Abstract
The impact of the gut microbiota on immune homeostasis within the gut and, importantly, also at systemic sites has gained tremendous research interest over the last few years. The intestinal microbiota is an integral component of a fascinating ecosystem that interacts with and benefits its host on several complex levels to achieve a mutualistic relationship. Host-microbial homeostasis involves appropriate immune regulation within the gut mucosa to maintain a healthy gut while preventing uncontrolled immune responses against the beneficial commensal microbiota potentially leading to chronic inflammatory bowel diseases (IBD). Furthermore, recent studies suggest that the microbiota composition might impact on the susceptibility to immune-mediated disorders such as autoimmunity and allergy. Understanding how the microbiota modulates susceptibility to these diseases is an important step toward better prevention or treatment options for such diseases.
Keywords: microbiota, homeostasis, regulatory T cells, microbial conditioning, IgA, innate lymphoid cells, autoimmunity, allergies
The Microbiota
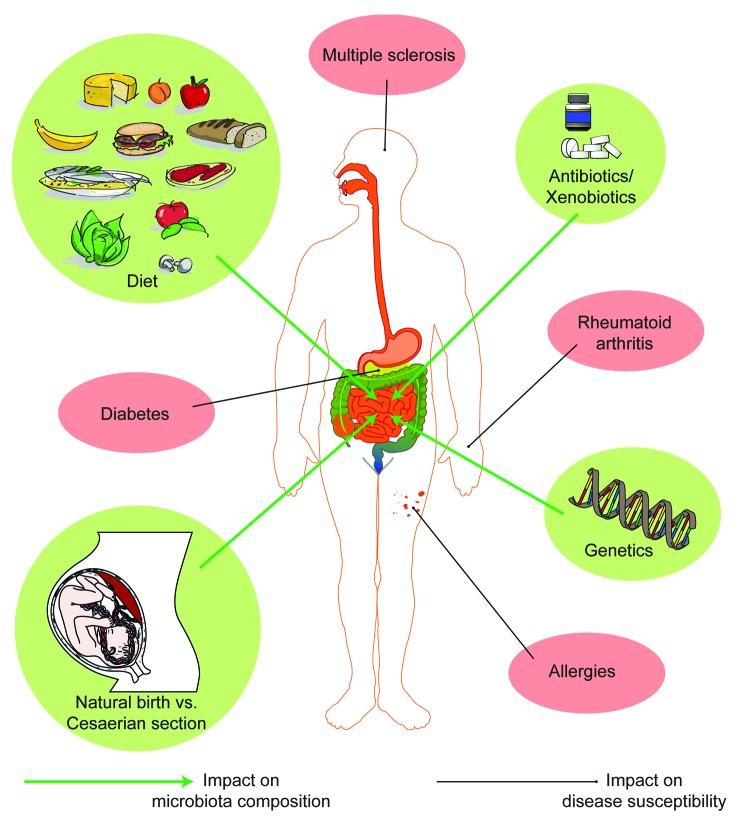
Under normal conditions the intrauterine environment is sterile and bacteria do not colonize fetal body surfaces, including the fetal intestine. Intestinal colonization with a commensal microbiota starts already during the birthing process. In the case of a natural delivery colonization occurs initially through contact with the maternal fecal and vaginal microbiota. This initial seeding with bacteria starts off a sequential and dynamic bacterial colonization process of the infant’s intestine.1 The microbiota is then shaped by sequential colonization events until a stable microbiota is established. It is clear that babies delivered by caesarian section can receive quite a different set of seeding bacteria due to the aseptic nature of caesarian deliveries.1,2 Contact with maternal fecal material is absent (or severely reduced) and first microbial contact will be with commensal microbes from other sources, such as the skin. Colonization during early life is also influenced by feeding practices in that babies that are breast-fed harbor a different microbiota than babies that are formula-fed.3-6 These changes may impact on susceptibility to immune-mediated diseases later in life,7 as discussed further below (Fig. 1).
Figure 1. Interplay between microbiota composition and disease susceptibility. Antibiotics, diet, mode of delivery at birth, and genetics all seem to have a significant impact on the microbiota composition, which in turn might affect the susceptibility to immune mediated disorders.
Establishment of a stable microbiota takes several years in humans (compared with several weeks in laboratory mice depending on the complexity of the microbiota). Events such as weaning off breast milk, introduction of solid foods, diet,8 and early use of high dose antibiotics9 can have a major impact on the composition of the microbiota. Other environmental influences, such as xenobiotics, can influence microbial physiology and gene expression without impacting on the composition of the microbiota.10
The ‘normal’ adult microbiota of humans or mice is extremely diverse and consists of hundreds (or thousands) of bacterial species reaching densities of up to 1012 bacteria per gram content in the large intestine. This is the highest density observed in any bacterial habitat analyzed so far including aqueous, sediment, or soil ecosystems. Despite the observed species richness, the vast majority of intestinal bacteria can be assigned to eight out of the 55 bacterial phyla described so far.11 Such conservation at the phyla level is found in most mammalian species. This alone indicates that formation of the intestinal microbiota is not a random event but rather an evolutionary established process that provides a rich and specialized niche for select members from only a few bacterial phyla.
Axenic and Gnotobiotic Mouse Models
The complexity of the intestinal microbiota of laboratory animals and the variability between individual vivaria is a huge experimental and technical challenge in current biomedical research. Specific pathogen-free (SPF) mice with extremely diverse undefined microbiotas have helped to gain an important initial understanding of the importance of the microbiota composition in a variety of disease models. This is demonstrated for example by the fact that experiments performed in different vivaria often yield conflicting results and one important experimental variable is likely to be differences in the microbiota. Conversely, demonstrating that immune defects can lead to alterations in the microbiota must rely on robust experimental design using co-housed littermate controls. This requirement was elegantly illustrated in a landmark study by Ubeda et al. showing that differences in the composition of the microbiota observed in TLR-deficient mouse strains reflected long-term divergence of the microbiota when the different mouse strains are housed in isolation from each other rather than defective innate immunity.12 Although recent advances in high throughput sequencing technology make it feasible to study very complex microbiotas in detail, to precisely define cause or consequence of an observed effect remains challenging.
Importantly, studying the effects of the intestinal microbiota on the host immune system on a functional and mechanistic level requires precisely defined experimental systems in terms of genetic background (which is achieved through inbreeding) but also at the level of microbiota composition. Axenic (germ-free) mice are reared and housed under absolutely sterile conditions in flexible film isolators.13 Germ-free mice can then be colonized with single or multiple defined bacterial species to obtain gnotobiotic mice. Importantly, gnotobiotic mice also need to be maintained under the same rigorous conditions as axenic mice in order to maintain the gnotobiotic status and prevent introduction of additional microbes from animal handlers or the environment.
Axenic embryo transfer into germ-free pseudopregnant recipient females allows for the efficient re-derivation of any genetically modified (Tg, KO, KI, reporter, fate-map) mouse strain from any hygiene status to germ-free status.13 This gives the researcher the ability to choose from a huge range of inbred isogenic mouse lines and in the future hopefully also of a range of standardized isobiotic microbiotas (or single bacterial species), which can be shared between different laboratories to allow for inter-lab data comparison with a never before reached level of confidence.
As an alternative approach to the addition of defined bacterial species, germ-free mice can also be colonized with a complex consortium of bacteria isolated from conventional or SPF mice or humans. This is also a powerful experimental technique for studying host-microbial interactions and has provided much information about microbial-mediated changes in host responses. For example, the addition of human microbiota to germ-free mice has been extremely informative and has provided the opportunity to study the impact of human disease-associated microbiotas in a controlled experimental setting.8,14-17
The use of gnotobiology is not limited to mouse models. For example, gnotobiotic zebrafish have successfully been used to study evolutionarily conserved responses to the microbiota,18 and germ-free and gnotobiotic Drosophila melanogaster is used to study host-microbial homeostasis.19
In this review we summarize recent developments in understanding host-microbial interactions mostly based on observations made in mouse model systems.
Immune Adaptations in Response to Colonization
Intestinal colonization induces a range of physiological, metabolic, and immune adaptations within the host. Here we focus primarily on the host immune adaptations that promote host-microbial mutualism and immune homeostasis.
Innate immune maturation
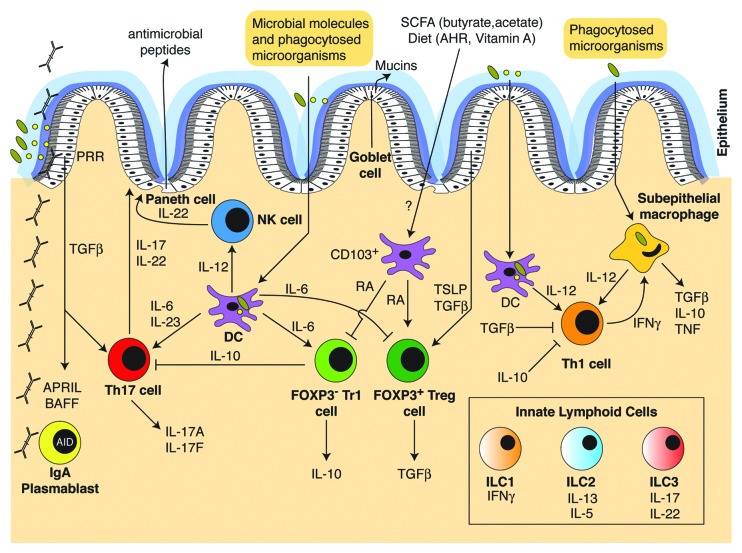
The intestinal epithelium provides a physical and biochemical barrier segregating the intestinal lumen from the inside of the body and intestinal epithelial cells are highly responsive to both microbial and immune-mediated signals. Goblet cells, specialized secretory epithelial cells, secrete mucins that form a tight mucus layer above the epithelial cell layer. The inner mucus layer is essentially devoid of bacteria,20 and the genetic deletion of mucin 2 leads to colitis,21 indicating the important role of mucus in maintaining the physical barrier. As the cells most closely located to the gut lumen, epithelial cells are potent producers of antimicrobial peptides that function as natural antibiotics by either directly killing or inactivating bacteria.22 Although some antimicrobial peptides such as α and β defensins are produced independently of the microbiota,23 others such as the C-type-Lectin Reg3γ or Ang4, which are controlled via pattern recognition receptors on host cells, are almost absent in germ-free mice.24,25 Enterocytes and paneth cells, a specialized epithelial cell that is located at the base of the crypts of Lieberkühn, have been shown to sense the density of the microbiota and become activated upon an increased bacterial load leading to a MyD88-dependent Reg3γ production.26,27 Indeed, knock out models of mice lacking Reg3γ or epithelial MyD88 provided evidence that Reg3γ is needed for the spatial segregation of bacteria and intestinal epithelium and suppression of adaptive immunity.27
In addition to anti-microbial peptides, intestinal epithelial cells also produce multiple cytokines in response to microbial-derived signals. For example, intestinal epithelial cells can produce thymic stromal lymphopoietin (TSLP), transforming growth factor-β (TGF-β), IL-25, a proliferation-inducing ligand (APRIL), and B cell activating factor (BAFF), which have downstream effects on both innate and adaptive immune cells (reviewed in ref. 28). Furthermore, epithelial cells express the receptor for IL-22, a key cytokine involved in intestinal homeostasis.29 Innate immune responses to commensal microbiota and the role of the gut microbiota in promoting host-microbial homeostasis has also been well studied in Drosophila melanogaster (reviewed in ref. 19).
Immunoglobulin A
One of the most prominent effects of intestinal colonization is the induction of secretory IgA (SIgA). With 40–60 mg/kg/day, IgA is the most abundantly produced antibody isotype in the body. IgA is especially important at mucosal surfaces where dimeric IgA is transported into the lumen via polymeric immunoglobulin receptor (pIgR)-dependent transcytosis through intestinal epithelial cells.30 Even though IgA is produced in such great quantities the lack of IgA in humans is quite common and mostly asymptomatic, much like IgA-deficient animals that can compensate for IgA by production of IgM.31 A functional role for IgA in mucosal infections has been clearly demonstrated for example for rotavirus, influenza, and cholera toxin.32 In addition, we have demonstrated that bacterial-specific IgA is induced following colonization and that the IgA repertoire is likely to adapt to changes in the microbial composition.33 The importance for IgA is furthermore demonstrated in activation-induced cytidine deaminase (AID)-deficient mice, which have a defective class switch recombination and somatic hypermutation. Those animals display anaerobic bacterial overgrowth in the proximal intestine and hyperplasia of intestinal isolated lymphoid follicles.34 The same authors have also investigated AID mutant mice in which the ability for class switch recombination is maintained, while, somatic hypermutation is severely impaired due to a single point mutation in AID (AIDG23S). The consequence is a dramatic reduction of intestinal high-affinity IgA and an altered microbial composition,35 thus indicating that high-affinity IgA can shape microbial composition. Nevertheless, the precise function of IgA in promoting host-microbial mutualism under homeostatic conditions (in the absence of infection or toxins) remains elusive.
CD4+ T cells
Another well-studied immune adaptation is the induction of different mucosal CD4+ T cell subsets following intestinal colonization. A variety of functionally distinct CD4+ T cells exist with the best-studied subsets in mucosal tissues being Foxp3+ regulatory T cells (Treg), Th1, Th2, Th17, and T follicular helper (Tfh) cells. A lot of progress has been made in identifying the role of these subsets in host-microbial mutualism by using more or less defined bacterial communities or individual species. While the work of Fiona Powrie and others has clearly demonstrated the role of intestinal Treg in controlling inflammation36 several studies have now demonstrated that normal intestinal colonization either with different bacterial communities37,38 or individual species such as Bacteroides fragilis39 also induces Treg. Even though it seems that some bacterial classes (e.g., Clostridia37,40) might be more potent than others in inducing intestinal Treg it has to be kept in mind that even segmented filamentous bacteria (SFB), which are the prototypical inducers of intestinal Th17 cells, induce Treg.41,42 Although the existence of SFB in humans is controversial, SFB-like organisms have been described in Ulcerative Colitis patients43 and therefore the biological effects of SFB may also be relevant in humans. Probiotic species have also been implicated in the induction of intestinal Treg cells. For instance, treatment of mice with the probiotic mixture VSL#3 (a mixture of bifidobacteria, lactobacilli, and Streptococcus salivarius) or the probiotic strain Lactobacillus reuteri increased the percentage of Treg cells.44,45 Therefore, induction of intestinal Treg following commensal colonization seems to be a hallmark of host-microbial immune adaptation.
B. fragilis-derived polysaccharide A (PSA) has been demonstrated to have immuno-modulatory functions46 but this seems to be a rather special case since PSA is also virulence factor of B. fragilis. Recently, short chain fatty acids (SCFA) have been described to be a more general bacterial metabolite involved in intestinal Treg induction.47-49
How the intestinal CD4+ T cell compartment reacts to changes in the microbiota composition and whether CD4+ T cell subset plasticity is involved in adapting to changes in microbiota composition is currently under investigation by a variety of laboratories.
Innate lymphoid cells (ILC)
Innate lymphoid cells are more recently discovered innate cell types that develop from an Id2-dependent common lymphoid progenitor and share functional characteristics with differentiated T cells (reviewed in ref. 50). ILC can be subdivided into three different groups referred to as Group 1, 2, and 3. Group 1 ILC typically produce IFN-γ and include classical NK cells as well as T-bet+ ILC, which are related to classical natural killer (NK) cells due to their expression of natural cytotoxicity receptors such as NKp46, but while NK cells depend on IL-15, ILC depend on IL-7 and lack granzymes and perforins.51 Group 2 ILC are GATA-3+ and mainly produce IL-13 and IL-5 whereas Group 3 ILC depend on the transcription factor RORγt and are important sources of IL-22 and IL-17. Group 1, 2, and 3 ILC share functional properties with Th1, Th2 and Th17 cells and the subdivision, functional plasticity and lineage conversion properties are not yet fully defined.52,53
At the present time, Group 3 RORγt+ ILC appear to be the most dependent on the presence of microbes or microbial products, although the extent of influence is still not completely understood.54-56 Differentiation of NKp46+ RORγt+ ILCs cells has been shown to depend on commensal microbes.54 Microbial stimulation also enhanced IL-22 production51 and stabilized expression of RORγt via intestinal epithelial cell-derived IL-7.57 The absence of microbiota resulted in reduced RORγt expression in intestinal ILC and preferential induction of IFNγ producing ILC that confer heightened susceptibility to inflammation.57 However, IL-22 production has also been reported to be suppressed in colonized mice due to intestinal epithelial-derived IL-25.56 Therefore RORγt+ ILC appear to be responsive to the presence and/or composition of the intestinal microbiota. Less is known about the impact of microbes on Group 1 and 2 ILC although they are clearly present in germ-free mice.
ILC may sense microbes through Toll-like receptors (TLR) and TLR2 expressed on RORγt+ ILC enhances IL-22 production via autocrine IL-2 signaling.58 However, it is likely that ILC also sense microbes indirectly and may even be better equipped to respond to environmental stimuli, like dietary and microbial metabolites via aryl-hydrocarbon receptor (AHR), NKp46,59 or other NCR.60 In response to microbial exposure intestinal epithelial cells and myeloid cells can secrete many regulatory cytokines and high expression levels of IL-25R, IL-33R, IL-23R, IL1βR, and other cytokine receptors52 poise ILC extremely well for the immediate innate response to changes in homeostasis that are first sensed by epithelial or myeloid cells.61,62
In summary, microbial colonization impacts many different immune cells present in the small and large intestine. There is then a complex interplay within the tissue microenvironment whereby cytokines and chemokines secreted by one cell type further impact the effector function of other cells types, and in turn, immune mediators can also feed back and impact the gut microbiota (Fig. 2).
Figure 2. Overview of the cytokine network regulating innate and adaptive immune-microbiota interactions. The immune cell types and cytokines involved in sensing the microbiota and controlling innate and adaptive immune homeostasis are illustrated.
Effects of Microbial Immune Conditioning Early in Life
Whether the composition of the developing microbiota early in life has an imprinting character on immunological events later in life is an attractive research question since it would potentially rationalize some parts of the hygiene hypothesis, first proposed by Strachan.63 In mouse models, this has been investigated in the context of invariant natural killer T (iNKT) cells and IgE.
Germ-free mice have elevated levels of iNKT cells in the lung and colonic lamina propria due to increased epithelial expression of the chemokine ligand CXCL16.64 iNKT cells express an invariant α chain of the TCR, recognize lipid antigens, and can release copious amounts of cytokines, including IL-4, IL-13, and IFN-γ following activation (reviewed in ref. 65). The increased numbers of iNKT cells in germ-free mice led to increased morbidity following experimental induction of IBD or allergic asthma. Even more striking was the observation that only neonatal colonization of germ-free mice could protect from the accumulation of iNKT cells, therefore indicating that exposure to microbes must occur within a short period of time after birth in order to establish iNKT cell tolerance later in life.
We have shown that elevated IgE levels observed in germ-free mice and mice with a limited microbial diversity is a result of immune dysregulation, which can be corrected by providing the appropriate microbial stimulus early in life.66 Microbial conditioning early in life was shown to be functionally relevant since mice that did not receive appropriate intestinal microbial stimulation during that time were much more prone to antigen-induced oral anaphylaxis later in life.
These findings may be very relevant to the human situation where the composition of the microbiota early in life can be influenced by a multitude of environmental factors, such as mode of delivery (natural birth vs. caesarian section), diet, or antibiotic use.
Impact of the Microbiota on Autoimmunity and Allergic Diseases
Autoimmune and allergic immune disorders such as inflammatory bowel disease, multiple sclerosis or asthma are rapidly increasing in westernized countries. All of these diseases have genetic susceptibility components that are usually identified by genome-wide association studies (GWAS) with more or less predictive value. However, the genetic susceptibility of the population cannot have changed so dramatically over just a few decades to explain the observed increase in incidence. Therefore, a non-genetic environmental (or epigenetic) component must be the driving force of the observed increase in incidence. While a link between the local cutaneous, gastric, or colonic microbiota with disorders of the skin, stomach, or colon, respectively, can easily be envisioned, we are only starting to appreciate the impact of the microbiota composition on systemic immune-mediated diseases. Importantly, there is strong emerging evidence for a functional link between the composition of the intestinal microbiota and susceptibility to several systemic immune disorders, such as Type 1 diabetes,67 rheumatoid arthritis,68 and allergic diseases.69 Animal models provide experimental evidence that changes in type and level of microbial stimulation can impact on disease outcome. The non-obese diabetic (NOD) mouse and the biobreeding diabetes-prone (BB-DP) rat serve as a model for Type 1 diabetes. The incidence of Type 1 diabetes in these animals was correlated with the hygiene conditions prevailing in the animal facility. Using the NOD mouse model a recent study revealed that sex differences in the gut microbiome could regulate autoimmunity in a hormone-dependent way.70 However, other mechanisms involved in microbiota-mediated systemic effects on the immune system remain poorly understood and are subject to intense investigation.
Diet-Microbiota-Immune Axis
The crosstalk between microbes and our immune system is well appreciated. However, it is unquestionable that the nutritional status of an individual impacts on the microbial community and therefore the immune system, through both direct and indirect pathways.71 It is now clear that the gut microbiota composition can shift in response to changes in the diet. In mice, changes can occur very rapidly after changing to a high-fat diet leading to altered microbiome gene expression and metabolic pathways.8 Changes in the microbiota can then influence immune homeostasis through a variety of different pathways.
Commensal microbes in the colon harvest energy from non-digestable polysaccharides like starch, cellulose, or xylans and thereby provide an additional source of energy that becomes accessible for the host. During this microbial fermentation process short chain fatty acids (SCFA) are generated as end products, with butyrate, propionate, and acetate comprising the three most abundantly generated SCFA. Germ-free mice have reduced levels of intestinal SCFA and accumulate non-digestible polysaccharides like raffinose,72 which accounts for enlarged cecum size and the black stool color. SCFA can have multiple effects on epithelial cells and immune cells and can profoundly affect inflammatory responses.73 Butyrate can provide an energy source for colonic epithelial cells74 and reinforce intestinal epithelial barrier integrity,75 but also impact T cell cytokine production.76 SCFA-induced signaling has been shown to inhibit histone-deacetylases,77 regulate autophagy in intestinal epithelial cells,78 modulate chemotaxis and function of neutrophils,79,80 and impact on the size and function of the colonic regulatory T cell pool.47-49 Furthermore, insulin-dependent fat accumulation can be reduced by GPR43 signaling on adipocytes via suppression of insulin-mediated fat uptake.81 Interestingly, since different microbial species produce different levels or types of SCFA,82 these metabolites may mediate some of the immunological effects that have been attributed to microbial diversity.
Vitamin A is an essential fat soluble vitamin that has been known for many years to promote immunity. Retinoic acid, a metabolite of Vitamin A, has potent immune effects (reviewed in ref. 83). Retinyl esters are hydrolyzed in the liver to retinol, which is released into the circulation or secreted in the bile. Retinol is then converted to retinoic acid (RA) within cells through the action of alcohol dehydrogenases (ADH) and retinaldehyde dehydrogenases (RALDH). Epithelial cells, stromal cells and dendritic cells all produce RA, which is controlled by expression of RALDH, which is in turn responsive to TLR signals and cytokines. RA promotes class switch recombination to IgA in B cells,84,85 imprints gut homing in T cells,86 and RA production by CD103+ dendritic cells drives induction of Treg87,88 and can promote Th17 differentiation.89 More recently, Vitamin A deficiency was shown to promote ILC2 induction and inhibit ILC3 activity, which enhanced immunity to worm infection90 and Vitamin A deficiency during pregnancy led to reduced formation of lymph nodes in the offspring and increased susceptibility to infection.91
The microbiota and/or diet can also influence the immune system through stimulating the aryl hydrocarbon receptor (AHR), which is a ligand-dependent transcription factor expressed by a wide range of cell types. The ligands for AHR can be derived from host cells, environmental toxins, bacterial metabolites, or naturally occurring plant-derived phytochemicals, such as flavonoids and glucosinolates from green vegetables like broccoli or brussel sprouts.92 Signaling through the AHR has been shown to regulate the postnatal expansion of intestinal RORγt+ group 3 ILC and the formation of intestinal lymphoid follicles,93-95 regulate Th17 and IL-22 production, Th1/Th2, Treg, and Tr1 cells, and promote the ability of DC to induce Treg differentiation through upregulation of idoleamine 2,3-dioxygenase (IDO) and RA.96 In addition to SCFA and AHR ligands, adenosine 5′-triphosphate (ATP) can also be microbial derived and ATP can induce polarization of Th17 cells. Indeed, low ATP levels in the intestinal lumen have been associated with the absence of Th17 cells in germ-free mice and administration of systemic or rectal ATP led to an increase in Th17 cells.97
Conclusion
Our microbial partners heavily influence the mucosal and systemic immune systems and the dynamics of colonization early in life are critically involved in educating the developing immune system. Changes in microbial composition, diversity, metabolism, and gene expression seem to have far-reaching effects on immunity and may be particularly relevant in the context of immune-mediated diseases such as autoimmunity or allergy. Understanding the mechanisms involved will help provide better treatment, or even prevention, protocols for such diseases in the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
K.D.M. received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC grant Agreement no. 281785 and the Swiss National Science Foundation. M.B.G. is a recipient of an Ambizione fellowship from the Swiss National Science Foundation.
Glossary
Abbreviations:
- IBD
Inflammatory bowel diseases
- Treg
regulatory T cells
- Th
T helper cells
- ILC
innate lymphoid cells
- T1D
Type 1 Diabetes
- EAE
Experimental Autoimmune Encephalomyelitis
- RA
retinoic acid (RA)
- SFB
segmented filamentous bacteria
- ASF
Altered Schaedler Flora
- DC
dendritic cell
- NK
natural killer cell
- PRR
pattern recognition receptor
- AHR
aryl hydrocarbon receptor
- SCFA
short chain fatty acids
- IL
interleukin
- TGFβ
tumor growth factor β
- TNF
tumor necrosis factor
- INFγ
interferon-γ
- TSLP
thymic stromal lymphopoietin
- RALDH
Retinaldehyde dehydrogenases
- BAFF
B cell activating factor
- APRIL
A proliferation-inducing ligand
References
- 1.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38:321–31. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmer SE, Wharton BA. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child. 1989;64:1672–7. doi: 10.1136/adc.64.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benno Y, Sawada K, Mitsuoka T. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol. 1984;28:975–86. doi: 10.1111/j.1348-0421.1984.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 5.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:478–82. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–45S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 7.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, Reijmerink NE, Dompeling E, van den Brandt PA, Ferreira I, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–, e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–56. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6:20ra11. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011;108:6252–7. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 18.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 2004;101:4596–601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615–26. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 20.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, Andersson M. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem. 2000;275:40478–82. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 24.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 26.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–8. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 29.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–63. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 30.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–21. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 31.Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–9. [PubMed] [Google Scholar]
- 32.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–32. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 33.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–7. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 35.Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12:264–70. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- 36.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–82. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 41.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caselli M, Tosini D, Gafà R, Gasbarrini A, Lanza G. Segmented filamentous bacteria-like organisms in histological slides of ileo-cecal valves in patients with ulcerative colitis. Am J Gastroenterol. 2013;108:860–1. doi: 10.1038/ajg.2013.61. [DOI] [PubMed] [Google Scholar]
- 44.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–46. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 45.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–93. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 46.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 47.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 49.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 51.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 53.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 54.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–9. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 56.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–6. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 57.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–64. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, Achdout H, Bachrach G, Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8:e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76:1719–27. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–87. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6:177–88. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 66.Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14:559–70. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathis D, Benoist C. The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol Rev. 2012;245:239–49. doi: 10.1111/j.1600-065X.2011.01084.x. [DOI] [PubMed] [Google Scholar]
- 68.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–78. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, Karisola P, Auvinen P, Paulin L, Mäkelä MJ, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012;109:8334–9. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 71.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, Rezzi S, Ross A, Kochhar S, Holmes E, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 75.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 76.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/S1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 77.Kim GW, Gocevski G, Wu CJ, Yang XJ. Dietary, metabolic, and potentially environmental modulation of the lysine acetylation machinery. Int J Cell Biol. 2010;2010:632739. doi: 10.1155/2010/632739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Chen Y, Jiang H, Nie D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy. 2011;7:235–7. doi: 10.4161/auto.7.2.14277. [DOI] [PubMed] [Google Scholar]
- 79.Vinolo MA, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct. 2009;27:48–55. doi: 10.1002/cbf.1533. [DOI] [PubMed] [Google Scholar]
- 80.Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH, Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond) 2009;117:331–8. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- 81.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011–6. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 87.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–47. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr., Wang J, Ramalingam TR, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–7. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–7. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013;65:1148–61. doi: 10.1124/pr.113.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–5. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 94.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–51. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiss EA, Diefenbach A. Role of the Aryl Hydrocarbon Receptor in Controlling Maintenance and Functional Programs of RORγt(+) Innate Lymphoid Cells and Intraepithelial Lymphocytes. Front Immunol. 2012;3:124. doi: 10.3389/fimmu.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]