Abstract
Introduction
Despite effective local therapy with surgery and radiation (RT), approximately 50% of patients with high grade soft tissue sarcoma (STS) will relapse and die of disease. Since experimental data suggest a significant synergistic effect when anti-angiogenic targeted therapies, such as sorafenib, are combined with RT, we chose to evaluate preoperative combined modality sorafenib and conformal RT in a Phase I/II trial among patients with extremity STS amenable to treatment with curative intent.
Methods
For the Phase I trial, eight patients with intermediate or high grade STS > 5 cm in maximal dimension or low grade STS > 8 cm in maximal dimension received concomitant sorafenib (dose escalation cohort 1:200 bid, cohort 2:200/400 daily) and preoperative RT (50 Gy in 25 fractions). Sorafenib was continued during entire period of RT as tolerated. Surgical resection was completed four to six weeks following completion of neoadjuvant sorafenib/RT. Three sorafenib dose levels were planned. Primary endpoints of the Phase I trial were maximal tolerated dose and dose-limiting toxicity (DLT).
Results
Eight patients were enrolled in the Phase I (5 female, median age 44, 3 high grade pleomorphic, 2 myxoid/round cell liposarcoma, 3 other). Median tumor size was 16 cm (range 8–29), and all tumors were located in the lower extremity. Two of 5 patients treated at dose level 2 developed DLT consisting of grade 3 rash not tolerating drug reintroduction. Other grade 3 side effects included anemia, perirectal abscess, and SVT. Radiation toxicity (grade 1 or 2 dermatitis, N=8) and post-surgical complications (3 grade 3 wound complications) were comparable to historical controls and other series of preoperative RT monotherapy. Complete pathologic reponse (≥ 95% tumor necrosis) was observed in 3 patients (38%).
Conclusion
Neoadjuvant sorafenib in combination with RT is tolerable and appears to demonstrate activity in locally advanced extremity STS. Further study to determine efficacy at dose level 1 is warranted. (NCT#00805727, ClinicalTrials.gov)
Keywords: Soft Tissue Sarcoma, Preoperative Radiotherapy, Sorafenib, Pathologic Response
Introduction
Although limb-sparing surgery combined with radiotherapy (RT) results in local control rates of approximately 90% among cases of extremity soft tissue sarcoma (STS), [1] patients with high grade tumors have a risk of distant recurrence and death approaching 50% within 5 years of diagnosis. Therefore, despite the recent FDA approval of pazopanib for metastatic STS, novel combined modality approaches are needed.
Angiogenesis is a fundamental component of tumor development and acquisition of the metastatic phenotype. [2, 3] Overexpression of vascular endothelial growth factor (VEGF) and its receptors has been observed as a near universal neoplastic phenomenon, and STS have been shown to overexpress angiogenic factors in both tumor tissue and serum. [4–6] In addition, early-stage clinical trials suggest that the combination of RT and anti-angiogenic agents may exhibit a synergistic effect. [7, 8] These data support the hypothesis that neoadjuvant/adjuvant therapy with TKIs in combination with RT may demonstrate enhanced activity for patients with STS.
Sorafenib is a multi-kinase inhibitor with anti-proliferative and anti-angiogenic effects. The safety and clinical activity of sorafenib has been examined in phase I studies conducted in patients with solid tumors. [9, 10] Phase III clinical trials have established the efficacy of sorafenib for patients with renal cell and hepatocellular carcinoma. [11, 12] Given its favorable toxicity profile, its potential synergistic effect with RT, and evidence for activity in the metastatic setting, we sought to test the hypothesis that preoperative combined modality sorafenib and conformal RT would be safe for patients with locally advanced STS of the extremity.
Patients and Methods
Patient Selection
Eligible patients were age ≥ 18, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, with pathologically confirmed STS of the extremity, either intermediate or high grade histology ≥ 5 cm in maximal-dimension or low grade histology ≥ 8 cm in maximal-dimension, with no evidence of metastatic disease. Additional inclusion and exclusion criteria are provided as a Supplemental Table. Both the UC Davis Scientific Review Committee and the Institutional Review Board reviewed and approved the study protocol and informed consent form prior to any patient being registered on this study. We obtained written informed consent from all patients prior to study entry.
Study Design
The primary objective of the phase I trial was to evaluate safety and toxicity, and the primary endpoint was maximal tolerated dose (MTD). A traditional dose-escalation design was used to determine MTD (NCT#00805727, ClinicalTrials.gov). For the determination of sorafenib-related adverse events (AEs), we based our evaluation on the time between initiation of concurrent sorafenib/RT and the date of surgery.
The MTD was defined as one dose level below that which produced dose-limiting toxicity (DLT) in 33% of patients. The study was designed to evaluate 3 dose levels, based on a starting dose of 200 mg bid, since the combination of sorafenib/RT had not been previously evaluated in a prospective clinical trial. Since 400 mg bid is the well-established MTD for sorafenib monotherapy, the study was not designed to escalate above this level. Dose level escalation was based on DLTs observed from the start of treatment until the date of definitive operation, but we also monitored DLTs in the postoperative period. A formal stopping rule, evaluating post-surgical AEs was pre-specified for the Phase II trial. We elected not to include a confirmation cohort for dose level 1 since we were assessing the novel combination of two previously FDA-approved drugs/modalities with well-described safety data. [13, 14]
Study Procedures
Sorafenib was initiated concomitant with RT on day 1 of study treatment. RT consisted of a total dose of 50 Gy delivered in 25 fractions of 200 cGy. RT treatment planning was based on a radial margin of approximately 2 cm and a longitudinal margin of approximately 3 cm beyond the gross disease.
Surgical resection occurred day 50–90 of the study to allow for the acute toxicities of neoadjuvant therapy to resolve. Routine perioperative care was provided, but careful documentation of wound complications was an integral part of this clinical trial. Wound complications were defined as described by O’Sullivan et al. [1]
Toxicity Assessment
Physical examinations, blood pressure (BP) monitoring, and routine laboratory studies were performed at study initiation. Physical examinations and BP monitoring were conducted weekly during concomitant sorafenib/RT treatments and then biweekly prior to surgical resection. Adverse events were graded according to CTCAE v3.0.
Definition of DLT
Per protocol, dose level escalation was based on DLTs observed from start of treatment until the date of surgery. DLT was defined as grade 4 anemia, grade 4 neutropenia lasting > 7 days, grade 3–4 thrombocytopenia with clinically significant bleeding or grade 4 thrombocytopenia lasting > 5 days, and grade 3–4 non-hematologic toxicity except for nausea/vomiting that responded to anti-emetic therapy, grade 3 neutropenic fever lasting ≤5 days, grade 3 encephalopathy lasting ≤2 days, grade 3–4 hypokalemia, hypophosphatemia, or hypomagnesemia unless hospitalization was required, grade 3 diarrhea controlled with medication within 2 days, and grade 3 hypertension that was not adequately controlled with medication (BP <150/100). We also prospectively articulated a treatment stop rule for the phase II portion of the trial after 10 patients have completed concurrent sorafenib, RT, and surgical resection to assess for a possible increased rate of overall AEs.
Radiologic and Pathologic Response
Radiologic response was evaluated by contrast-enhanced magnetic resonance imaging using the Response Evaluation Criteria in Solid Tumors (RECIST). Post-treatment imaging was obtained 1–2 weeks prior to surgical resection.
All pathology slides were subjected to central review by a subspecialty sarcoma pathologist (D.B.). Specimens were examined for determination of percentage tumor necrosis as defined previously. [15, 16] Complete or near-complete pathologic response (path CR) was defined as ≥ 95% tumor necrosis. [16, 17]
Serum Biomarkers
Exploratory molecular correlative analyses were conducted on serial plasma specimens. Blood samples were collected into a tube containing potassium EDTA and were centrifuged at 4°C within 10–15 minutes. Multiplex analysis using Luminex-technology was used to determine plasma levels of growth and angiogenic factors. Samples were measured in duplicate.
The rationale for the assessment of biomarkers was based on the pleotropic effects of sorafenib which affects tumor cell proliferation through the Raf/MEK/ERK signaling pathway and angiogenesis through the VEGFR pathway. [18–20] In addition, hypoxia has profound effects on radioresistance, mediated in part through the HIF-1α transcription factor. [5, 6, 21] Therefore, we evaluated changes in the serum expression of these proteins as predictors of response and/or resistance to treatment.
Statistical Considerations
Summary statistics were reported as mean ± standard error with median/range where appropriate. We analyzed serum biomarker levels using a repeated measures analysis of variance (ANOVA) to compare repeated measurements in individual biomarkers over time between responders (path CR) and non-responders. We first tested the interaction terms between the time points and the groups. The Kaplan-Meier method was used to estimate survival curves. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). Significance was set at P < 0.05.
Results
Patient and Tumor Characteristics
Between July 2009 and November 2011, 8 patients underwent treatment. The baseline patient and tumor characteristics are depicted in Table 1. There were 2 patients with low grade tumors, including an 8 cm myxoid liposarcoma involving the sciatic nerve and a 29 cm well-differentiated liposarcoma with concern on preoperative imaging for a dedifferentiated component. Two patients (25%) underwent R1 resections, including 1 patient with a gross complete resection but a microscopic positive margin along the sciatic nerve and 1 patient with well-differentiated liposarcoma with gross complete resection with tumor capsule intact, but a < 1 mm microscopic margin.
TABLE 1.
Clinicopathologic Characteristics of Soft Tissue Sarcoma Patients Undergoing Neoadjuvant Conformal Radiotherapy plus Sorafenib
| Characteristic | Number (N=8) | % | |
|---|---|---|---|
| Gender | Male | 3 | 38 |
| Female | 5 | 62 | |
|
| |||
| Age at Diagnosis, median (range) | 44 (33–74) | ||
|
| |||
| Site | Lower Extremity | 8 | 100 |
|
| |||
| Grade | High | 6 | 75 |
| Low | 2 | 25 | |
|
| |||
| Depth | Deep | 7 | 88 |
| Superficial | 1 | 12 | |
|
| |||
| Histology | High Grade Undifferentiated Pleomorphic | 2 | 38 |
| Myxoid/Round Cell Liposarcoma | 2 | 25 | |
| Clear Cell Sarcoma | 1 | 12 | |
| Myxofibrosarcoma | 1 | 12 | |
| Myxoid Liposarcoma | 1 | 12 | |
| Well-Differentiated Liposarcoma | 1 | ||
|
| |||
| Maximal Tumor Diameter median (range) | 16.3 cm (8.2–29.4) | ||
|
| |||
| Margin Status | R0 | 6 | 75 |
| R1 | 2 | 25 | |
Toxicity
Toxicity from combined modality therapy is depicted in Table 2. Two of 5 patients treated at sorafenib dose level 2 experienced DLT consisting of grade 3 macular-papular rash not tolerating drug reintroduction. Other grade 3 side effects included anemia, perirectal abscess, and supra-ventricular tachycardia (SVT). Patient 6 developed grade 3 anemia (hemoglobin 6.9 g/dL) with concomitant SVT. This event was classified as possibly related to protocol therapy, but not considered a DLT given the protocol specifications regarding hematologic toxicities. Patient 8 developed a peri-rectal abscess after completion of neoadjuvant treatment but prior to surgical resection. This was classified as not related to protocol therapy. One patient (12%) developed hand-foot syndrome/palmar-plantar erythrodysesthesia.
Table 2.
Toxicity from Combined Modality Therapy
| A. | |||
|---|---|---|---|
| Sorafenib Dose Level* | Patient¶ | Sorafenib | |
| Any Toxicity | ≥ Grade 3 Toxicity | ||
| 1 | 1 | Hypertension | None |
| 3 | Hypertension | None | |
| 4 | Hand-Foot Syndrome | None | |
| 2 | 5 | None | None |
| 6 | Anemia, Supraventricular Tachycardia | Anemia, Supraventricular Tachycardia | |
| 7 | Rash | Rash** | |
| 8 | Perirectal abscess | Perirectal abscess | |
| 9 | Rash | Rash** | |
| B. | |||
|---|---|---|---|
| Sorafenib Dose Level | Patient | Radiotherapy | |
| Any Toxicity | ≥ Grade 3 Toxicity | ||
| 1 | 1 | Dermatitis | None |
| 3 | Dermatitis | None | |
| 4 | Dermatitis | None | |
| 2 | 5 | Dermatitis | None |
| 6 | Dermatitis | None | |
| 7 | Dermatitis | None | |
| 8 | Dermatitis | None | |
| 9 | Dermatitis | None | |
| C. | |||
|---|---|---|---|
| Sorafenib Dose Level | Patient | Surgery | |
| Any Toxicity | ≥ Grade 3 Toxicity | ||
| 1 | 1 | Surgical Site | None |
| 3 | Surgical Site | Surgical Site | |
| 4 | None | None | |
| 2 | 5 | None | None |
| 6 | Surgical Site | Surgical Site | |
| 7 | None | None | |
| 8 | Surgical Site | Surgical Site | |
| 9 | None | None | |
Dose level 1 = 200 mg BID. Dose level 2 = 200 mg Q AM/400 mg Q PM
Patient 2 was screened and consented, but withdrew consent prior to initiating treatment.
Per protocol, grade 3 rash classified as > 30% body surface area macular/papular rash not tolerating drug reintroduction after resolution of rash to grade 2 or below. Grade 3 non-hematologic toxicities were classified as dose-limiting toxicities (DLT).
Radiation-related toxicity and post-surgical wound complications are depicted in Table 2. Radiation toxicity (grade 1 or 2 dermatitis, N=8) and post-surgical complications (38% grade 3 wound complications) were comparable to rates anticipated with preoperative RT monotherapy.
Radiographic and Pathologic Response to Treatment
There were 7 patients who experienced stable disease by RECIST criteria and 1 patient with a partial response. Three study patients (38%) demonstrated path CR (Table 2), including one patient (1/3) at dose level 1 and two patients at dose level 2 (2/5). The association of tumor size, tumor histology, and tumor necrosis is depicted in Table 3.
Table 3.
Individual Patient Treatment Response
| Sorafenib Dose Level* | Patient¶ | Histology | Tumor Size (cm) | Percent Necrosis |
|---|---|---|---|---|
| 1 | 1 | Myxoid/Round Cell Liposarcoma | 22.1 | 96 |
| 3 | Clear Cell Sarcoma | 18.9 | 5 | |
| 4 | Myxoid Liposarcoma | 8.2 | 5 | |
| 2 | 5 | Myxoid/Round Cell Liposarcoma | 9.8 | 95 |
| 6 | High Grade Undifferentiated Pleomorphic Sarcoma | 14.6 | 55 | |
| 7 | High Grade Undifferentiated Pleomorphic Sarcoma | 12.9 | 95 | |
| 8 | Myxofibrosarcoma | 18.0 | 85 | |
| 9 | Well-differentiated Liposarcoma | 29.4 | 5 |
Dose level 1 = 200 mg BID. Dose level 2 = 200 mg Q AM/400 mg Q PM
Patient 2 was screened and consented, but withdrew consent prior to initiating treatment.
Serum Biomarkers
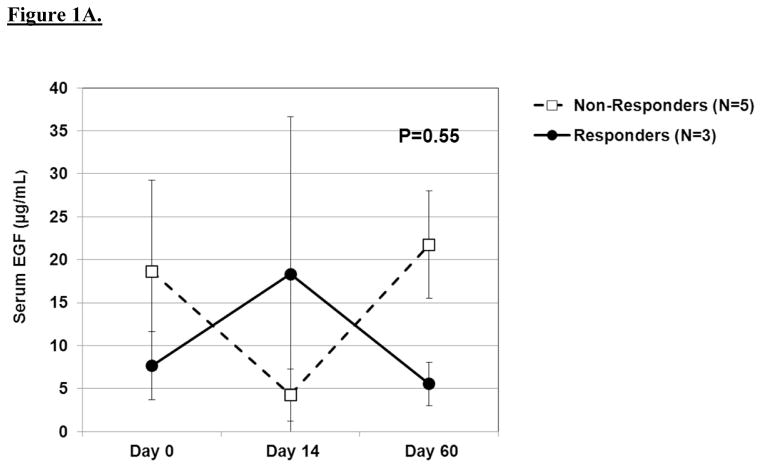
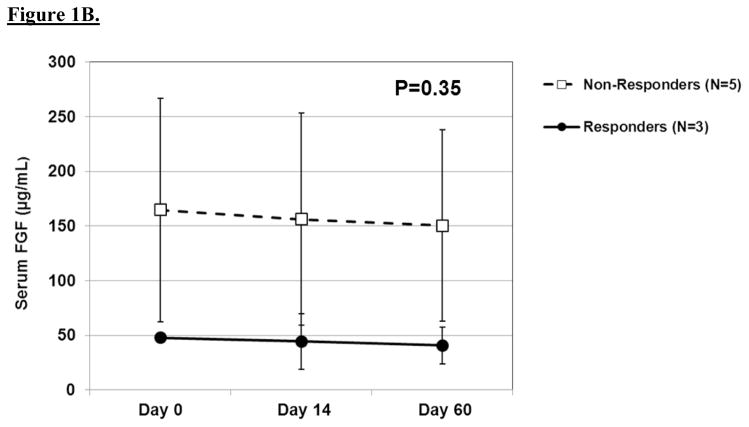
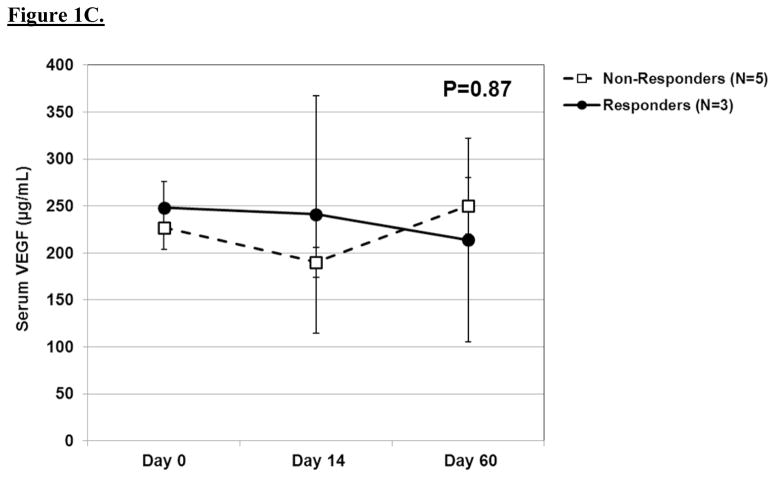
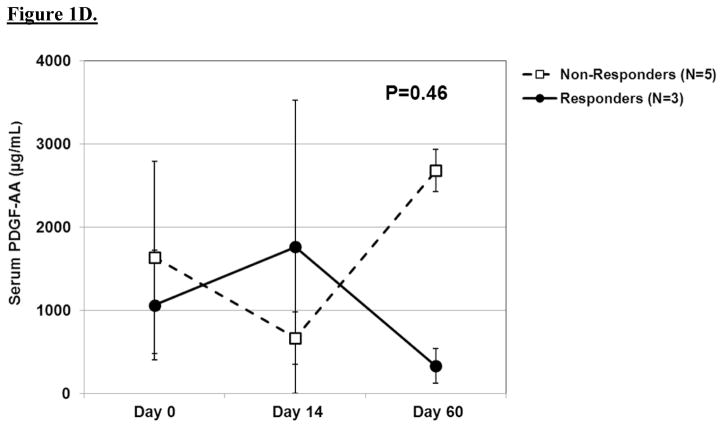
The results of serial biochemical testing for angiogenic and growth factors are depicted in Figures 2A–D. Overall, there were no significant differences in the serum levels of these biomarkers among pathologic responders and non-responders at any of the time points. VEGF (Figure 2c), in particular, showed no appreciable differences among responders and non-responders at any time point. In contrast, there was a trend among non-responders to have rising levels of epidermal growth factor and platelet-derived growth factor-AA at day 60 as well as persistently elevated levels of basic fibroblast growth factor at each time point.
Oncologic Outcome
With a median follow-up of 30 months, three-year overall survival, local recurrence-free survival, and distant recurrence-free survival were 75%, 100%, and 42%, respectively. There were 4 distant recurrences. These occurred at 3, 3, 6, and 31 months, respectively. Two of these patients (clear cell sarcoma and HGUPS) died of disease, while one patient (HGUPS) was diagnosed with stable disease following a combination of systemic chemotherapy and stereotactic body radiation for pulmonary metastases. The other patient (myxoid/round cell liposarcoma) was rendered disease-free following resection of an oligo-metastatic lesion.
Discussion
Although limb-sparing surgery combined with RT is highly effective in the local management of extremity STS, distant disease control remains a significant problem for these patients, particularly those with high grade histologic subtypes. [22] Because of the high risk nature of American Joint Committee on Cancer (AJCC) stage 3 STS, adjuvant and neoadjuvant chemotherapy has frequently been advocated for patients with locally advanced STS. However, studies utilizing traditional cytotoxic chemotherapeutic agents, either alone or in combination with RT, have been equivocal, and prospective studies which have demonstrated improved outcomes following adjuvant chemotherapy are mitigated by other studies demonstrating no benefit or loss of benefit with longer follow up. [23–27] A recent updated meta-analysis of the role of perioperative chemotherapy in the management of locally advanced, but non-metastatic STS, observed a modest, but statistically significant, benefit in favor of Adriamycin/ifosfamide-based chemotherapy. [28] However, there is significant concern about the potential toxicities of cytotoxic chemotherapy in the management of localized STS, particularly when the oncologic benefits appear to be modest. Therefore, the evaluation of novel therapeutic agents for the treatment of STS appears warranted. One of the benefits of neo-adjuvant therapy is the ability to monitor disease response, including pathologic response, as a surrogate marker of oncologic outcome.
Sorafenib is pleotropic tyrosine kinase inhibitor with anti-angiogenic and anti-proliferative properties. [18] It is FDA-approved in the management of metastatic renal cell carcinoma and hepatocellular carcinoma. Phase II studies have demonstrated activity and clinical benefit for patients with metastatic STS. [29, 30] Maki et al., for example, observed a clinical benefit rate (complete response + partial response + stable disease) of 56% at 3 months among 122 patients with recurrent or metastatic STS. Patients with angiosarcoma and leiomyosarcoma experienced the most favorable responses. [29] Similarly, von Mehren et al. evaluated sorafenib in a multi-institutional Phase II study of patients with metastatic STS. Although there were no patients with radiographic response, 6 patients (75%) with vascular sarcomas experienced clinical benefit for ≥ 6 months. [30]
Furthermore, pre-clinical data suggest a significant synergistic effect when anti-angiogenic targeted therapiesare combined with RT. [19, 31] Anti-angiogenic therapies appear to “normalize” the tumor vasculature, thereby rendering them less hypoxic and more sensitive to RT. [2, 3, 32] This potential radio-sensitization is significant in STS since complete and near-complete pathologic response have been associated with improved oncologic outcomes in STS patients treated with neoadjuvant therapy. [17, 33, 34] Furthermore, a key randomized clinical trial evaluating preoperative versus postoperative RT for extremity STS patients undergoing resection with curative intent demonstrated that overall survival was statistically improved in the patients who received preoperative radiotherapy (85% vs. 75% at 3 years, P=0.048). [1] However, it is important to note that the survival data from this trial were a secondary endpoint., Given that no differences were observed in progression-free survival or regional/distant recurrences between the preoperative and postoperative radiotherapy cohorts, these survival data should be viewed with caution. Nevertheless, it is reasonable to hypothesize that neoadjuvant RT in combination with novel systemic agents, such as sorafenib, may improve both local and distant disease-control in patients with STS, potentially through immune-mediated effects. [22, 35]
The primary objective of this phase I trial was to evaluate safety and toxicity and thereby determine the maximal tolerated dose (MTD). Our data demonstrate that sorafenib can be administered safely in the neoadjuvant setting in combination with concomitant conformal RT. Side effects were consistent with standard toxicities observed with sorafenib monotherapy, including hypertension and macular-papular rash, [29, 30] while the incidence of hand-foot syndrome was low (12%),. No unanticipated side effects were observed, and the rates of RT and post-surgical morbidities were consistent with those observed in other studies of these modalities. [1, 33]
Although this Phase I study was not designed to evaluate therapeutic response, we did observe a 38% rate of path CR, including one patient (1/3) at dose level 1 and two patients at dose level 2 (2/5). Conversely, we observed 4 distant recurrences and 2 disease-related deaths during follow up of our cohort, and these oncologic outcomes do not appear superior to historical controls. [33, 36] This discrepancy between pathologic response and oncologic outcome may be related to the relatively short period of exposure to sorafenib as systemic therapy in our study. Other early phase clinical trials of novel neoadjuvant therapies have resumed therapy for responding patients in the postoperative period. This approach may translate into superior distant disease control and long term oncologic outcome.
Another potential limitation of our study is the lack of stratification based on histology. As has been observed in the metastatic setting, there are differences in STS response rates to sorafenib based on histologic type, and differences in radio-sensitivity among STS histologies have also been demonstrated. [33, 37–39] We intend to address this important issue in the phase II setting by employing a histology-based clinical trial design which includes both a myxoid and non-myxoid histology arm.
In summary, among patients with locally advanced extremity STS, neoadjuvant sorafenib in combination with conformal RT is tolerable and appears to show activity. The results of this phase I trial indicate that dose level 1 (200 mg bid) is the appropriate MTD. Further study to determine efficacy at this level appears warranted.
Supplementary Material
Figure 1.
A. Line graph demonstrating baseline and post-treatment levels of serum EGF among pathologic responders and non-responders over time. Mean ± standard error are shown. A repeated measures ANOVA was used to compare repeated measurements over time. B. Line graph demonstrating baseline and post-treatment levels of serum bFGF among pathologic responders and non-responders over time. C. Line graph demonstrating baseline and post-treatment levels of serum VEGF among pathologic responders and non-responders over time. D. Line graph demonstrating baseline and post-treatment levels of serum PDGF-AA among pathologic responders and non-responders over time.
Acknowledgments
The study described was supported by Bayer-Onyx as well as the National Center for Advancing Translational Sciences (NCATS) and the National Institutes of Health (NIH) through grant #UL1 TR000002. We also thank Dr. Philip Mack and William S. Holland from the Molecular Pharmacology Core Laboratory at the University of California Davis Comprehensive Cancer Center for support in the development and conduct of the serum biomarker correlative studies.
Footnotes
Presented in part at the Society of Surgical Oncology 65th Annual Cancer Symposium, Orlando, FL, March 21–24, 2012.
Clinical Trial Information: NCT#00805727
References
- 1.O’Sullivan B, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29(6 Suppl 16):3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Angiogenesis and lymphangiogenesis in tumors: insights from intravital microscopy. Cold Spring Harb Symp Quant Biol. 2002;67:239–48. doi: 10.1101/sqb.2002.67.239. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SS, et al. Circulating angiogenic factor levels correlate with extent of disease and risk of recurrence in patients with soft tissue sarcoma. Ann Oncol. 2004;15(8):1261–6. doi: 10.1093/annonc/mdh309. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SS, et al. Angiogenic profile of soft tissue sarcomas based on analysis of circulating factors and microarray gene expression. J Surg Res. 2006;135(2):282–90. doi: 10.1016/j.jss.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Yudoh K, et al. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br J Cancer. 2001;84(12):1610–5. doi: 10.1054/bjoc.2001.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12(4):465–77. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 8.Czito BG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys. 2007;68(2):472–8. doi: 10.1016/j.ijrobp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Awada A, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92(10):1855–61. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strumberg D, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 11.Ratain MJ, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(16):2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 13.Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96(13):990–7. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- 14.Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352(9):930–2. doi: 10.1056/NEJMe058007. [DOI] [PubMed] [Google Scholar]
- 15.Shah D, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32(9):3911–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Canter RJ, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17(10):2578–84. doi: 10.1245/s10434-010-1156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eilber FC, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–9. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm SM, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 19.Plastaras JP, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67(19):9443–54. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 20.Chang YS, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–74. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 21.Lim JH, et al. Ras-dependent induction of HIF-1alpha785 via the Raf/MEK/ERK pathway: a novel mechanism of Ras-mediated tumor promotion. Oncogene. 2004;23(58):9427–31. doi: 10.1038/sj.onc.1208003. [DOI] [PubMed] [Google Scholar]
- 22.Wunder JS, et al. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8(6):513–24. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- 23.Frustaci S, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238–47. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 24.Frustaci S, et al. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65(Suppl 2):80–4. doi: 10.1159/000073366. [DOI] [PubMed] [Google Scholar]
- 25.Gortzak E, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096–103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 26.Petrioli R, et al. Adjuvant epirubicin with or without Ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol. 2002;25(5):468–73. doi: 10.1097/00000421-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Brodowicz T, et al. Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma. 2000;4(4):151–60. doi: 10.1155/2000/126837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pervaiz N, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573–81. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 29.Maki RG, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Mehren M, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118(3):770–6. doi: 10.1002/cncr.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, et al. Angiogenesis and radiation response modulation after vascular endothelial growth factor receptor-2 (VEGFR2) blockade. Int J Radiat Oncol Biol Phys. 2005;62(5):1477–85. doi: 10.1016/j.ijrobp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19(4 Suppl 3):7–16. [PubMed] [Google Scholar]
- 33.Canter RJ, et al. Radiographic and Histologic Response to Neoadjuvant Radiotherapy in Patients With Soft Tissue Sarcoma. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wunder JS, et al. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80(7):1020–33. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Romero AI, et al. Regulation of CD4+NKG2D+ Th1 Cells in Patients with Metastatic Melanoma Treated with Sorafenib: Role of IL-15Ralpha and NKG2D Triggering. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1186. [DOI] [PubMed] [Google Scholar]
- 36.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–6. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 37.Pitson G, et al. Radiation response: an additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys. 2004;60(2):522–6. doi: 10.1016/j.ijrobp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Roberge D, et al. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97(3):404–7. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Chung PW, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115(14):3254–61. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.