. pylori. However, the development of CD8+ T cell responses has barely been reported in studies employing mouse or gerbil models of infection. This could be because the response has been unnoticed, perhaps because CD4+ T cells outnumber CD8+ T cells in the gastric mucosa, or due to species-specific differences, where mice and gerbils do not easily mount a CD8+ T cell response following colonization with H. pylori.
In contrast to human H. pylori infections, the relevance of CD8+ T cells during the host response to H. pylori in mice has been mostly described in the absence of CD4+ T cells.20 Results from studies with H. pylori infected GK1.5 mice21,22 and H. felis infected MHC-II−/− mice,22 both lacking CD4+ T cells, show elevated levels of gastric CD8+ T cells that contribute to the development of severe gastric lesions, a property traditionally attributed to effector CD4+ T cells.23,24 Immunization studies using MHC-II−/− mice demonstrate no protective effect against H. pylori infection and high frequencies of CD8+ T cells in the stomach.25 These results indicate that CD8+ T cells are main contributors to gastritis and CD4+ T cells exert a dominant regulatory role thereby limiting the severity of infection. Indeed, clinical studies in humans, both in children and adults, suggest the association between H. pylori colonization with increased CD8+ T cells and development of gastric ulcers.26-28 In addition, human patients with fewer functional Treg cells are more likely to develop peptic ulcers and are afflicted by more intense gastritis.16 Only recently, Ruiz et al.29 demonstrated the increased infiltration of CD8+ T cells into the gastric mucosa of H. pylori infected immunocompetent mice, which were characterized by their ability to produce IFNγ.
A New Pig Model of Helicobacter pylori Infection
We have recently developed a novel pig model of H. pylori infection. In two independent studies we found a reproducible induction of a Th1 response followed by a cytotoxic T cell response. This response was induced by both J99 and SS1 H. pylori strains. Analysis of the systemic response over a 50-day period showed initial expansion of CD4+Tbet+ cells, pointing toward the development of a Th1 response, which is consistent with what has been previously reported in mouse models. Interestingly, this CD4+ T cell response preceded the expansion of CD8+Tbet+ T cells. Of note, we recorded that on day 35 post-infection up to 80% of circulating CD8+ T cells in infected pigs expressed this transcription factor compared with only 10% on average in the control non-infected group. These phenotypic changes correlated with upregulation of IFNγ, and markers of cytotoxic function like granzyme A, B, perforin, and CD16.30
Similar to H. pylori-mediated chronic gastritis in humans, our pig model shows that bacteria persist in the pig stomach at the expense of lesion development. One of the main features observed in this novel H. pylori pig model was the development of numerous large tertiary structures consisting of lymphoid aggregates in the stomach mucosa.30 These changes were described in the stomachs of humans that were experimentally infected with H. pylori, and were still detectable, although of smaller size, after antibiotic therapy to eliminate the bacteria.28 Moreover, a recent study describes the recruitment of DC-LAMP+ dendritic cells to gastric lymphoid follicles in stomach specimens obtained from H. pylori carriers. These cells were in close proximity to Foxp3+ cells and thus it is suggested that lymphoid follicles might be important sites where immune responses to H. pylori are regulated.31 Comparable to immune responses observed in humans, our pig model also shows the infiltration of Foxp3+ T cells into the gastric mucosa.30
Overall these findings corroborate that the pig model of H. pylori infection closely resembles human pathology. Thus, it has the potential to shed new light on host-H. pylori interactions and help developing novel treatment modalities. Especially our findings on CD8+ T cells raise several questions and will require further investigation. The first and most relevant aspect is what is the role of CD8+ T cells and how do they contribute to the pathogenesis of H. pylori-induced gastric disease, particularly to gastritis and ulcer development. The second is how does H. pylori induce MHC-I restricted immune responses. Of notable interest is the identification of the antigenic determinants from H. pylori that are recognized by CD8+ T cells and the pathways involved in processing and presentation of H. pylori antigens through the MHC-I pathway. Finally, are there differences among H. pylori strains in their ability to induce CD8+ responses?
While additional work will be needed to provide answers to these questions, a literature review and our own data provide support for the induction of cytotoxic responses to H. pylori.
Helicobacter pylori as Intracellular Pathogen
Traditionally, H. pylori has been considered an extracellular bacterium found as free-swimming in the mucus lining of the stomach or in close association with gastric epithelial cells.20 More recent data suggest that H. pylori can survive inside cells. Several studies have demonstrated that H. pylori can persist in hepatocytes and replicate in macrophages, bone marrow-derived dendritic cells,32 and gastric epithelial cells in vitro, thus providing evidence for its role as facultative intracellular organism with the ability to reside, replicate, and successfully evade antibiotic therapy within host cells.33 Recent in vivo studies have further strengthened the role of H. pylori as an intracellular pathogen in mice and humans. More specifically, H. pylori was not only localized to murine gastric epithelial progenitor cells34 but was also identified in human tissue specifically residing within metaplastic, dysplastic, and neoplastic gastric epithelial cells, parietal cells, and lamina propria macrophages.35,36 Furthermore, H. pylori has been found in gastric lymph nodes suggesting lymphatic dissemination37 and providing in vivo evidence that H. pylori can spread beyond the gastric mucosa most likely within migratory phagocytic cells.
Special attention has been given to the infection of epithelial and phagocytic cells by H. pylori. Although the mechanisms of invasion are not well understood, it was determined that in epithelial cells the process was dependent on c-Met and the Type IV secretion system.38 Recent findings suggest that H. pylori survives in the intracellular environment by manipulating phagosome and autophagy maturation.32,39 It has been shown that strains carrying s1 isoforms of VacA have a dual effect on autophagy by promoting it in initial phases of contact with epithelial cells, although prolonged exposure to the toxin results in the disruption of autophagosome maturation.40 Mechanistic insights on the molecular pathways affecting autophagosome formation, maturation, and degradation, and how these are manipulated by H. pylori are given in several recent publications.40-44 During chronic phases of infection, defects in components of the autophagosome machinery, such as ATG12 by MIR30B, or the presence of the ATG16L1*300A allele result in increased bacterial survival and persistence within cells.45 In primary human macrophages, virulent H. pylori strains can promote the formation of megasomes, large structures arising from homotypic fusion of phagosomes. Megasomes have limited degradative capacity and, consequently, H. pylori can survive for an extended time interval. These megasomes were localized by immunocytochemistry to the perinuclear region and differences were noted among strains with regards to the time of megasome formation.46 The presence of H. pylori within phagosomes does not explain the induction of cytotoxic responses since antigens originating form phagocytosed bacteria are mainly processed through the endocytic pathway for MHC-II presentation. In fact, there is no evidence linking phagosome and autosome manipulation with MHC-I antigen processing in H. pylori infections. However, a new mechanism of amphisomal route of MHC-I cross-presentation has been described in dendritic cells infected with Clamydia, another gram-negative intracellular bacteria.47 This particular mechanism of antigen processing requires the release of Clamydia into the cytoplasm after vacuole desintegration. In the cytoplasm clamydial antigens undergo proteosomal degradation and are subsequently transported into recycling endosomes where they are loaded into MHC-I peptides. It is well established that, through its Type IV secretion system, H. pylori can inject toxins like VacA and CagA directly into the cytosol.48 Thus, the development of cytotoxic responses could alternatively result from proteosomal degradation of H. pylori proteins released directly into the cytosolic compartment, rather than from the presence of H. pylori within cellular compartments. Also, CD8+ T cell responses could arise as a result of cross-presentation. In this regard, a study by Azem et al.49 demonstrates that H. pylori antigen pulsed B cells were able to elicit a CD8+ response via MHC-I through cross-priming. The majority of mucosal CD8+ cells (>80%) in gastric biopsies from H. pylori infected individuals were shown to be of a memory phenotype. The proliferative memory response of peripheral CD8+ cells to urease was significantly higher in infected vs. uninfected individuals. In addition, more than half of the H. pylori infected individuals showed strong memory responses to H. pylori lipoprotein A (HpaA) ex vivo which was not the case for cells from uninfected individuals. Binding of HpaA to TLR2 on NK cells has also been implicated in the induction of IFNγ secretion by NK cells.50,51
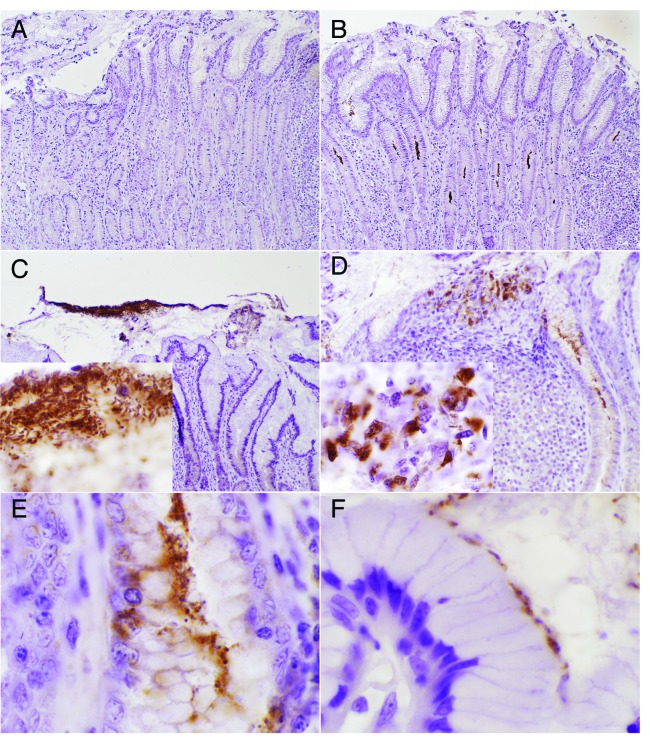
Macrophages are recruited to the gastric mucosa following H. pylori colonization. This process is a critical step in the acute response to the bacterium.52 In our pig model, H. pylori still persists in the stomach on day 50 post-infection. Bacterial localization was assessed by immunohistochemistry, and most of it was found in the mucus layer, or in the gastric pits (Fig. 1B and C). In addition, a smaller fraction of H. pylori organisms were localized in the lamina propria. Of particular interest was the presence of H. pylori within lymphoid aggregates (Fig. 1D), in an area close to the lumen, which is enriched in myeloid cells and lymphocytes. At larger magnification, H. pylori can be observed in the extracellular compartment as free swimming (Fig. 1C, insert) or overlaying the apical side of epithelial cells (Fig. 1F). However, the H. pylori found in lymphoid aggregates was intracellular (Fig. 1D, insert). In addition, we also observed some bacteria in the gastric pits invading an epithelial cell (Fig. 1E). Overall, these new data demonstrate an intimate association between the development of CD8+ T cell responses and the presence of a small fraction of H. pylori in the gastric mucosa within cells of the lamina propria and epithelial cells. Still, there is a need for a substantial effort ahead to directly demonstrate the presence of H. pylori-specific CD8+ T cells and the exact mechanisms by which these responses are induced.
Figure 1. Localization of Helicobacter pylori strain SS1 in the mucosa of the pig stomach at day 50 post-infection. Bacterial detection was performed in formalin fixed stomach sections stained with an anti-H. pylori polyclonal antibody from Cell Marqe, or with secondary only as a negative control (A). Most of the H. pylori was localized in the mucus layer and gastric pits (B, C), however, a small fraction was found in the lamina propria (E). At high power magnification (1000×) H. pylori can be observed in the extracellular compartment as free swimming (C, insert) or overlying the apical side of epithelial cells (E). Some H. pylori positive staining was detected in the intracellular compartment of cells in lymphoid aggregates (D, insert) and within epithelial cells (E). Original magnification, 100× (A, B), 200× (C, D), and 1000× (C, insert; D, insert; E and F).
Conclusions
Although traditionally viewed as extracellular bacterium, recent findings demonstrate the presence of H. pylori in the intracellular environment of epithelial and myeloid cells. In concordance with this, CD8+ T cell responses have been detected in infected humans. However, the role of CD8+ T cells in the immune response to H. pylori has been poorly characterized thus far. A novel animal model of H. pylori infection demonstrates for the first time the induction of cytotoxic CD8+ T cell responses in pigs and the presence of H. pylori within cells of the gastric mucosa. Recent findings on the interaction between H. pylori, gastric epithelial cells, and macrophages have unveiled the bacterium’s ability to interfere with intracellular processes such as autophagy, membrane trafficking, or phagosome maturation, which are crucial in antigen processing and presentation. However, mechanistic links between these molecular changes and the induction of CD8+ T cell responses remain elusive. A deeper mechanistic understanding of the immune responses induced following chronic colonization of the stomach with H. pylori in relevant models of infection is needed to accelerate the design of optimal interventions against H. pylori-associated pathologies.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by the NIAID Contract No. HHSN272201000056C to J.B.-R. and funds from the Nutritional Immunology and Molecular Medicine Laboratory.
References
- 1.Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter. 2008;13(Suppl 1):1–6. doi: 10.1111/j.1523-5378.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851–6. doi: 10.1046/j.1365-2036.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Permin H, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter infection. Helicobacter. 2005;10(Suppl 1):21–5. doi: 10.1111/j.1523-5378.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561–7. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am J Gastroenterol. 2000;95:2206–11. doi: 10.1111/j.1572-0241.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 6.Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–90. [PubMed] [Google Scholar]
- 7.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology 2010; 139:1894-901 e2; quiz e12. [DOI] [PubMed]
- 8.Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev Res (Phila) 2008;1:308–11. doi: 10.1158/1940-6207.CAPR-08-0170. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–7. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda S. Infection: Helicobacter pylori may protect against IBD--a mechanistic insight. Nat Rev Gastroenterol Hepatol. 2011;8:299. doi: 10.1038/nrgastro.2011.77. [DOI] [PubMed] [Google Scholar]
- 12.Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol. 2011;17:1383–99. doi: 10.3748/wjg.v17.i11.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peek RM, Jr., Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 14.Sewald X, Jiménez-Soto L, Haas R. PKC-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary T lymphocytes. Cell Microbiol. 2011;13:482–96. doi: 10.1111/j.1462-5822.2010.01551.x. [DOI] [PubMed] [Google Scholar]
- 15.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci U S A. 2004;101:7727–32. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 17.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 19.Zhang M, Liu M, Luther J, Kao JY. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes. 2010;1:325–9. doi: 10.4161/gmic.1.5.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan S, Noto JM, Romero-Gallo J, Peek RM, Jr., Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan MP, Pedersen J, Zhan Y, Lew AM, Pearse MJ, Wijburg OL, Strugnell RA. CD8+ T cells are associated with severe gastritis in Helicobacter pylori-infected mice in the absence of CD4+ T cells. Infect Immun. 2008;76:1289–97. doi: 10.1128/IAI.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui T, Nishio A, Okazaki K, Kasahara K, Saga K, Tanaka J, Uza N, Ueno S, Kido M, Ohashi S, et al. Cross-primed CD8+ cytotoxic T cells induce severe Helicobacter-associated gastritis in the absence of CD4+ T cells. Helicobacter. 2007;12:486–97. doi: 10.1111/j.1523-5378.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 23.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–61. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 24.Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Müller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182:7085–101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 25.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–41. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmin-Basa A, Michalkiewicz J, Gackowska L, Kubiszewska I, Eljaszewicz A, Mierzwa G, Bala G, Czerwionka-Szaflarska M, Prokurat A, Marszalek A. Pediatric Helicobacter pylori infection and circulating T-lymphocyte activation and differentiation. Helicobacter. 2011;16:27–35. doi: 10.1111/j.1523-5378.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 27.Kotłowska-Kmieć A, Bakowska A, Szarszewski A, Kamińska B, Łuczak G, Radys W, Landowski P, Brodzicki J, Korzon M, Liberek A. Helicobacter pylori increases expression of proapoptotic markers Fas and FasL on CD4 lymphocytes in children. Acta Biochim Pol. 2009;56:433–8. [PubMed] [Google Scholar]
- 28.Graham DY, Opekun AR, Osato MS, El-Zimaity HM, Lee CK, Yamaoka Y, Qureshi WA, Cadoz M, Monath TP. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235–43. doi: 10.1136/gut.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz VE, Sachdev M, Zhang S, Wen S, Moss SF. Isolating, immunophenotyping and ex vivo stimulation of CD4+ and CD8+ gastric lymphocytes during murine Helicobacter pylori infection. J Immunol Methods. 2012;384:157–63. doi: 10.1016/j.jim.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronsteiner B, Bassaganya-Riera J, Philipson C, Viladomiu M, Carbo A, Pedragosa M, Vento S, Hontecillas R. Helicobacter pylori infection in a pig model is dominated by Th1 and cytotoxic CD8+ T cell responses. Infect Immun. 2013;81:3803–13. doi: 10.1128/IAI.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansson M, Sundquist M, Hering S, Lundin BS, Hermansson M, Quiding-Järbrink M. DC-LAMP+ dendritic cells are recruited to gastric lymphoid follicles in Helicobacter pylori-infected individuals. Infect Immun. 2013;81:3684–92. doi: 10.1128/IAI.00801-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YH, Wu JJ, Lei HY. The autophagic induction in Helicobacter pylori-infected macrophage. Exp Biol Med (Maywood) 2009;234:171–80. doi: 10.3181/0808-RM-252. [DOI] [PubMed] [Google Scholar]
- 33.Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157–65. doi: 10.1128/IAI.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh JD, Karam SM, Gordon JI. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc Natl Acad Sci U S A. 2005;102:5186–91. doi: 10.1073/pnas.0407657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187:1165–77. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozbek A, Ozbek E, Dursun H, Kalkan Y, Demirci T. Can Helicobacter pylori invade human gastric mucosa?: an in vivo study using electron microscopy, immunohistochemical methods, and real-time polymerase chain reaction. J Clin Gastroenterol. 2010;44:416–22. doi: 10.1097/MCG.0b013e3181c21c69. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N, et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88:664–81. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira MJ, Costa AC, Costa AM, Henriques L, Suriano G, Atherton JC, Machado JC, Carneiro F, Seruca R, Mareel M, et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem. 2006;281:34888–96. doi: 10.1074/jbc.M607067200. [DOI] [PubMed] [Google Scholar]
- 39.Greenfield LK, Jones NL. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013;21:602–12. doi: 10.1016/j.tim.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–77. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A, Romero-Gallo J, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–71. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yahiro K, Satoh M, Nakano M, Hisatsune J, Isomoto H, Sap J, Suzuki H, Nomura F, Noda M, Moss J, et al. Low-density lipoprotein receptor-related protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. J Biol Chem. 2012;287:31104–15. doi: 10.1074/jbc.M112.387498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deen NS, Huang SJ, Gong L, Kwok T, Devenish RJ. The impact of autophagic processes on the intracellular fate of Helicobacter pylori: more tricks from an enigmatic pathogen? Autophagy. 2013;9:639–52. doi: 10.4161/auto.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terebiznik MR, Raju D, Vázquez CL, Torbricki K, Kulkarni R, Blanke SR, Yoshimori T, Colombo MI, Jones NL. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5:370–9. doi: 10.4161/auto.5.3.7663. [DOI] [PubMed] [Google Scholar]
- 45.Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang HG, Fang Y, Yu B, Zhang JY, Xie QH, et al. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy. 2012;8:1045–57. doi: 10.4161/auto.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borlace GN, Jones HF, Keep SJ, Butler RN, Brooks DA. Helicobacter pylori phagosome maturation in primary human macrophages. Gut Pathog. 2011;3:3. doi: 10.1186/1757-4749-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiegl D, Kägebein D, Liebler-Tenorio EM, Weisser T, Sens M, Gutjahr M, Knittler MR. Amphisomal route of MHC class I cross-presentation in bacteria-infected dendritic cells. J Immunol. 2013;190:2791–806. doi: 10.4049/jimmunol.1202741. [DOI] [PubMed] [Google Scholar]
- 48.Kim IJ, Blanke SR. Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA) Front Cell Infect Microbiol. 2012;2:37. doi: 10.3389/fcimb.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azem J, Svennerholm AM, Lundin BS. B cells pulsed with Helicobacter pylori antigen efficiently activate memory CD8+ T cells from H. pylori-infected individuals. Clin Immunol. 2006;118:284–91. doi: 10.1016/j.clim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Yun CH, Lundgren A, Azem J, Sjöling A, Holmgren J, Svennerholm AM, Lundin BS. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infect Immun. 2005;73:1482–90. doi: 10.1128/IAI.73.3.1482-1490.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindgren A, Pavlovic V, Flach CF, Sjöling A, Lundin S. Interferon-gamma secretion is induced in IL-12 stimulated human NK cells by recognition of Helicobacter pylori or TLR2 ligands. Innate Immun. 2011;17:191–203. doi: 10.1177/1753425909357970. [DOI] [PubMed] [Google Scholar]
- 52.Schumacher MA, Donnelly JM, Engevik AC, Xiao C, Yang L, Kenny S, Varro A, Hollande F, Samuelson LC, Zavros Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142:1150–9, e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]