Abstract
The ability of microorganisms, whether present as commensals within the microbiota or introduced as part of a therapeutic regimen, to influence behavior has been demonstrated by numerous laboratories over the last few years. Our understanding of the mechanisms that are responsible for microbiota-gut-brain interactions is, however, lacking. The complexity of the microbiota is, of course, a contributing factor. Nonetheless, while microbiologists approaching the issue of microbiota-gut-brain interactions in the behavior well recognize such complexity, what is often overlooked is the equal complexity of the host neurophysiological system, especially within the gut which is differentially innervated by the enteric nervous system. As such, in the search for common mechanisms by which the microbiota may influence behavior one may look for mechanisms which are shared by both host and microbiota. Such interkingdom signaling can be found in the shared production of neurochemical mediators that are found in both eukaryotes and prokaryotes. The study of the production and recognition of neurochemicals that are exactly the same in structure to those produced in the vertebrate organisms is known as microbial endocrinology. The examination of the microbiota from the vantage point of host-microbiota neuroendocrine interactions cannot only identify new microbial endocrinology-based mechanisms by which the microbiota can influence host behavior, but also lead to the design of interventions in which the composition of the microbiota may be modulated in order to achieve a specific microbial endocrinology-based profile beneficial to overall host behavior.
Keywords: hormones, neuroendocrine, enteric nervous system, signaling, behavior, microbial endocrinology
Introduction
The complexity of the host-microbiota interface is increasingly being recognized to impact nearly every aspect of health and disease from the degradation of complex carbohydrates in the gut to provide energy to the host1 to the modulation and induction of host behavior.2,3 At present, the majority of efforts directed at the identification of the relevant mechanisms that underlie and regulate host-microbiota interactions involve cataloguing of the changes in the microbiota either prior to or subsequent to the development of a specific disease state. For example, the role of the microbiota in the causation and continued pathology of inflammatory bowel disease has generated numerous critical studies that have attempted to associate one specific family or species with the disease.4 In large part, general agreement between published studies as to a specific disease-associated microbiota profile has not yet been fully achieved. Differences in methodology and the ever evolving complexity of the bioinformatics programs used to interrogate the enormous databases obtained from analysis of specimens, whether fecal or mucosally-associated microbial communities, may be one of many reasons.5 Regardless of the reasons, we are undoubtedly at the very early stages of identifying relevant mechanisms that regulate host-microbiota interactions.
Additionally, the vast majority of investigations that investigate host-microbiota interrelationships do so from the vantage point of the microbiota influencing the host. While the host can certainly influence the composition of the microbiota as best exemplified from the ingestion of wide-spectrum antibiotics6 or changes in diet,1 in general most studies are concerned with how the specific composition of the microbiota influences the host and do not examine how the host can influence the microbiota (other than changes in dietary preferences). Further, while it is acknowledged that many studies do examine the host immune response to alterations in the microbiota, such as in the pathogenesis of inflammatory-mediated diseases in the gut,4 these are more properly perceived as a host reaction to the consequences of altered diversity in the gut, which can result in, for example, increased bacterial invasion of the intestinal epithelium thereby invoking an immune response. As such, they are not as much a study of how the host may control the microbiota, but instead are a host reaction to changes in the gut microbiota due to as still unknown mechanisms (which may be due to alterations in the host immune system itself).
This focused examination will therefore center on a specific directed mechanism that is proposed to be central to the interaction of host and microbiota. It is based on the shared neuroendocrine signaling between bacteria and host. This intersection of microbiology with host neurophysiology is the field known as microbial endocrinology (for review see refs. 7, 8). There is growing recognition that bacteria and yeasts, for example, contain many of the same neurochemicals that are present in the host. For example, production of γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter found in the mammalian brain9 as well as exhibiting immunomodulatory properties,10 is produced in large milligram quantity by a number of bacteria contained within the gastrointestinal tract.11,12 For the purposes of this review, GABA will serve as a prototypical example of a neuroactive compound produced by microbiota as it has been most extensively investigated to date and for which both the molecular mechanisms governing its production and potential use as a therapeutic agent are well-understood. With that said, GABA represents only a very small part of the ever-increasing spectrum of neuroactive chemicals that have been isolated from the microbiota (for a review see 12) For example, production of dopamine which is needed for proper neuronal functioning as well as being the substrate for the production of the stress hormone norepinephrine is made by Escherichia spp.12,13 and produced within the gut.14 Acetylcholine,15 histamine,16 serotonin,17 and even more newly described neurotransmitters such as agmatine18-20 have all been shown to be produced by microorganisms. Of prime importance in recognizing the ubiquitous nature of neurotransmitters, produced by members of the microbiota and its relationship to the host, is that the biochemical pathways used by the microbiota to produce these neurochemicals are exactly the same as found in the host tissues (Fig. 1).21 As such, it has been proposed that the development of these pathways first occurred in bacteria and by late horizontal gene transfer were acquired by the eukaryotic cell systems.21 Recent results from the human microbiome project has shown that such bacterial-mammalian cell lateral gene transfer of bacterial DNA into the human somatic genome occurs via integration of a RNA intermediate and is more common than previously recognized.22
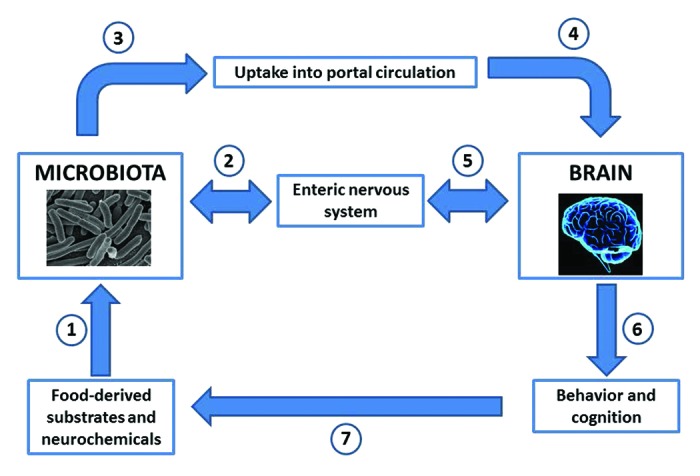
Figure 1. The microbial endocrinology-based pathways by which neuroactive compounds produced by both the host and the microbiota can serve as a mechanism by which the brain and behavior can be modulated within the microbiota-gut-brain axis. Food ingested by the host contains both the substrates needed for neurochemical production by the host and the microbiota as well as fully functional neuroactive components (1). The microbiota in the gut is capable of either forming neurochemicals from the substrates present in the ingested food; or responding to the neuroactive food components themselves; or responding to neurochemicals secreted into the gut by components of the host enteric nervous system (2). Neurochemicals produced by the microbiota in the gut have two pathways by which to influence the host; they can either be taken up from the gut into the portal circulation (3) or they can directly interact with receptors found on components of the enteric nervous system which innervates the complete length of the gastrointestinal tract (2). Once in the portal circulation, microbiota-derived neurochemicals can influence components of the nervous system and ultimately the brain (4). Microbiota-derived neurochemicals can also influence components of the nervous system such as the brain through enteric nervous system-central nervous system communication (5). The result of either pathway (4) or (5) on the brain may result in an alteration of behavior or cognition (6) as well as food preferences and appetite (7).23,71 As described in the text, this should not be viewed as a one-way direction of only gut-to-brain since the brain may influence the composition of the microbiota through the specific release of neurochemicals into the gut lumen (2).
As part of this focused examination, I will also examine and propose under what conditions the host neurophysiological system can influence the microbiota.23 In this fashion, the concept of microbial endocrinology will be employed as a global mechanism which can permit and enable bi-directional communication between the host and the microbiota whose ultimate effect is the “health” of not only the host, but also the microbiota.23 It is also important for the reader to note that this examination is not meant to be a comprehensive review of the literature. Instead it is the specific intent of this review to employ selected studies (often reviews in their respective fields to point the reader to wider databases of studies not cited in the present paper) spanning a number of decades to demonstrate the basis for considering microbial endocrinology as a bi-directional mechanism by which the microbiota and the host can influence one another. As such, the author readily acknowledges the contributions of others who are not cited in this review.
Microbiota and Neurohormones
The recognition that microorganisms actively produce, as well as possess cognate receptors for, a wide range of neuroendocrine hormones has been reported for decades (for reviews see refs. 12, 24). The range of hormones that are found in microorganisms is extremely diverse extending from somatostatin to acetylcholine to progesterone. Critically, microorganisms which inhabit the gastrointestinal tract are capable of producing neuroendocrine hormones that have cognate host receptors which can easily be found both intra- and extra-intestinally to which these neurohormones, in sufficient quantities, can effect neurophysiological changes in the host. For example, certain Lactobacillus and Bifidobacterium strains isolated from the human gastrointestinal tract can produce in vitro over 20 000 µg ml−1 of GABA in the presence of a suitable substrate.11
Until recently, the vast majority of reports dealing with microbes and neuroendocrine hormone production were confined mainly to in vitro studies. The ground-breaking study by Asano and colleagues14 was the first to establish that the microbiota was capable of the in situ production of biologically active neuroendocrine hormones. In this study, luminal levels of catecholamines in the gastrointestinal tract were measured in specific pathogen-free, germ-free, and gnotobiotic mice. Asano et al. reported that the catecholamines, norepinephrine, and dopamine, were produced in appreciable physiological amounts in specific pathogen free mice.14 However, in germ-free animals substantially lower amounts were detected in luminal contents. Critically, whereas the majority of catecholamines in pathogen-free animals were structurally determined to be free and biologically active, those found in germ-free animals were present in a biologically inactive, conjugated form. Inoculation of germ-free animals with the flora from specific pathogen free mice resulted in the production of free, biologically active, catecholamines within the gut lumen. As such, this report14 clearly established that in vivo the microbiota is capable of producing neuroendocrine hormones that are commonly only associated with host production. That these substances also are intimately involved in host neurophysiology provides solid evidence that the fields of microbiology and neurophysiology do intersect with attendant consequences for both host and microbiota as further discussed below.
As written in the first comprehensive study of the intestinal luminal metabolome in mice, Matsumoto and colleagues25 noted in their otherwise excellent and elegant study that “For clarifying the relationship between health and/or disease and intestinal bacterial metabolites, only free bacterial metabolites in the intestinal luminal content should be analyzed.” This statement was directed at previous targeted metabolomics studies of the intestine in which intracellular material was also analyzed from sonicated fecal samples.26 While the assertion that only free bacterial metabolites should be analyzed does on first consideration seem reasonable, a more careful consideration can conclude otherwise. First, the intracellular concentrations of neuroactive compounds within specific bacteria are largely unknown as most studies tend to concentrate on the amount released into in vitro culture. Experiments which have utilized sonicated bacteria such as Lactobacillus have revealed high concentrations of intracellular neurochemicals such as GABA.27 As such, release of bacterial contents within a specific local environment within the gut may be taken up rapidly be neurochemical-responsive elements within the gut, such as enteric neurons or may be directly absorbed into the portal circulation via passive diffusion through the gut. Further, it is not unreasonable to assume that upon death the intracellular contents of the microorganism may be released into the surrounding area, especially if those microorganisms are located luminal epithelial chemosensors which can respond to and transmit information regarding bacterial metabolites such as neuroactive compounds.28 Thus, any neurochemicals would not be present in the luminal fluid for subsequent metabolomic measurement. Second, at this still very early stage of understanding the role of neurochemical production and recognition by the microbiota, we don’t whether or how such production is in fact used by the bacteria themselves as a form of intercellular communication. If this is the case as has been proposed23, then released neuroendocrine hormones would be quickly taken up by other targeted bacteria and thus would not be available for measurement. Thus, it would seem that the best approach would be to analyze the sonicated cellular sample as well as the fluid itself to ascertain the capacity of the microbiota to produce neuroactive compounds.
Critical analyses of the scientific literature going back over nearly a century reveals numerous reports that suggested that the ability of microorganisms to both produce and respond to neuroendocrine hormones could have potent physiological consequences for the host.8,12,24 A typical example were the reports of substantial GABA production by both normal colonic bacteria29 as well as bacterial pathogens commonly associated with blood-borne sepsis in humans.30 While the physiological significance of such a finding was initially postulated to be a factor in the altered consciousness that accompanies the development of hepatic encephalopathy, subsequent studies did not substantiate such a role. In the case of GABA, subsequent reports continued to appear to indicate that the ability of bacteria to produce it had often unexpected consequences for neurobiological research. For example, the finding of that a bacterially contaminated distilled water apparatus contained “GABA-receptor binding sites” called into question a number of findings with mammalian tissues such as brain homogenates.31 The initial demonstration of a high affinity binding site for a neurotransmitter in a bacterium, namely GABA in a strain of Pseudomonas fluorescens, utilized the same radioligand binding techniques that were employed at the time for the study of such binding sites in brain membranes.32
It still remains a mystery as to why the scientific community at the time was unable to fully appreciate the clinical consequences for the host when members of the microbiota were capable of producing a neuroendocrine hormone within the gut lumen that could directly exert physiological effects by interactions with host receptors within the gut or following uptake into the circulation. Contrary to the impression that is often given by reviews that examine shared signaling systems in what has been termed interkingdom signaling, the realization that such shared signaling molecules existed between vertebrate host and microbe, especially neuroendocrine hormones, was perceived decades ago to be a prime determinant of health and disease. Most notable was a period in the 1980s that saw a flurry of reports reporting the existence of a number of neuroendocrine hormones in bacteria that had been previously only associated with vertebrates.33 As evidence that these findings of shared molecules and their receptors did find wide dissemination in the scientific literature at the time was the publication of reviews in high impact journals such as the New England Journal of Medicine.34 For whatever reasons, this intensive period of investigation in the 1980s was not followed in the immediate subsequent years by a similar effort and as such the observations of shared hormonal signaling pathways did not make sustained inroads into the question of health and disease within the scientific and medical community at large.
It was not until the first report of direct stimulation of bacterial growth of bacteria by neuroendocrine hormones in 199235,36 that reports began to appear that ascribed a specific role to the production and recognition of neuroendocrine hormones by bacteria that led to the creation of the field of microbial endocrinology.37 At present, the majority of reports concerned with microbial endocrinology have concentrated on the role it plays in the pathogenesis of infectious disease. Insights from these studies, such as the ability of catecholamines to alter gene expression in a number of pathogens38-41 as well as conjugative transfer between enteric bacteria,42 will undoubtedly inform subsequent studies examining the role of the microbiota in its ability to its role in alteration of host behavior.23,43
Regardless, it is apparent from even a cursory overview of the past literature that we are just at the very beginning of understanding the ability of the microbiota to produce neurochemicals that may influence the host. Critically, consideration of microbial endocrinology as a mechanism to account for the ability of the microbiota to influence host behavior raises an evolutionary question that is not usually asked in discussions of the gut-microbiota-brain axis. Specifically, if the microbiota can influence the brain, can the reverse occur? Given that production of neuroendocrine hormones by the host is also prevalent in the gut then the answer would seem to be self-evident according to a microbial endocrinology-based approach since members of the microbiota not only produce the very same neurochemicals as the host but also possess the cognate receptors for them. Thus, a microbial organ within the gut exists in which bacteria communicate not only with the host, but also with each other through the production and recognition of neuroendocrine hormones which have a long shared evolutionary history. This concept of a microbial organ based on microbial endocrinology-based principles has already been proposed.23 Animal studies which point to such host neurophysiological directed alterations of the microbiota are discussed below.
Neurochemicals in Microorganisms: To What End?
Probably the most fascinating question that arises when one considers the plethora of neurochemicals found within microorganisms is the question of what purpose do they serve. It is perhaps somewhat surprising to learn that the presence of what are thought to be almost exclusively vertebrate neurotransmitters, neurohormones, and related receptors are in fact widely dispersed throughout nature. For example, in addition to its presence in vertebrates, neuroendocrine hormones such as the catecholamines have been additionally identified in plants as diverse as tomatoes and bananas,44,45 insects,46 and fish.47
As detailed above, the role of neurochemicals has been most extensively studied in the realm of infectious disease where its role has been shown to extend from food-borne infections48-50 to bacterial-mediated lung disease51 to indwelling medical devices where the administration of exogenous neuroendocrine hormones, such as the catecholamine pressors to maintain cardiac and kidney function, stimulate the development of bacterial biofilms on devices such as catheters.52 While such observations support a role for microbial endocrinology interactions in the pathogenesis of infectious disease, it would be reasonable to argue that in the context of host homeostasis that the existence and capacity for such neuroendocrine-microbiota interactions serves a much larger, and evolutionarily relevant, function.
This widespread presence of neurohormones throughout nature suggests that microorganisms in general have had ample time preceding the evolution of man to come into contact with a wide spectrum of neurohormones and develop mechanisms by which to synthesize as well as recognize neurohormones.53,54 While this ubiquitous distribution of neurohormones and receptors throughout nature is often little recognized in current medical thought,34 their existence means that these prokaryotic systems are most likely evolutionary precursors to the more complex vertebrate systems such as the central nervous system. In fact, the presence of neurochemicals in mammals as cell-to-cell signaling compounds has been suggested to have resulted from lateral gene transfer from bacteria, especially when one considers that the same exact biochemical synthetic pathways are found in both bacteria and mammalian cells.21
Interface of Host Neurophysiology and Microbiota: Infectious Disease Consequences
In proposing that neuroendocrine-microbiota interactions, otherwise known as microbial endocrinology, serve as a mechanism by which the host communicates with the microbiota in a bi-directional manner, it would be expected that a dramatic change in host neurophysiology would be detected by the microbiota that then would respond accordingly. A study that demonstrated this employed a neurotoxin to immediately impact nerves within the body causing a sudden release of the “fight-or-flight” stress-related hormone norepinephrine.55 The neurotoxin 6-hydroxydopamine (6-OHDA) was employed to cause the sudden release of norepinephrine stores from sympathetic noradrenergic nerves that constitute part of the autonomic nervous system. A critical element of the experimental design was that the effect of 6-OHDA-induced changes in neurophysiological function on the composition of the microbiota could be examined over time since the damage caused to nerve terminals by 6-OHDA was fully reversible with complete healing of the nerves being achieved over 14 days. Within 24 hours following 6-OHDA administration, a dramatic shift in the composition of the microbiota occurred in which Gram-negative bacteria increased over 5 logs in total population.56 This observation was supported by previous in vitro work by a number of groups, which had shown that Gram-negative bacteria were much more responsive to catecholamines such as norepinephrine than Gram-positive bacteria.57 As the catecholaminergic nerves re-healed over a two-week period, the composition of the microbiota returned to the same distribution as present pre-6-OHDA administration56 thereby demonstrating the ability of the microbiota to respond to neuroendocrine signaling from the host. Other in vivo studies, such as those by Vlisidou et al. demonstrated in bovine ligated ileal loops that the luminal concentration of the catecholamine norepinephrine could directly influence the ability of Escherichia coli O157:H7 to form attaching and effacing lesions in the intestinal mucosa.58
More recent studies that have employed sequence analysis of the microbiota have demonstrated that the composition of the microbiota is sensitive to stress conditions that are experienced by the host. Bailey et al.59 demonstrated that mice subjected to restraint stress experienced a dramatic alteration in the microbiota characterized by a stressor-induced reduction in the relative abundance of bacteria in the family Porphyromonadaceae. Significantly, this alteration in the normal composition of the microbiota allowed for the emergence of pathogens establishing a productive infection.
Experimental Issues and Assumptions
Microbial endocrinology as a mechanism by the microbiota to affect host neurophysiological function and ultimately behavior, and in a similar fashion how the host may regulate the composition of the microbiota, a number of experimental issues, and assumptions, need to be addressed. There are three main areas that are of immediate, primary importance.
Can microbial-produced neuroendocrine hormones actually change host behavior?
This is a question that at present can only be answered by inference from other related studies. For example, it is well recognized that Lactobacillus spp. are prodigious producers of GABA.11 Recently, a functional food study employed the GABA-producing Lactobacillus brevis FPA 3709 strain as a means to enrich black soybean milk with GABA which was then fed to rats subjected to a forced swim behavioral test.60 Forced swim tests, in which animals are placed in a water-containing glass cylinder and the duration of immobility before the animals begin to swim is measured, is a well-recognized test of depressive-like behavior. In this study, it was shown that GABA-enriched soybean milk significantly reduced the immobility time before rats began to swim and was as effective as the selective serotonin reuptake inhibitor fluoxetine as an antidepressant.60 In another study, the ability of per oral fed L. rhamnosus to reduce anxiety- and depressive-like behavior in mice was shown to be mediated via central GABA receptor expression in the brain with specific levels of GABAAα2 mRNA altered in those brain regions associated with the specific behavior.61 Animals in which the vagus nerve (the longest of the cranial nerves that innervates the gut as well as other visceral organs) had been severed (vagotomized animals) did not show any behavioral or brain GABA mRNA-related changes. This demonstrated that communication between the gut and brain as mediated by the vagus nerves was one of the pathways by which probiotic bacteria could exert its behavioral effects in the host.61
Recently it has been proposed that, given their ability to produce high amounts of neuroendocrine hormones such as GABA and histamine, probiotics should functionally be viewed as neurochemical drug-like delivery vehicles.27 Production of such bacterial-derived neuroactive compounds in the gut could be expected to not only influence host neurophysiological function both intra- and extra-intestinally as well as influence the pathogenic potential of other bacteria, but also affect host immune cell function. For example, GABA is the predominant inhibitory neurotransmitter in the nervous system, and exerts anti-inflammatory actions in the immune system as well. Because GABA can inhibit pro-inflammatory peptide release from viscerosensory neurons and inhibits activity of inflammatory immune cells, the production of GABA by gut probiotic bacteria could constitute a powerful mechanism for prophylaxis of gastrointestinal inflammatory conditions. It should be noted that GABA receptors have been localized on pro-inflammatory immune cells, which function to downregulate inflammatory responses such as cytokine release.10 Thus, production of GABA by probiotic bacteria could reduce inflammation that is associated with the pathogenesis of gut-related disorders such as inflammatory bowel disease.
The ability of immune cells to modulate behavior represents a well-documented axis by which behavior may be modified.62 As such, the modulation of immune activity by neuroendocrine hormones secreted by gut bacteria may represent an indirect pathway by which neuroendocrine hormone-secreting bacteria first influence immune cells that then in turn influence neuronal elements within the gut that communicate with the brain and drive behavior. However, as with all reports that have utilized per oral administered bacteria whether probiotic or not, no chemical measurements of the gut luminal fluid were performed following measurement of behavior to quantify whether any neuroactive compounds were being produced that could account for the observed changes in behavior as well as any changes measured in gene expression or mRNA formation in the brain. Further, more simple in vitro measures of the neurochemical synthesizing potential of exogenously administered bacteria were also not routinely performed in nearly all studies. The increasing use of metabolomics does afford the opportunity to perform such neuroactive screening of microbial products within the gut lumen such as the cecum and as well as from in vitro-based growth studies.25 Directed metabolomics panels which target neurochemicals whose production by the intestinal microbiota within the gut would influence host cells, such as immune cells, has already been proposed and utilized.27
While the use of metabolomics to measure actual concentrations of neuroactive compounds present in the gut lumen at the time of behavioral observation will prove a crucial step in demonstrating microbial endocrinology as a mechanism mediating gut-microbiota-brain communication, it still does not yet completely prove cause and effect. As has been noted previously in a step-by-step methodological outline to evaluate the ability of neurochemical-producing probiotics to influence health and disease,27 the use of non-neurochemical secreting mutants of the same bacterial strain will be needed to observe if the same behavioral outcome occurs in the absence of the neurochemical or not. Until such studies are performed that rigorously utilize such a cause-and-effect approach, the demonstration of microbial endocrinology as a viable mechanism governing gut-microbiota-brain communication will remain largely hypothetical.
What is the impact of diet on the production of neuroactive compounds by the microbiota?
Many of the bacteria which produce neuroendocrine hormones utilize the exact pathway that is observed in eukaryotic cells.21 As such, as in eukaryotic cells the initial substrate for the synthesis of a neuroactive compound is a dietary component. If that dietary component is not present, then it should not be expected that the neuroactive compound in question will be produced. While on the face of it this seems to be self-evident, the composition of diets that are used vary greatly and no effort is currently made to control or even assay for such substrates. Since plants are capable of the production of a wide variety of neuroendocrine hormones,63,64 it can safely be assumed that any foods that rely on plant-based material will also contain these neurochemicals which interestingly are not altered even when the food is baked at high temperatures.65 The importance in characterizing as completely as possible the chemical makeup of the food can be seen in the study of Matsumoto et al.25 which analyzed both the food metabolome and luminal metabolome at the same time. By comparing the two metabolomes, they demonstrated that many of the neuroactive compounds found in the gut luminal may not have a gut microbial origin but may be due to the food itself.25 Thus, any interpretation of luminal metabolomic results cannot assume that any neuroactive compounds were necessarily produced by the gut microbiota but may in fact be of food origin.
That changes in diet can impact the composition of the microbiota is well recognized. Whether such diet-induced changes in the microbiota can also result in alteration of the gut-microbiota-brain axis that could lead to changes in behavior or cognition is less well understood. One study that has examined this possibility involved the use of a meat-based diet to alter the microbiota and at the same time examine learning and memory in mice.66 Animals were fed diets consisting of either standard laboratory chow or chow supplemented with 50% lean ground beef. Diets were tightly controlled and matched for a number of food-related properties and substances that could potentially independently affect memory and learning. Over a 3 month feeding period, bacterial diversity as measured by 454 pyrosequencing was increased in meat-fed animals as compared with standard chow.66 Measures of learning and memory performed at multiple times during the 3 month feeding period demonstrated that meat-fed mice displayed improved working and reference memory compared with normal chow fed animals that was a function of increased diversity of the microbiota. As such, the design of diets to include a wider spectrum of dietary components that may serve as neuroendocrine substrates may be needed if a fuller understanding of the potential for the gut microbiota to produce neurochemicals that impact the gut-microbiota-brain axis, and possibly higher brain functions such as memory and learning, is to be achieved. Further, in vitro assays which simply employ standard microbiological media that does not reflect the food components that comprise a particular diet and then attempt to assay for neuroendocrine hormone production will more than likely obtain an incorrect answer for the same reason.
Location, location, location
Current among the various methodological issues that accompany examination of a microbial endocrinology-based approach to understanding the gut-microbiota-brain axis is the question of the juxtaposition of host neuroanatomy with the microbiota. There is the assumption, similar to the one that seems to dominate studies of the microbiota, that single samples are reflective of the whole. These comments are not to suggest that microbiota researchers are unaware of this—only that it is not discussed at length, or at all, in many of the reports. For example, microbiota studies that report out the distribution of bacteria from a single fecal specimen often extrapolate such results to be reflective of the distribution of the microbiota as a whole within the gut. Given the existence of bacteria as biofilms adhered to the mucosal surface throughout the gut, or dispersed within the mucus layer, such an assumption that one fecal specimen would capture all of these ecological niches in an equal manner is questionable to say the least. Similarly, in the study of microbiota influencing components of the enteric nervous system, it is often assumed that innervation is somewhat similar throughout the length of the gut which neuroanatomists have recognized for decades but which microbiology-oriented researchers, including the present author, do not adequately address in their studies. Innervation along the gastrointestinal tract is extensive with both the central nervous system (CNS) (for example, through the extrinsic innervation by the vagus nerve) and the enteric nervous system (ENS) (with elements within the wall of the gastrointestinal tract and innervation extending into the crypts). Most importantly, anatomical sections of the gastrointestinal tract are differentially innervated by components of the CNS and ENS (it is beyond the scope of this review to discuss the neuroanatomy and the reader is pointed to a number of excellent reviews67,68).
As such, considering that both components of the microbiota and the host neuroanatomy are differentially located throughout the gastrointestinal tract, then the number and complexity of possible interactions grows exponentially. And most importantly is that the task of dissecting and identifying out the relevant mechanisms becomes that much harder. For example, could microbiota-induced neuronal activation within the brain resulting in a quantifiable behavior be traced to a bacterial species that inhabits a mucus layer immediately adjacent to a specific part of the gut from which sensory information obtained by ENS elements travels to the CNS via extrinsic primary afferent neurons that track along either vagal or spinal afferent routes? Can we distinguish that from bacteria that specifically inhabit the proximal gut instead of the distal gut and communication to the brain in that region occurs instead via the vagus nerve? If we are to reach definitive proof of microbial endocrinology as a mechanism by which the microbiota can influence the brain, and ultimately behavior, then it will be necessary to identify those bacteria which interface with elements of the CNS and ENS and the mediators that they produce that enable such communication. One way to approach this would be to work backward—in other words, by knowing the identity of the specific neuronal elements one would utilize a metabolomics-based assay to evaluate whether bacteria in adjoin gut sections are capable of producing those neurochemicals that would interact with the neuronal elements.
Of course, adding to the complexity is the fact that consideration of location also needs to consider direct uptake of microbially-produced neurochemicals into the systemic circulation in effect by-passing interaction with elements of the ENS and instead interacting with extra-intestinal elements in modulating behavior.
Concluding Thoughts: Speculation into the Unknown of the Gut-Microbiota-Brain Axis
Much of the literature that approaches the gut-microbiota-brain axis is concerned with it as a “one-way street.” While the overwhelming majority of investigators do, in fact, recognize that there is bi-directional communication there are few studies which examine if the brain can influence the microbiota. Reports which utilize stressors, such as that by Bailey et al.,59 do suggest this is a possibility, but since stress is a host-wide physiological event, it is difficult (and dangerous) to make such a conclusion.
However, the question does remain to be asked especially given what we do know of the microbiota following certain periods in which its composition is radically altered. For example, one of the great mysteries in gastroenterology is how the microbiota reestablishes itself following administration of wide-spectrum antimicrobials. Antibiotic-associated diarrhea is a well-recognized consequence of antimicrobial administration, but following cessation of antimicrobial consumption the microbiota does in the majority of cases, with time, return to its pre-antimicrobial exposure state. Is this, in part, due to neuroendocrine hormone-based signals from the CNS to the ENS? For example, is the presence of serotonin in the gut lumen which is under the control of the host a microbial endocrinology-based means by which the brain can influence the prevalence of certain microbial species?
The long evolutionary symbiosis between host and the microbial inhabitants in the gastrointestinal tract, necessitate that the host’s nervous system must have developed the means by which to not only monitor, but also influence the composition of the microbial organ. This recognition of such active monitoring by the host also implies that certain gastrointestinal-related clinical conditions involving microbes can be viewed anew. For example, the ability of the gastrointestinal tract to eventually repopulate itself following the development of antibiotic-associated diarrhea with essentially the same microbial diversity that was present before the initiation of antibiotic therapy remains one of the biggest mysteries in modern medicine.69 Could the restoration of the indigenous microbial flora in antibiotic-associated diarrhea be driven in part by the host providing the neuroendocrine signals that favor the proliferation of certain microbial species that ultimately help restore the microbial diversity within the gastrointestinal tract back to what the host deems most beneficial? These interactions may be from the direct release of neuroendocrine hormones that certain bacteria, in a microbial endocrinology-driven fashion, can utilize to initiate proliferation and elaboration of quorum sensing molecules. Analogously, could the emergence of certain indigenous bacteria, notably Clostridium difficile that may occur as an untoward result of antibiotic therapy70 be due to a failure of the host’s nervous system to provide the proper signals to allow the indigenous flora to recover quickly enough to prevent such pathogen overgrowth?
The microbial organ itself possesses its own nervous system
The discovery that microbes can actively respond to catecholamines coupled with the extensive and varied microbial possession of a wide-ranging spectrum of neuroendocrine hormones raises the obvious paradigm-shifting question whether the development of microbial neuroendocrine production and recognition systems means that within the gastrointestinal tract the microbial organ itself may possess its own nervous system. Since the majority of the microbial flora within the gut exists as a biofilm, there is a need for intra- and inter-species communication. Further, given that a preponderance of what are usually considered to be exclusively mammalian neuroendocrine synthesis pathways also exist in many of the microbial species within the gastrointestinal tract, the question naturally arises that if such common synthesis pathways exist, then why couldn’t the production of the same neurochemicals allow for intercellular communication between the members of the microbiota and hence function as a nervous system? The demonstration of such a microbial organ-specific nervous system would understandably have profound implications both on basic scientific as well as clinical thought since this would suggest that such a functioning “cognitive” system within the gastrointestinal tract must interface with the host to enable and perpetuate its own existence.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 3.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 4.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu Rev Pathol. 2012;7:99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- 5.Fraher MH, O’Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9:312–22. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- 6.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyte M, Freestone PPE. Microbial endocrinology: interkingdom signaling in infectious disease and health. New York: Springer, 2010. [Google Scholar]
- 8.Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 2004;12:14–20. doi: 10.1016/j.tim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Obata K. Synaptic inhibition and γ-aminobutyric acid in the mammalian central nervous system. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:139–56. doi: 10.2183/pjab.89.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjurstöm H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 12.Roshchina VV. Evolutionary considerations of neurotransmitters in microbial, plant and animal cells. In: Lyte M, Freestone PP, eds. Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health. New York: Springer, 2010:17-52. [Google Scholar]
- 13.Tsavkelova EA, Botvinko IV, Kudrin VS, Oleskin AV. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem. 2000;372:115–7. [PubMed] [Google Scholar]
- 14.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–95. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson M, Rowatt E. The production of acetylcholine by a strain of Lactobacillus plantarum. J Gen Microbiol. 1947;1:279–98. doi: 10.1099/00221287-1-3-279. [DOI] [PubMed] [Google Scholar]
- 16.Devalia JL, Grady D, Harmanyeri Y, Tabaqchali S, Davies RJ. Histamine synthesis by respiratory tract micro-organisms: possible role in pathogenicity. J Clin Pathol. 1989;42:516–22. doi: 10.1136/jcp.42.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahkolahi AM, Donahue MJ. Bacterial flora, a possible source of serotonin in the intestine of adult female Ascaris suum. J Parasitol. 1993;79:17–22. doi: 10.2307/3283271. [DOI] [PubMed] [Google Scholar]
- 18.Uzbay TI. The pharmacological importance of agmatine in the brain. Neurosci Biobehav Rev. 2012;36:502–19. doi: 10.1016/j.neubiorev.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Arena ME, Manca de Nadra MC. Biogenic amine production by Lactobacillus. J Appl Microbiol. 2001;90:158–62. doi: 10.1046/j.1365-2672.2001.01223.x. [DOI] [PubMed] [Google Scholar]
- 20.Gale EF. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940;34:392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292–9. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Riley DR, Sieber KB, Robinson KM, White JR, Ganesan A, Nourbakhsh S, Dunning Hotopp JC. Bacteria-human somatic cell lateral gene transfer is enriched in cancer samples. PLoS Comput Biol. 2013;9:e1003107. doi: 10.1371/journal.pcbi.1003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyte M. The microbial organ in the gut as a driver of homeostasis and disease. Med Hypotheses. 2010;74:634–8. doi: 10.1016/j.mehy.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Lenard J. Mammalian hormones in microbial cells. Trends Biochem Sci. 1992;17:147–50. doi: 10.1016/0968-0004(92)90323-2. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, Benno Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep. 2012;2:233. doi: 10.1038/srep00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–81. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 28.Breer H, Eberle J, Frick C, Haid D, Widmayer P. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem Cell Biol. 2012;138:13–24. doi: 10.1007/s00418-012-0954-z. [DOI] [PubMed] [Google Scholar]
- 29.Schafer DF, Fowler JM, Jones EA. Colonic bacteria: a source of gamma-aminobutyric acid in blood. Proc Soc Exp Biol Med. 1981;167:301–3. doi: 10.3181/00379727-167-41169. [DOI] [PubMed] [Google Scholar]
- 30.Minuk GY. Gamma-aminobutyric acid (GABA) production by eight common bacterial pathogens. Scand J Infect Dis. 1986;18:465–7. doi: 10.3109/00365548609032366. [DOI] [PubMed] [Google Scholar]
- 31.Balcar VJ. Presence of a highly efficient “binding” to bacterial contamination can distort data from binding studies. Neurochem Res. 1990;15:1237–8. doi: 10.1007/BF01208585. [DOI] [PubMed] [Google Scholar]
- 32.Guthrie GD, Nicholson-Guthrie CS. gamma-Aminobutyric acid uptake by a bacterial system with neurotransmitter binding characteristics. Proc Natl Acad Sci U S A. 1989;86:7378–81. doi: 10.1073/pnas.86.19.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeRoith D, Roberts C, Jr., Lesniak MA, Roth J. Receptors for intercellular messenger molecules in microbes: similarities to vertebrate receptors and possible implications for diseases in man. Experientia. 1986;42:782–8. doi: 10.1007/BF01941525. [DOI] [PubMed] [Google Scholar]
- 34.Roth J, LeRoith D, Shiloach J, Rosenzweig JL, Lesniak MA, Havrankova J. The evolutionary origins of hormones, neurotransmitters, and other extracellular chemical messengers: implications for mammalian biology. N Engl J Med. 1982;306:523–7. doi: 10.1056/NEJM198203043060907. [DOI] [PubMed] [Google Scholar]
- 35.Lyte M. The role of catecholamines in gram-negative sepsis. Med Hypotheses. 1992;37:255–8. doi: 10.1016/0306-9877(92)90197-K. [DOI] [PubMed] [Google Scholar]
- 36.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50:203–12. doi: 10.1016/0024-3205(92)90273-R. [DOI] [PubMed] [Google Scholar]
- 37.Lyte M. Microbial Endocrinology: A Personal Journey In: Lyte M, Freestone PPE, eds. Microbial endocrinology: interkingdom signaling in infectious disease and health. New York: Springer, 2010:1-16. [Google Scholar]
- 38.Pullinger GD, Carnell SC, Sharaff FF, van Diemen PM, Dziva F, Morgan E, Lyte M, Freestone PP, Stevens MP. Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE. Infect Immun. 2010;78:372–80. doi: 10.1128/IAI.01203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bearson BL, Bearson SM, Uthe JJ, Dowd SE, Houghton JO, Lee I, Toscano MJ, Lay DC., Jr. Iron regulated genes of Salmonella enterica serovar Typhimurium in response to norepinephrine and the requirement of fepDGC for norepinephrine-enhanced growth. Microbes Infect. 2008;10:807–16. doi: 10.1016/j.micinf.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Hegde M, Wood TK, Jayaraman A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol. 2009;84:763–76. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- 41.Oneal MJ, Schafer ER, Madsen ML, Minion FC. Global transcriptional analysis of Mycoplasma hyopneumoniae following exposure to norepinephrine. Microbiology. 2008;154:2581–8. doi: 10.1099/mic.0.2008/020230-0. [DOI] [PubMed] [Google Scholar]
- 42.Peterson G, Kumar A, Gart E, Narayanan S. Catecholamines increase conjugative gene transfer between enteric bacteria. Microb Pathog. 2011;51:1–8. doi: 10.1016/j.micpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Lyte M, Gaykema R, Goehler L. Behavior modification of host by microbes. In: Schaechter M, ed. Encyclopedia of Microbiology. Oxford: Elsevier, 2009:121-7. [Google Scholar]
- 44.Udenfriend S, Lovenberg W, Sjoerdsma A. Physiologically active amines in common fruits and vegetables. Arch Biochem Biophys. 1959;85:487–90. doi: 10.1016/0003-9861(59)90516-8. [DOI] [PubMed] [Google Scholar]
- 45.Von Roepenack-Lahaye E, Newman MA, Schornack S, Hammond-Kosack KE, Lahaye T, Jones JD, Daniels MJ, Dow JM. p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J Biol Chem. 2003;278:43373–83. doi: 10.1074/jbc.M305084200. [DOI] [PubMed] [Google Scholar]
- 46.Pitman RM. Transmitter substances in insects: a review. Comp Gen Pharmacol. 1971;2:347–71. doi: 10.1016/0010-4035(71)90060-7. [DOI] [PubMed] [Google Scholar]
- 47.Guerrero HY, Caceres G, Paiva CL, Marcano D. Hypothalamic and telencephalic catecholamine content in the brain of the teleost fish, Pygocentrus notatus, during the annual reproductive cycle. Gen Comp Endocrinol. 1990;80:257–63. doi: 10.1016/0016-6480(90)90170-Q. [DOI] [PubMed] [Google Scholar]
- 48.Moreira CG, Weinshenker D, Sperandio V. QseC mediates Salmonella enterica serovar typhimurium virulence in vitro and in vivo. Infect Immun. 2010;78:914–26. doi: 10.1128/IAI.01038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Methner U, Rabsch W, Reissbrodt R, Williams PH. Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int J Med Microbiol. 2008;298:429–39. doi: 10.1016/j.ijmm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Reissbrodt R, Rienaecker I, Romanova JM, Freestone PP, Haigh RD, Lyte M, Tschäpe H, Williams PH. Resuscitation of Salmonella enterica serovar typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl Environ Microbiol. 2002;68:4788–94. doi: 10.1128/AEM.68.10.4788-4794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson MT, Armstrong SK. Norepinephrine mediates acquisition of transferrin-iron in Bordetella bronchiseptica. J Bacteriol. 2008;190:3940–7. doi: 10.1128/JB.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyte M, Freestone PP, Neal CP, Olson BA, Haigh RD, Bayston R, Williams PH. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet. 2003;361:130–5. doi: 10.1016/S0140-6736(03)12231-3. [DOI] [PubMed] [Google Scholar]
- 53.Le Roith D, Shiloach J, Berelowitz M, Frohman LA, Liotta AS, Krieger DT, Roth J. Are messenger molecules in microbes the ancestors of the vertebrate hormones and tissue factors? Fed Proc. 1983;42:2602–7. [PubMed] [Google Scholar]
- 54.LeRoith D. Vertebrate hormones and neuropeptides in microbes: evolutionary origin of intercellular communication. In: Martini L, Ganong WF, eds. Front Neuroendocrinol. New York: Raven Press, 1984:1-25. [Google Scholar]
- 55.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 56.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 57.Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol Lett. 1999;172:53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- 58.Vlisidou I, Lyte M, van Diemen PM, Hawes P, Monaghan P, Wallis TS, Stevens MP. The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect Immun. 2004;72:5446–51. doi: 10.1128/IAI.72.9.5446-5451.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78:1509–19. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko CY, Lin HTV, Tsai GJ. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013;48:559–68. doi: 10.1016/j.procbio.2013.02.021. [DOI] [Google Scholar]
- 61.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin MR, Rothermundt M. Clinical psychoneuroimmunology. Handb Clin Neurol. 2012;106:211–25. doi: 10.1016/B978-0-444-52002-9.00012-7. [DOI] [PubMed] [Google Scholar]
- 63.Smith TA. The occurrence, metabolism and functions of amines in plants. Biol Rev Camb Philos Soc. 1971;46:201–41. doi: 10.1111/j.1469-185X.1971.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 64.Kuklin AI, Conger BV. CATECHOLAMINES IN PLANTS. J Plant Growth Regul. 1995;14:91–7. doi: 10.1007/BF00203119. [DOI] [Google Scholar]
- 65.Vijayakumari K, Siddhuraju P, Janardhanan K. Effect of different post-harvest treatments on antinutritional factors in seeds of the tribal pulse, Mucuna pruriens (L.) DC. Int J Food Sci Nutr. 1996;47:263–72. doi: 10.3109/09637489609012587. [DOI] [PubMed] [Google Scholar]
- 66.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96:557–67. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 68.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–57. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 69.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–78. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 70.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 71.Norris V, Molina F, Gewirtz AT. Hypothesis: bacteria control host appetites. J Bacteriol. 2013;195:411–6. doi: 10.1128/JB.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


