Abstract
Citrobacter rodentium infection is a murine model of pathogenic Escherichia coli infection that allows investigation of the cellular and molecular mechanisms involved in host-protective immunity and bacterial-induced intestinal inflammation. We recently demonstrated that following C. rodentium infection, the absence of Resistin-Like Molecule (RELM) α resulted in attenuated Th17 cell responses and reduced intestinal inflammation with minimal effects on bacterial clearance. In this addendum, we investigated the cytokine modulatory effects of RELMα and RELMα expression in the intestinal mucosa following C. rodentium infection. We show that in addition to promoting Th17 cytokine responses, RELMα inhibits Th2 cytokine expression and Th2-cytokine effector macrophage responses in the C. rodentium-infected colons. Second, utilizing reporter C. rodentium, we examined RELMα expression and macrophage recruitment at the host pathogen interface. We observed infection-induced macrophage infiltration and RELMα expression by intestinal epithelial cells. The influence of infection-induced RELMα on macrophage recruitment in the intestine is discussed.
Keywords: Citrobacter, T helper 17, T helper 2, infection, inflammation, macrophage, resistin-like molecule α
Introduction
Mucosal surfaces, such as the intestine, are constantly exposed to the external environment, and development of a balanced immune response is essential to prevent pathogen invasion while controlling excessive or unnecessary inflammation. Notably, macrophages, which constitute a significant proportion of the leukocytes within the gut, serve as initiators to polarize immune effector or regulatory responses following a variety of infectious or inflammatory stimuli.1 In Citrobacter rodentium infection, a mouse model for enteropathogenic and enterhemorrhagic infections of humans,2 we recently showed that Resistin-Like Molecule (RELM) α promoted infection-induced intestinal inflammation via effects on macrophages.3
RELMα is a secreted protein that is most commonly associated with alternatively activated macrophages (AAMac), which are recruited in T helper type (Th) 2 cytokine-dominated environments, such as helminth infection and allergy.4-6 In Th2 cytokine-biased immune responses, RELMα exhibited critical immunomodulatory functions.7-9 In contrast, studies by Rothenberg and colleagues uncovered a pro-inflammatory function for RELMα in mouse models of inflammatory bowel disease.10,11
Our recent study focused on examining the role of RELMα in bacterial infection-induced inflammation.3 Following C. rodentium infection, we observed that in comparison to wild-type (WT) mice, RELMα−/− mice were protected from infection-induced intestinal inflammation. The ameliorated response observed in RELMα−/− mice was associated with reduced Th17 cell-derived IL-17A. The Th17 cell-associated immune response, characterized by the production of cytokines IL-17 and IL-22, is critical for host immunity to several gastrointestinal pathogens including C. rodentium, Helicobacter pylori, and Salmonella enterica.12-14 Infection-induced IL-17 promotes the recruitment of neutrophils and other effector cells, and IL-22 induces critical host defense mechanisms including anti-microbial peptide synthesis and mucus production.15-17 Dysregulated Th17 cell responses are also associated with multiple inflammatory disorders, such as multiple sclerosis and inflammatory bowel disease (IBD).18 Following C. rodentium infection, RELMα−/− mice successfully cleared C. rodentium despite decreased accumulation of Th17 cells, suggesting that RELMα-induced Th17 cell responses promoted immunopathology. We identified that one mechanism of RELMα-mediated regulation was through macrophage production of the Th17 polarizing cytokine IL-23. In this addendum, we investigated the implications of RELMα-mediated regulation by examining the local cytokine environment in the C. rodentium-infected intestine, and by measuring macrophage infiltration and RELMα expression in the C. rodentium-colonized mucosa. Following C. rodentium infection, we show that RELMα−/− mice exhibit significantly increased expression of Th2 cytokines and the Th2-effector AAMac gene Arg1 that encodes for arginase. Given the counterbalance between Th2 and Th17 cell activation,19 our data suggests that the RELMα-mediated stimulation of Th17 cell responses may be through the inhibition of Th2 immune cytokines. Second, we characterized RELMα expression and macrophage responses in the infected intestinal mucosa by immunofluorescent staining and green fluorescent protein (GFP) reporter C. rodentium. In the C. rodentium-infected mucosa, we observed potent RELMα expression by intestinal epithelial cells. This was correlated with significant intestinal crypt elongation and increased macrophage accumulation at the interface with GFP-C. rodentium. In conclusion, these new studies reveal RELMα inhibition of the Th2 cytokine response at the site of C. rodentium infection and demonstrate increased macrophage infiltration and RELMα expression at the site of C. rodentium colonization.
Results
RELMα effects on the IL-23/Th17 Axis: involvement of the Th2 cytokine response
Antigen presenting cells in the colon are critical initiators of the T helper cell response during infection.20 Monocytes and macrophages can further shape the scope and magnitude of local innate and adaptive immune responses via cytokine production and polarization of innate lymphoid cells (ILCs) and CD4+ T helper cells. A specific subset of macrophages, the CX3CR1+ macrophages, have been ascribed a critical role in supporting host-protective ILC-derived IL-22 following C. rodentium infection.21 Further, lineage-specific deletion of monocytes/macrophages resulted in impaired accumulation of C. rodentium infection-induced IFNγ+ and IFNγ+IL-17+ CD4+ T cells and host-protection.22 Our recent data demonstrating that RELMα can promote expression of IL-12p40 and IL-23p19, cytokines that are critical for Th1/Th17 polarization, and the accumulation of Th17 CD4+ T cells further highlights the importance of macrophages in shaping the local cytokine milieu.3
RELMα is most commonly associated with AAMacs and helminth infection, where it functions to negatively regulate expression of Th2 cytokines such as IL-13.7,8 Given the existence of cross-regulation between Th2 and Th17 cytokine production,19 we hypothesized that an additional mechanism by which RELMα promotes Th17 cytokine responses may involve indirect effects on the local cytokine environment. Consistent with this hypothesis, C. rodentium-infected RELMα−/− mice exhibited significantly reduced colon IL-17 levels and increased colon IL-5 and IL-13 levels compared with WT controls (Fig. 1A). This switch to a Th2-associated cytokine response in RELMα−/− mice correlated with increased expression of arginase1 (Fig. 1B), an enzyme that is expressed by Th2 cytokine-induced AAMacs. Previous helminth infection studies have shown that macrophage-derived arginase can inhibit Th2 cell cytokine production and proliferation and arginase-expressing macrophages can ameliorate intestinal inflammation.23,24 Therefore, we hypothesize that C. rodentium-induced RELMα contributes to Th17 cell polarization both directly, through induction of IL-12p40 and IL-23p19, and indirectly by the inhibition of Th2 cytokines. In the absence of RELMα, the increased induction of Th2 cytokines and AAMac-derived arginase may act to limit effector CD4+ T cell proliferation and contribute to intestinal tissue protection. Since studies originally identified RELMα, arg1, and ym1 as coordinately expressed by AAMacs,5 our data suggesting that RELMα may inhibit AAMac activation and arg1 expression is unexpected. These data suggests that RELMα provides a cell-intrinsic negative feedback mechanism to inhibit AAMac activation. Alternatively, during C. rodentium infection RELMα is expressed by cell types other than macrophages, including epithelial cells and eosinophils, and the cellular source of RELMα may influence functional outcomes. RELMα−/− bone marrow chimeras may shed light on these differences and are currently being investigated. These studies have the potential to deepen our understanding of how RELMα regulates initiation and resolution of inflammation to various pathogenic insults at mucosal barrier surfaces, and may have implications for the understanding of the pathogenesis of human bacterial infection and intestinal inflammatory disorders.
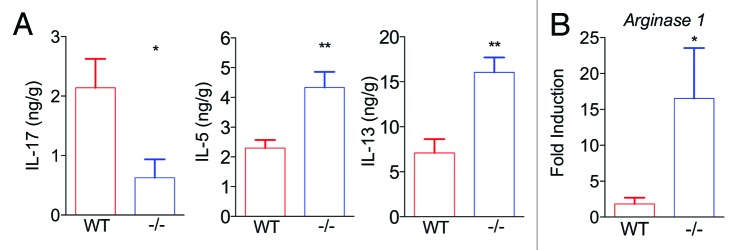
Figure 1. RELMα promotes IL-17 expression while inhibiting Th2 effector responses in Citrobacter rodentium-infected colons. WT and RELMα−/− mice were left naïve or infected for 10 d with C. rodentium followed by recovery of distal colon tissue for ELISA, plotted as ng cytokine per g colon tissue (A), or real-time RT-PCR, plotted as fold induction over naïve controls (B).
RELMα belongs to the RELM family of secreted proteins that includes two human proteins (Resistin and RELMβ), and investigating murine RELM proteins may give insight into understanding the function of human RELM proteins in health and disease.25 Interestingly, a recent transcriptional profiling study by Loke and colleagues revealed a positive correlation between human resistin expression and Th17 cells in human ceca biopsies.26 Taken together with our recent studies on RELMα, it is possible that murine RELMα is functionally comparable to resistin in humans. Although this study was performed on biopsies from healthy individuals, Th17 cell-associated responses have previously been associated with the pathogenesis of human IBD.27 It is possible that resistin expression in the human intestine may contribute to this inflammatory environment and future studies on biopsies from IBD patients may give insight into a potential cytokine-stimulatory effect of resistin in human inflamed tissue.
RELMα expression and macrophage responses at the host-pathogen interface
Given our findings of RELMα-mediated effects on macrophages at the site of infection, we examined RELMα expression and macrophage recruitment at the C. rodentium-host interface. Using a GFP-expressing C. rodentium, we undertook imaging studies to investigate the cell types expressing C. rodentium-induced RELMα, localization of C. rodentium in the intestine, and the kinetics of colonic macrophage accumulation. Similar to the infection kinetics that we previously reported, infection of BL/6 mice with GFP-C. rodentium resulted in bacterial growth in the colon that peaked at day 10 post-infection, followed by clearance from the colon by day 25 post-infection (data not shown). Colon tissue cryosections from naïve or infected mice were stained with anti-RELMα (purple) and the macrophage binding lectin GSL (red) (Fig. 2A). We previously observed RELMα expression by macrophages, eosinophils, and intestinal epithelial cells by flow cytometry and immunohistochemistry. Surprisingly, these new localization studies demonstrate that in the C. rodentium-colonized crypts, RELMα was predominantly expressed by cells of goblet cell morphology rather than GSL+ macrophages. The potential effect of goblet cell-derived RELMα on macrophage function at the host-pathogen interface is a new research avenue that we are investigating.
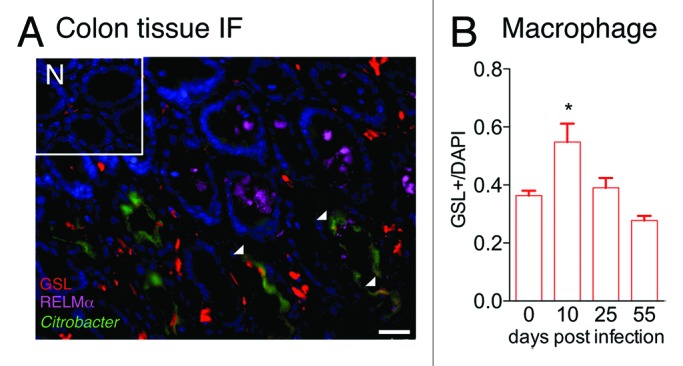
Figure 2. Macrophage recruitment and RELMα expression in C. rodentium-infected mice. (A) Immunofluorescent stained colon tissue sections from naïve (N) or day 10-infected WT mice reveal GSL+ macrophages (red), RELMα (purple), C. rodentium-GFP (green), and DAPI (blue). Scale bar 25 μm. (B) Quantification of GSL+ macrophage frequency in naïve or infected mice was performed.
Previous reports have demonstrated C. rodentium-induced increase in CX3CR1+ macrophages in the intestine.21 We hypothesized that macrophages localized in the crypts are influenced by the proximity of the bacterial infection and by infection-induced factors such as epithelial-derived RELMα. At day 10 post-infection with C. rodentium, there was a marked increase in infiltrating GSL+ macrophages in the colonized crypts. We also observed areas of GFP (green) and GSL (red) co-localization (Fig. 2A, white arrows), implicating macrophage phagocytosis of C. rodentium as a host defense mechanism. Quantification of macrophage infiltration at the host-pathogen interface revealed that there was a significant increase in macrophage frequency at the peak of C. rodentium infection (day 10) that was resolved when C. rodentium had been cleared at day 25 and day 55 post-infection (Fig. 2B). The recruitment of macrophages to the host-pathogen interface has previously been shown to be critical for bacterial phagocytosis and clearance.21 In addition, the infection-induced macrophages may also play a homeostatic role for the resolution of intestinal inflammation following bacterial clearance. For instance, Stappenbeck and colleagues reported the critical importance of macrophages in DSS-induced colitis.28 Here, activated macrophages in the colonic crypts of DSS-treated mice produced regenerative factors that stimulated epithelial cell proliferation. Future studies in our lab will investigate the role of macrophages and macrophage-derived factors such as RELMα or arginase in the resolution of C. rodentium-induced inflammation, and the consequence of selective macrophage depletion at different time points post-infection.
Discussion
In our previous studies, we demonstrated a novel role for RELMα in contributing to a pathogenic Th17 response in the colon following infection with C. rodentium through effects on macrophages.3 Here, we relate these findings to our other previous work demonstrating that RELMα limits Th2 inflammation.7 These studies suggested that the RELMα-mediated effects observed might be a consequence of the counter-regulation of Th2/Th17 cytokine responses. Here, we show in new data that C. rodentium-infected RELMα−/− mice have increased Th2 cytokine responses in the intestine. Additionally, employing reporter GFP-C. rodentium, we observed infection-induced RELMα expression by goblet cells and significant increases in infection-induced macrophages in the C. rodentium-colonized mucosa. Real-time RT-PCR analysis of the infected intestine in WT or RELMα−/− mice revealed increased expression of arginase1, suggesting that RELMα−/− mice exhibited polarized alternative macrophage activation.
The importance of macrophage activation in bacterial infection has been the focus of several recent studies showing that classically activated macrophages (CAMacs) can limit bacterial burdens while AAMacs are detrimental to the host and sustain chronic bacterial infection.29-32 CAMacs differentiate in response to toll-like receptor ligands such as LPS and Th1 cytokines, and promote bacterial killing through the production of proinflammatory cytokines and microbicidal products. In contrast, AAMacs that differentiate in response to Th2 cytokines are considered anti-inflammatory and express regulatory molecules such as Transforming Growth Factor β and arginase1. Following infection with several different bacterial pathogens including Salmonella typhimurium, Brucella abortus, or C. rodentium, AAMacs impaired bacterial clearance by a variety of cell-intrinsic and cell-extrinsic mechanisms. First, compared with CAMacs, AAMacs exhibit a vastly different metabolic profile, resulting in a glucose-rich intracellular environment conducive for bacterial growth.30,32 Additionally, AAMacs have deficient autophagy responses that are necessary for intracellular bacterial killing.31 AAMacs may also impair bacterial clearance via cell-extrinsic mechanisms such as the inhibition of T cell activation or antibacterial CAMac responses.29 However, whether AAMacs or their effector molecules mediate effects on infection-induced intestinal inflammation independently of effects on bacterial growth is less well defined. Our finding that the ameliorated intestinal inflammation in RELMα−/− mice was associated with increased arginase1 expression suggests that AAMac activation in the intestine may have a beneficial effect on C. rodentium-induced inflammation once the bacterial burdens are cleared. Consistent with this hypothesis, previous studies have shown beneficial effects of SHIP−/− AAMacs in ameliorating intestinal inflammation induced by DSS.33 Together with our results, these studies suggest that examination of AAMac responses during acute C. rodentium infection, as well as following clearance during the resolution of inflammation, is warranted.
In summary, studies in this addendum reveal that C. rodentium colonization of the mucosa promotes goblet cell expression of RELMα (Fig. 3 part 1). In addition to our previous report showing that RELMα stimulates inflammatory macrophage-derived IL-23 (Fig. 3 part 2), our new data in this addendum implicates RELMα inhibition of Th2 cytokines as an additional mechanism that promotes Th17 cell responses (Fig. 3 part 3). Finally, we show infection-induced macrophage responses in the intestine, and increased AAMac activation in the RELMα−/− mice that is associated with ameliorated intestinal inflammation (Fig. 3 part 4). The functional significance of macrophage activation and infiltration at the host-pathogen interface and the role that these macrophages may play in bacterial clearance or intestinal inflammation remain critical avenues of investigation that may help the treatment of bacterial-induced colitis.
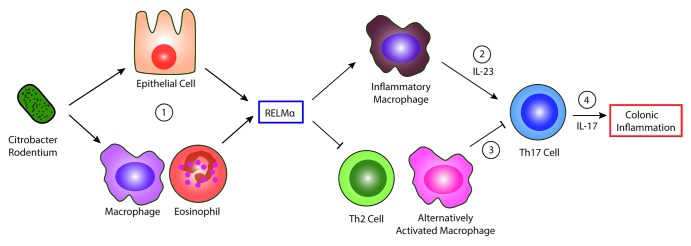
Figure 3. RELMα regulation of bacteria-induced intestinal inflammation. (1) Citrobacter rodentium infection induces RELMα expression by epithelial cells and leukocytes. (2) RELMα activates inflammatory macrophages to secrete IL-23 and activate Th17 cells. (3) RELMα inhibits Th2 cells and alternatively activated macrophages, which may counter-regulate the Th17 immune response and/or inhibit intestinal inflammation. (4) The elicited Th17 response causes colonic inflammation in the host.
Materials and Methods
Mice and Infection: C57BL/6 mice were purchased from Jackson Laboratories and bred in-house. RELMα−/− mice were backcrossed onto Jackson C57BL/6 mice as previously described.7 Mice were maintained in a specific pathogen-free facility at the University of California, Riverside, and all procedures were performed under the guidelines of the Institutional Animal Care and Use Committee. WT and GFP-C. rodentium (DBS-100) were provided by Bruce Vallance,15 and infection was performed as previously described. In brief, mice were orally gavaged with 0.2 mL of ~5 × 108 colony forming units.
Histology: One-cm distal colon was removed, flushed with PBS, and was submerged in 4% formaldehyde for 4 h, followed by 30% sucrose overnight. Tissue was embedded in OCT (Biotek) and 8μm cryosections were cut using a cryostat (Leica). For immunofluorescent staining, sections were blocked with normal goat serum and then stained with rabbit anti-mouse RELMα antibody (Peprotech) at 4 °C overnight, followed by Cy3 conjugated anti-rabbit IgG (Abcam) for 30 min at room temperature. After washing with PBS/0.1% Tween-20, the sections were blocked with Streptavidin Blocker, Biotin Blocker (Vector laboratories), and StartingBlock T20 block buffer (Thermo Scientific), 15 min each at room temperature, then stained with biotinylated GSL (Vector laboratories) for 1 h at room temperature and followed by Cy5 conjugated streptavidin (Jackson ImmunoResearch Laboratories) for 30 min at room temperature. The sections were washed and covered with ProLong® Gold Antifade Reagent with DAPI and coverslip (Cell Signaling Technology). Microscopy was performed using a Leica immunofluorescent microscope with AF6000 software at 200× and 400× magnification. The gray-scale values of GSL+ macrophages was measured and corrected with that of DAPI (nuclei) from 3–5 random fields of each immunofluorescent section.
Real-time RT PCR and ELISA Analysis: 1 cm of distal colon tissue was recovered in RNAlater (Qiagen) followed by RNA extraction and real-time RT-PCR, as previously described. For cytokine ELISAs, 1cm of distal colon tissue was weighed and homogenized in 0.5mL PBS, followed by ELISA with anti IL-17, anti IL-13, and anti IL-5 capture and detection antibodies (eBioscience).
Statistical Analysis: Graphs are analyzed with Graphpad Prism and are presented as mean +/− SEM. Statistical significance was confirmed when P < 0.05, using an unpaired two-tailed student T-test or when >2 groups, a one way ANOVA test followed by the Dunnett’s post-test.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We would like to thank Bruce Vallance for providing the C. rodentium strains, and David Artis for reagents and helpful discussions. We also thank Kaila Bennett and Olivia Sakhon for critical reading of this manuscript. We are thankful for the support of the Crohn’s and Colitis Foundation of America’s William and Shelby Model Family Foundation Postdoctoral Fellowship (M.G.N.), the National Institute of Health (R01AI091759 to M.G.N.), and the Irvington Institute Postdoctoral Fellowship of the Cancer Research Institute (L.C.O.).
Glossary
Abbreviations:
- AAMac
alternatively activated macrophage
- CAMac
classically activated macrophage
- DSS
dextran sodium sulfate
- IBD
inflammatory bowel disease
- RELM
resistin-like molecule
- Th
T helper type
- WT
wild-type
References
- 1.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–64. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 3.Osborne LC, Joyce KL, Alenghat T, Sonnenberg GF, Giacomin PR, Du Y, Bergstrom KS, Vallance BA, Nair MG. Resistin-like molecule α promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. J Immunol. 2013;190:2292–300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 7.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, et al. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–52. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr., Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truscott M, Evans DA, Gunn M, Hoffmann KF. Schistosoma mansoni hemozoin modulates alternative activation of macrophages via specific suppression of Retnla expression and secretion. Infect Immun. 2013;81:133–42. doi: 10.1128/IAI.00701-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–7, e1. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–63. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–86. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136:2237–46, e1. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187:4440–50. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 21.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6:177–88. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med. 2013;210:2025–39. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher JL, Hacker A, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–17. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 24.Weisser SB, McLarren KW, Voglmaier N, van Netten-Thomas CJ, Antov A, Flavell RA, Sly LM. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol. 2011;41:1742–53. doi: 10.1002/eji.201041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22:259–65. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff MJ, Leung JM, Davenport M, Poles MA, Cho I, Loke P. TH17, TH22 and Treg cells are enriched in the healthy human cecum. PLoS One. 2012;7:e41373. doi: 10.1371/journal.pone.0041373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 30.Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. Salmonella require the fatty acid regulator PPARδ for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14:171–82. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su CW, Cao Y, Zhang M, Kaplan J, Su L, Fu Y, Walker WA, Xavier R, Cherayil BJ, Shi HN. Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages. J Immunol. 2012;189:1459–66. doi: 10.4049/jimmunol.1200484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xavier MN, Winter MG, Spees AM, den Hartigh AB, Nguyen K, Roux CM, Silva TM, Atluri VL, Kerrinnes T, Keestra AM, et al. PPARγ-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages. Cell Host Microbe. 2013;14:159–70. doi: 10.1016/j.chom.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisser SB, Brugger HK, Voglmaier NS, McLarren KW, van Rooijen N, Sly LM. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90:483–92. doi: 10.1189/jlb.0311124. [DOI] [PubMed] [Google Scholar]


