Abstract
Yoga, an increasingly popular discipline among Westerners, is frequently used to improve painful conditions. We investigated possible neuroanatomical underpinnings of the beneficial effects of yoga using sensory testing and magnetic resonance imaging techniques. North American yogis tolerated pain more than twice as long as individually matched controls and had more gray matter (GM) in multiple brain regions. Across subjects, insular GM uniquely correlated with pain tolerance. Insular GM volume in yogis positively correlated with yoga experience, suggesting a causal relationship between yoga and insular size. Yogis also had increased left intrainsular white matter integrity, consistent with a strengthened insular integration of nociceptive input and parasympathetic autonomic regulation. Yogis, as opposed to controls, used cognitive strategies involving parasympathetic activation and interoceptive awareness to tolerate pain, which could have led to use-dependent hypertrophy of insular cortex. Together, these findings suggest that regular and long-term yoga practice improves pain tolerance in typical North Americans by teaching different ways to deal with sensory inputs and the potential emotional reactions attached to those inputs leading to a change in insular brain anatomy and connectivity.
Keywords: DTI, insula, pain tolerance, VBM, yoga practice
Introduction
Yoga practice integrates physical discipline, mental training, and moral principles to encourage a healthy and holistic way of living. Currently, several types of yoga are practiced in western societies and most of them encompass the practice of physical postures (termed asana in Sanskrit), breathing exercises (termed pranayama), concentration exercises that focus and stabilize attention (termed dharana) and meditation (termed dhyana).
Several studies, including randomized controlled trials, have directly examined yoga as a potential treatment for pain and found evidence for the beneficial and safe use of yoga to alleviate different painful conditions (reviewed by Wren et al. 2011). These studies have often assumed that the benefits of yoga stem from its effect on the musculoskeletal system (e.g. increase in strength and flexibility). However, yoga also involves focused attention and has been shown to improve mood and depression (Woolery et al. 2004; Lavey et al. 2005; Shapiro et al. 2007). Both attentional and emotional factors influence pain perception (Rhudy and Meagher 2001; Villemure and Bushnell 2002; Wiech et al. 2008). Furthermore, yoga practitioners are encouraged to adopt an emotionally detached observation of the present moment and, accordingly, yoga has been shown to improve mindfulness scores (Brisbon and Lowery 2011), which are also associated with improved pain tolerance (Kingston et al. 2007). The emotional and cognitive tools developed in yoga practice could potentially alter a person's relationship with pain, particularly by strengthening control over affective reaction to pain. However, the effects of long-term and regular yoga practice on experimental pain perception and the underlying neuroanatomical basis of altered pain perception have yet to be explored.
In the current study, we examine thermal detection and pain thresholds and cold pain tolerance in experienced North American yoga practitioners and individually matched controls as well as the strategies employed by the 2 groups to tolerate pain. To examine the neuroanatomical underpinnings of perceptual changes, we also examine structural differences in brain gray matter (GM) and white matter (WM) between these yogis and controls and correlate these differences with perceptual factors.
Materials and Methods
Participants
We recruited 14 experienced North American yoga practitioners (mean ± SD: 9.6 ± 2.8 years of regular yoga practice; 8.6 ± 4.1 h/week). The study was open to all types of yoga that had a philosophical basis routed in mind–body interconnectedness and integrated physical postures, meditation, and breathe control exercises. We elected to enroll practitioners of different yoga styles in order to capture common effects of yoga practice rather than the effects of a specific type of yoga. Five subjects reported practicing only 1 style of yoga. Of those, 2 subjects practiced Vinysasa yoga, 1 Ashtanga, 1 Iyengar, and 1 Sivananda. Nine subjects reported practicing more than 1 style of yoga. Of those, 7 mentioned practicing Ashtanga, 5 Vinyasa, 4 Iyengar, 2 Kripalu, and 1 Sivananda. On average, subjects reported devoting 66.1% (±SD = 21.1) of their yoga practice to yoga postures (asana), 11.4% (±12.2) to breath control exercises (pranayama), 16.1% (±11.9) to yoga-related concentration and meditation practices (dharana and dhyana), and 6.4% (±6.7) to chanting. To further describe our sample subjects were questioned about the reasons why they practiced yoga and their interest in yoga philosophy. Subjects were given a list of 12 potential reasons for practicing yoga and were told to indicate all the reasons that applied to them. Among the most frequently cited reasons were: To improve health (n = 14), relax (n = 12), increase the ability to focus (n = 12), get exercise (n = 11), have time to oneself (n = 10), and improve mood (n = 10). Other reasons included: Develop or maintain flexibility (n = 9), be fit and attractive (n = 8), cope with stress (n = 7), help with depression or anxiety (n = 6), relieve pain (n = 4), and spend time with friends (n = 1). Subjects were questioned about their interest in yoga philosophy using the following question: “Do you spend time reading yoga texts or studying yoga philosophy?” They were given the following choices: (1) Not interested in yoga philosophy and do not spend time studying it; (2) not exposed to yoga philosophy; (3) only exposed to yoga philosophy while practicing in a studio; (4) occasionally read a book on yoga philosophy (this could include reading topics in a yoga magazine); (5) continuously read/study yoga philosophy and often engage in discussions on the topic. All subjects reported being interested and exposed to yoga philosophy. Eight of the 14 subjects reported that they continuously read/studied yoga philosophy and often engaged in discussions on the topic. Two subjects reported that they occasionally read a book on yoga philosophy, while the remaining 4 subjects reported being somewhat between occasionally and continuously reading about yoga philosophy. Controls (n = 14) were individually matched to yogis for sex (5 males/group), age (± 2 years) [yogis: 37.0 ± 6.6 years, controls: 36.7 ± 7.3 years; t(df = 26) = 0.11; P = 0.913], body mass index [BMI; yogis: 21.6 ± 2.1, controls: 22.6 ± 2.8; t(df = 26) = 1.12; P = 0.271], handedness (9 right-handed subjects/group), education [yogis: 15.9 ± 1.6 years; controls: 15.5 ± 2.1 years; t(df = 26) = 0.61; P = 0.54]), and exercise level other than yoga postures [yogis: 5.2 ± 3.1 h/week; controls: 4.7 ± 3.5 h/week; t(df = 26) = 0.41; P = 0.684]. Thus, none of these variables were used as covariates of no interest for magnetic resonance imaging (MRI) group analyses. Applicants were excluded if they currently suffered from or had a history of claustrophobia, chronic pain, chronic systemic diseases, psychiatric, or neurological disorders, or if they were pregnant or breast-feeding, regular smokers or regular users of marijuana, alcohol, or any other recreational drugs, or taking analgesics or antidepressants. Controls were excluded if they had any previous experience with yoga, meditation, or martial arts. All participants provided written inform consent and received monetary compensation for their participation. All behavioral tests were administered by the same female experimenter (V.C.).
Behavioral Measures
Expectation Measures
We assessed yogis' expectations pertaining to the potential effect of yoga practice on pain perception in everyday life (outside of yoga practice) by asking them the following question: “How confident would you be that practicing yoga regularly would be successful in changing how a person perceives pain outside of yoga practice?” Subjects had to place a mark on a 100-mm visual analog scale divided in increments of 10 with “0” labeled “not at all confident” and “100” labeled “very confident” to indicate their answer. They were then asked: “If you answered anything other than 0 for question 1, please indicate whether you think regular yoga practice would (1) increase pain perception outside of yoga practice; (2) decrease pain perception outside of yoga practice; (3) I don't know whether yoga would increase or decrease pain perception outside yoga practice.”
Thermal Detection and Pain Thresholds
Thermal stimulation was produced by a computer-controlled thermode with a 3 × 3 cm contact surface (TSA II, Medoc Ltd., Advanced Medical System, Israel). Heat and cold detection and pain thresholds were tested on the volar surface of the nondominant forearm at the midpoint between the crease of the elbow and the wrist. For all tests, the baseline temperature was 32°C. For thermal detection thresholds, temperature changed at a rate of 0.5°C/s (both ascending and descending ramps) and the interstimulus interval varied randomly between 5 and 8 s. For thermal pain thresholds, we used an ascending ramp of 1°C/s and a descending ramp of 8°C/s and the interstimulus interval was kept constant at 30 s to avoid sensitization. Subjects kept their eyes closed during stimulation. Tests were first practiced on the dominant forearm to familiarize the subjects with the procedures. For detection thresholds, subjects were instructed to press a mouse button as soon as they felt the slightest change in the thermode temperature. For pain thresholds, subjects were instructed to press a mouse button immediately at the first sign of an aching, stinging, pinching, or burning sensation. Three trials were performed for each threshold, and the geometric mean of the 3 trials was taken as the threshold for all tests.
Cold Pain Tolerance
The water in a circulating water bath was kept at 5°C. Subjects were instructed to immerse their nondominant hand up to the wrist in the cold water until they could no longer tolerate the pain. Although they were not told in advance, subjects were asked to remove their hand from the water after 120 s. Two trials separated by about 3 min were performed, and the average time spent in the water was used as the tolerance measure.
Mental Strategies Used During the Tolerance Task
After the tolerance task, the subjects were asked what type of strategies they used to tolerate the pain and keep their hand in the water. The responses were compiled in a document that displayed each subject in a random order under a code that did not reveal to which group the subject belonged. Nine categories of responses were identified from the sample: “Makes use of or focuses on breathing,” “demonstrates negative emotions,” “focuses on sensation – observes it without reacting,” “relaxes the mind or the body or parts of the body,” “ignores the pain,” “accepts the sensation, does not reject it,” “distracts the self from pain,” “reinterprets the sensation,” and “makes use of positive imagery.” These categories were listed in a separate document. A group of 6 evaluators not involved in the study were given the list of 9 possible choices plus an additional “none of the above” option. They were asked to carefully read the response transcriptions of the 28 subjects and to indicate, using the list of 10 choices, under which categories the responses of each subject would fit. They could use as many categories as they saw fit for a given subject. The identity of the subjects was decoded, and the strategies used by each group were compiled. The use of a given strategy was attributed to a subject if a majority of evaluators (at least 4 of the 6) agreed that the strategy was used.
Statistical Analysis
T-tests for independent samples were performed on the thermal detection, thermal pain, and pain tolerance measures using SPSS PASW Statistics 18.0.
MRI Acquisition
Subjects participated in 1 MRI scanning session, which included a 10-min T1-weighted anatomical scan [inversion time (TI) = 900 ms, repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, flip angle = 9°, field of view = 256 mm; voxel size 1 mm3), and 15 min of diffusion tensor imaging (DTI) scanning [TR = 8300 ms, TE = 89 ms, FOV = 256 mm; voxel size 2 mm3; b0 = 1000 s/mm2; 99 directions; additional 10 images with no diffusion weighting (b = 0 s/mm2)]. Throughout the session, participants wore earplugs to protect them from the scanner noise, and their heads were immobilized. Brain images were acquired using a 3-T Siemens Trio (Siemens, Erlangen, Germany) with a standard 12-channel head coil.
Voxel-based Morphometry
Preprocessing
Anatomical images were preprocessed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), running on Matlab (R2007a). Images were bias corrected, tissue classified [GM, WM and cerebrospinal fluid (CSF)], and spatially normalized to the MNI space using linear (12-parameter affine) and nonlinear transformations (warping). The nonlinear transformation parameters were calculated via the high-dimensional DARTEL algorithm (Ashburner 2007) with the supplied standard template. Subsequently, the voxel values were multiplied by the nonlinear components derived from the spatial normalization to allow for the comparison of the absolute amount of tissue (i.e. GM volume) corrected for individual brain sizes. Finally, the modulated volumes were smoothed with a Gaussian kernel of 8-mm full-width at half-maximum.
Comparison of GM Volume Between Yogis and Controls
Total GM volume [expressed as % of total intracranial volume (TIV)] was compared between groups using an independent sample t-test. Voxel-wise GM differences were examined using independent sample t-tests in SPM8. To avoid possible edge effects between different tissue types, all voxels with GM values of <0.1 were excluded from the analysis (absolute threshold masking). We examined the whole-brain statistical maps using a voxel-wise threshold of P < 0.01, cluster corrected for multiple comparisons at P < 0.05 using Gaussian random field theory (RFT; Worsley et al. 1996). Cluster size correction determines whether the spatial extent of a given cluster (defined as a set of contiguous voxels exceeding a preselected cluster defining threshold) is unusually large by chance alone (Hayasaka and Nichols 2003). Smoothness of voxel-based morphometry (VBM) data is not uniform across the image and this can lead to cluster sizes that are under- or overestimated based on the degree of local smoothness, potentially rendering cluster size inference invalid. We therefore report clusters corrected for nonstationary cluster extent using the NS toolbox in SPM5 (http://fmri.wfubmc.edu/cms/software). Mean GM values in clusters showing a significant group difference were correlated with heat pain threshold and pain tolerance score. In significant clusters, the correlation analysis was repeated using mean GM extracted from an unbiased anatomical mask of that particular brain region (provided by the Harvard-Oxford Atlas in Fslview). Finally, for the yogis, we performed an exploratory whole-brain VBM regression analysis (voxel-wise threshold P < 0.001, cluster corrected at P < 0.05 using RFT) with the number of years of yoga practice as the predictor. Since the duration of yoga practice and the age of yoga practitioners showed a trend for positive correlation (r = 0.59, P = 0.078), age was used as a covariate of no interest in the whole-brain regression analysis.
Cortical Thickness Analysis
To verify the GM volume results using an alternative metric of cortical GM, we also performed cortical thickness analysis (CTA).
Preprocessing
Anatomical images were submitted to the CIVET pipeline (version 1.1.9), in-house software developed at the Montreal Neurological Institute (MNI) for fully automated structural image analysis (Ad-Dab'bagh et al. 2006). Briefly, each scan first underwent an automated intensity nonuniformity correction using the N3 software package (Sled et al. 1998). The intensity-corrected volumes were transformed both linearly (12-parameter affine) and nonlinearly to the MNI space (Collins et al. 1994). Each voxel was then automatically classified into GM, WM, CSF, or backgroud (Zijdenbos et al. 2002). Partial volumes were calculated in each voxel, that is, for each voxel a percentage of GM, WM, CSF, and background was attributed depending both on the voxel's intensity and on the intensity of the neighboring voxels (Tohka et al. 2004). A cortical fitting stage registered the brain surfaces to a model that calculates 81 924 vertices, which were then back-transformed to the original brains to calculate thickness in millimeter at each vertex for each brain (MacDonald et al. 2000; Kim et al. 2005). A final surface registration step was performed (Lyttelton et al. 2007), followed by applying a surface-based smoothing kernel of 30 mm (Chung et al. 2003).
Comparison of Cortical Thickness Between Yogis and Controls
Mean cortical thickness was compared between groups using an independent sample t-test. Vertex-wise differences in cortical thickness were analyzed using independent sample t-tests in SurfStat (http://www.math.mcgill.ca/keith/surfstat/). A whole-brain analysis was conducted with cluster correction for multiple comparisons at P < 0.05 using RFT, with a liberal cluster-forming threshold of P < 0.05 in order to detect subtle spatially extended cortical changes (Bermudez et al. 2009; Seminowicz et al. 2010). Total number of vertices included in the whole-brain analysis excluding cerebellum, and the interhemispheric cut was 78 166.
DTI Analysis and Tractography
Preprocessing
Diffusion-weighted images were preprocessed with FSL 4.1 (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html). Briefly, raw data were corrected for eddy currents and head motion, and skull and nonbrain tissue were removed (Smith 2002). Fractional anisotropy (FA) images, which reflect the degree of water diffusion anisotropy in each brain voxel, and therefore the degree of directionality in WM, and eigenvalue (L) images, which describe the direction of water diffusivity, were created by fitting a tensor model to the diffusion data using FSL's Diffusion Toolbox (FDT). All subjects' FA data were then aligned into a common space via the supplied FMRIB58_FA standard-space image using FSL's nonlinear registration tool FNIRT, followed by affine registration into the MNI space. Next, a mean FA image was built and thinned to create a mean FA skeleton representing the centers of all tracts common to the study sample. Finally, each subject's aligned FA image was projected onto this skeleton. Group maps of mean diffusivity [MD = (L1 + L2 + L3)/3], axial diffusivity (AD; L1; parallel to principal diffusion direction), and radial diffusivity [RD = (L2 + L3)/2; perpendicular to principal diffusion direction] were processed the same way.
Comparison of WM Between Yogis and Controls
Voxel-wise statistical analysis of the FA data was carried out using Tract-Based Spatial Statistics (TBSS) in FSL (Smith et al. 2006). FA values were compared between groups, first across the whole-brain WM, and then restricted to the bilateral insular cortex based on the GM correlation analysis with pain tolerance. Insular cortex was defined using the Harvard-Oxford Atlas in Fslview and dilated to include the adjacent WM. TBSS was performed using a permutation-based inference tool for nonparametric statistical thresholding. The number of permutations was set at 10 000. All voxel-wise group comparisons were performed using independent sample t-tests. The significance threshold for between-group differences was set at P < 0.05, cluster corrected using RFT (cluster-forming threshold was set at P < 0.01). For areas of significant FA difference, we tested how the groups differed in component diffusivities such as AD and RD using independent sample t-tests. We then examined the correlation between mean FA extracted from the left insular cortex (dilated mask defined by the Harvard-Oxford Atlas in Fslview) and heat pain threshold and pain tolerance score across both groups. In the significant insula cluster, we examined the relationship between FA and the duration of yoga practice in the yoga group, while controlling for age.
Tractography
To visualize the connectivity profile of WM pathways passing through the area where FA differed between groups, we performed probabilistic tractography (Behrens et al. 2007) using FDT to produce an estimate of the most likely location of a pathway from the cluster of significant FA increase. Briefly, a local model of fiber orientation, capable of resolving crossing fibers, was inferred from the data, followed by building up distributions on the diffusion parameters at each voxel in the individual subject's space by repetitive sampling. Seed (FA cluster) was transformed from MNI standard space into DTI space of each subject, and 5000 sample tracts were generated from each seed voxel. The tractography was unrestricted, allowing tracts to propagate in each direction (i.e. no targets or masks were specified). Average connectivity maps were generated in the MNI standard space by back-transforming individual connectivity distributions. In each subject, we set an arbitrary threshold of 50 of 5000 samples to determine the presence of a tract at a given voxel and to eliminate spurious connections, consistent with common practice (Bridge et al. 2008; Rilling et al. 2008; Moayedi et al. 2012). We then added these thresholded subjects' maps across groups. In the resulting map, a value of 28 indicated a voxel in which each subject had a suprathreshold probability of connection from the seed.
Results
A Majority of Yoga Practitioners Expected Yoga Practice Would Decrease Their Reactivity to Pain in Everyday Life
Half of the yogis (n = 7) considered that regular yoga practice increased pain perception outside of yoga practice. They were on average 89% confident about their statement (SD = 9). However, most of them (n = 6) explained that although yoga would increase pain perception, in the sense of making a person more aware of pain, it would make pain more neutral, allow more control over it, increase tolerance, decrease reactivity, or change the response to pain. One person mentioned that this would depend on the type of yoga being practiced. Three yogis considered that regular yoga practice decreased pain perception outside of yoga practice and were on average 80% confident about their statement (SD = 10). The remaining 4 subjects reported not knowing whether yoga practice increased or decreased pain perception outside yoga practice and were 70% confident about their statement (SD = 11). One of the yogis who reported not knowing whether yoga practice would increase or decrease pain perception added that yoga would, however, change how a person would react to pain.
Yoga Practitioners Have Higher Pain Tolerance Than Controls
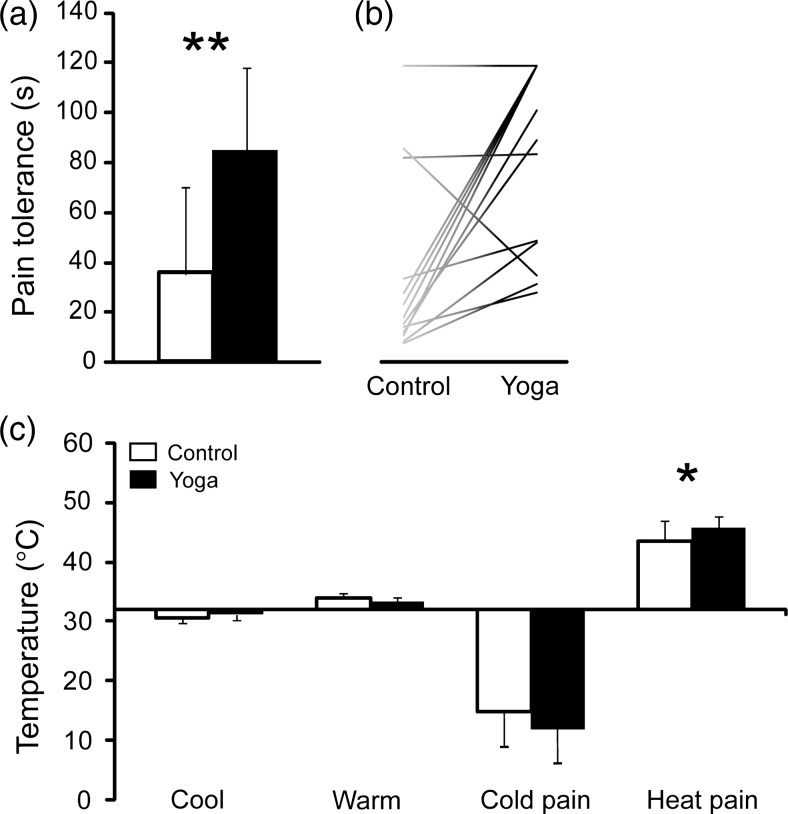
Yoga practitioners tolerated cold pain more than twice as long as the controls (Fig. 1a). All but 2 yogis tolerated pain longer than their individually matched control (Fig. 1b).
Figure 1.
(a) shows that cold pain tolerance times for yogis were more than twice as long as those for controls [yogis: 85.08 ± 37.86 s; controls: 35.43 ± 34.93 s; t(df = 26) = 3.61; P = 0.001 (**P < 0.01)]. (b) shows tolerance times for individual controls and yogis. Each yogi was individually matched to a control for age, sex, handedness, education, BMI, and exercise level other than yoga postures. All but 2 yogis tolerated pain longer than their matched control. (c) Heat pain threshold was slightly higher in yogis [yogis:45.74 ± 1.91°C; controls: 43.42 ± 3.54°C; t(df = 26) = 2.16; P = 0.040], while there was no significant group difference in cold pain [yogis 11.38 ± 6.71°C, controls 14.27 ± 7.01°C, t(df = 26) = −1.11, P = 0.276], cold detection [yogis 31.13 ± 0.50°C, controls 30.70 ± 0.71°C, t(df = 26) = 1.84, P = 0.077], or warm detection thresholds [yogis 33.42 ± 0.72°C, controls 33.87 ± 0.77°C, t(df = 26) = −1.60, P = 0.122]. Black bars, yogis; white bars, controls.
We also compared sensory detection and thermal thresholds between groups using a contact thermode on the volar forearm. Heat pain threshold was slightly higher in yogis, while there was no significant group difference in cold pain, cold detection, or warm detection thresholds (Fig. 1c).
Yoga Practitioners Have More GM in Multiple Cortical Regions, but Only Insular GM Is Correlated with Pain Tolerance
Total GM volume (expressed as % of TIV) did not differ significantly between groups [yogis 50.91 ± 1.72; controls 50.26 ± 2.00, t(df = 26) = −0.914, P = 0.369], nor did mean cortical thickness across the whole brain [yogis: 3.23 ± 0.08 mm; controls: 3.19 ± 0.06 mm; t(df = 26) = −1.65; P = 0.111]. However, using VBM, we found that yoga practitioners had greater GM volume than controls in multiple cortical regions (Table 1), whereas there were no regions where controls had more GM than yogis. CTA identified 3 regions that were thicker in yoga practitioners, and these regions largely overlapped with the VBM findings (Table 1).
Table 1.
VBM and CTA
| Brain region | MNI co-ordinates (x, y, z) | Cluster sizea | P-value of cluster | Peak t-value | Correlation of mean GM in cluster with pain tolerance across all subjects |
|---|---|---|---|---|---|
| VBM yoga > control | |||||
| Right cingulate/SMA/S1 | 6, −15, 46 | 5420 | <0.001 | 5.12 | r = 0.27, P = 0.156 |
| Left insula/S2/temporal | −46, −9, −3 | 3048 | <0.001 | 5.00 | r = 0.53, P = 0.004 |
| r = 0.41, P = 0.032b | |||||
| Left dorsal MPFC | −3, 50, 28 | 1564 | 0.005 | 3.85 | r = 0.14, P = 0.477 |
| Left IPL/SPL | −38, −66, 39 | 526 | 0.038 | 4.63 | r = 0.22, P = 0.260 |
| Right insula/S2 | 42, −24, 15 | 1339 | 0.039 | 3.53 | r = 0.442, P = 0.019 |
| r = 0.42, P = 0.026b | |||||
| VBM control > yoga | |||||
| No regions | |||||
| Cortical thickness yoga > control | |||||
| Right cingulate/S1/SPL | 23, −25,63 | 2946 | <0.001 | 4.16 | |
| Right insula/S2/temporal | 58, −21, 40 | 891 | 0.013 | 3.42 | |
| Left S1/S2 | −16, −26, 69 | 976 | 0.039 | 3.08 | |
| Cortical thickness control > yoga | |||||
| No regions | |||||
Note: Results shown are significant at P < 0.05, cluster corrected. For VBM results, cluster extent is corrected for nonstationarity.
S1: primary somatosensory cortex; S2: secondary somatosensory cortex; MPFC: medial prefrontal cortex; SMA: supplementary motor area; IPL: inferior parietal lobule; SPL: superior parietal lobule.
aFor VBM results, cluster size is expressed as the number of voxels, and for cortical thickness results, cluster size is expressed as the number of vertices.
bCorrelation analysis with cold pain tolerance repeated using mean GM values extracted from an independent anatomical mask of the insula cortex (the Harvard-Oxford Atlas supplied with Fslview).
We next evaluated whether volumetric GM in brain areas found to differ between yogis and controls were related to cold pain tolerance and heat pain threshold across subjects. The only brain regions for which cold pain tolerance was positively correlated with GM volumes were in the left and right insular regions (Fig. 2a,b insets, respectively; Table 1). These correlations remained significant when instead of using mean GM from the group difference cluster we used mean GM extracted from an independent anatomically defined insular cortex mask (Fig. 2a,b scatter plots, respectively; Table 1 bold). Heat pain threshold did not correlate with GM volume in any of the clusters (all r < 0.2, P > 0.25).
Figure 2.
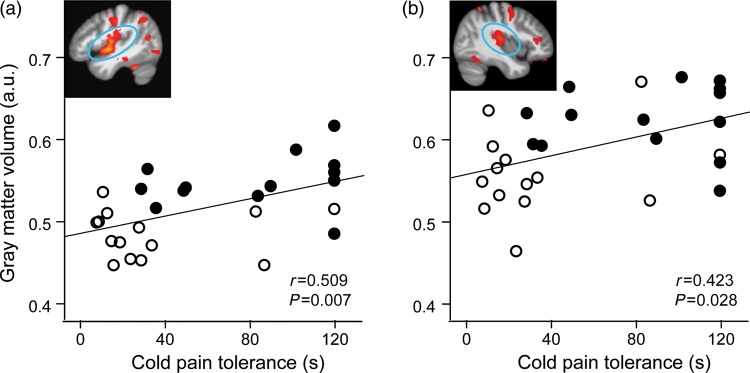
Cold pain tolerance (time in seconds) was positively correlated with GM volumes across all subjects in the left insula (a) and the right insula (b). GM values used in correlation are mean values from the left and right insular cortices, respectively, independently defined using the Harvard-Oxford Atlas in Fslview. Full circles, yogis; open circles, controls; fit line, regression line across groups. a.u.: arbitrary units.
GM Volume of the Left Insular Cortex Is Positively Correlated with the Duration of Yoga Practice
To evaluate whether the increased insular size in yoga practitioners could at least partially be caused by the yoga practice, we correlated the number of years of yoga practice with GM in the yoga practitioners. This analysis revealed that among the areas of significant GM increase the GM volume in the left, but not in the right, insular cortex did in fact correlate with the duration of yoga practice (Fig. 3).
Figure 3.
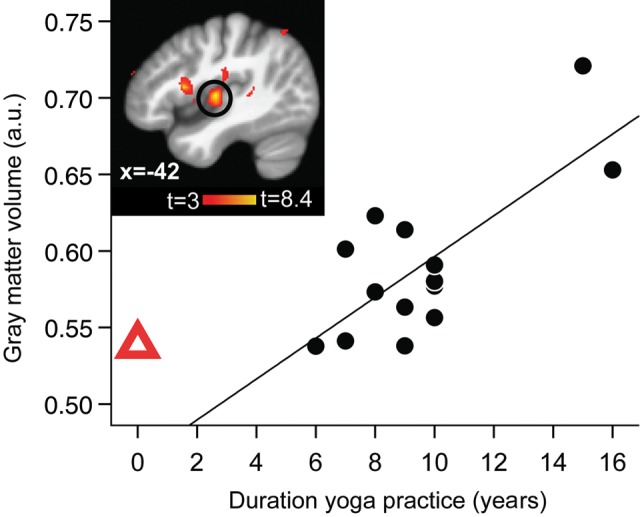
Left insular GM volume was positively correlated with the number of years of yoga practice in yogis. Inset shows the region of the left insular cortex with the significant correlation circled (whole-brain VBM regression with years of yoga practice, controlling for age; peak x, y, z MNI coordinates: −44, −12, 3; t(peak) = 8.38; cluster size 407 voxels, P(cluster) = 0.002). Displayed on study average brain (n = 28). Scatterplot shows the extracted mean GM values in the significant insular cluster plotted against years of practice for each yogi. a.u.: arbitrary units. Full black circles, yogis and fit line shows regression line across the yoga group. Red triangle shows the mean of control values extracted from the same GM cluster (i.e. “0 years yoga practice”). The red triangle is not included in the regression with GM, but shows the natural extension at 0 years of yoga practice (i.e. control subjects) and is displayed here to address the issue of undersampling in this study by showing that the longest-term practitioners (>14 years) and the nonpractitioners (0 years) fall onto the opposite ends of the same spectrum.
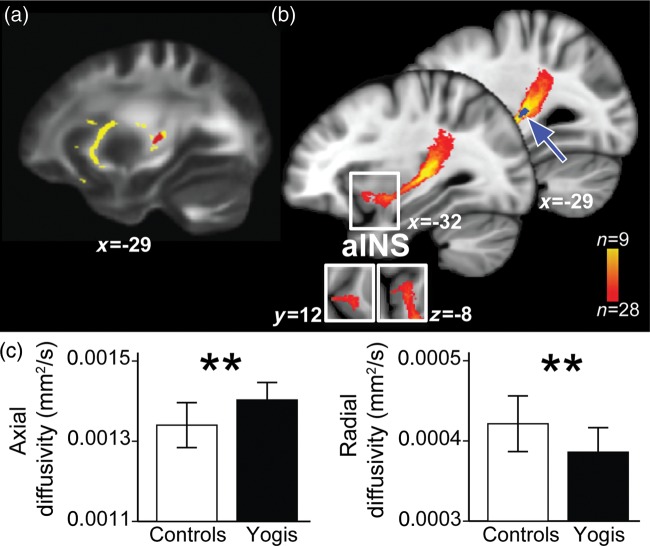
Yoga Practitioners Have Greater Left Intrainsular White Matter Connectivity
Whole-brain WM analysis of FA yielded no significant group differences. Evidence shows that parts of insular cortex are involved in pain processing and other parts in pain modulation (Schweinhardt and Bushnell 2010). Thus, to examine whether the insular GM increases in yoga practitioners could be associated with an increased intrainsular connectivity, we specifically examined the WM characteristics of the insular cortex and adjacent WM by comparing FA values between yogis and controls. In the region-of-interest analysis of insular cortices based on GM findings, yogis showed a cluster of higher FA than controls in the WM adjacent to the left insula (Fig. 4a). We then used probabilistic tractography to determine the connectivity pattern of the WM pathway passing through the area of altered FA. The WM tracing was performed in an unrestricted manner (allowing for tract propagation in all directions). The WM pathway identified using this method showed connectivity between posterior and anterior insular regions (Fig. 4b).
Figure 4.
DTI analysis revealed an increased intrainsular connectivity in the left insular cortex that correlated with cold pain tolerance. (a) shows a cluster of higher FA for yogis than controls in the left retrolenticular internal capsule/superior longitudinal fasciculus [rlC/SLF; t(peak) 4.52, P < 0.05 cluster corrected, cluster size 78 voxels, MNI coordinates at peak −29, −24, 6], the WM adjacent to the left insula. The cluster is displayed on the WM mask (yellow). (b) shows the results of probabilistic tractography, which was used to track the WM pathway passing through the area of significant FA increase (blue cluster and blue arrow). The WM tracing was performed in an unrestricted manner (allowing for tract propagation in all directions). The WM pathway identified using probabilistic tractography projects from the seed onto more anterior parts of the insular cortex [insets are showing the coronal (y = 12) and axial (z = −8) view]. For easier visualization, the WM pathway is thresholded to show a portion of the tract present in at least 9 of 28 subjects (colorbar). Displayed on study average brain. (c) shows the AD and RD for yogis (black bars) and controls (white bars). AD was significantly higher in yogis than controls [yogis 0.00140 ± 0.00004, controls 0.00134 ± 0.00006, t(df = 26) = 3.29, P = 0.003], and RD was lower in yogis than controls [yogis 0.00039 ± 0.00003, controls 0.00042 ± 0.00003, t(df = 26) = 2.87, P = 0.008]. Bars represent mean values, and error bars represent SD.
Next, we tested how yogis and controls differed in FA component diffusivities in this tract. We found that AD, that is, diffusivity parallel to the principal fiber direction, was higher in yogis than controls, while RD, that is, diffusivity perpendicular to the principal fiber direction, was lower in yogis (Fig. 4c).
We then examined the relationship between mean FA and cold pain tolerance across all subjects and found no significant correlation (r = 0.25, P = 0.194). As with the other measures, we did not find a significant correlation between FA and heat pain threshold (r = 0.04, P = 0.846). Finally, we examined the relationship between mean FA and the duration of yoga practice in yoga practitioners while controlling for age and found no significant correlation (r = −0.14, P = 0.645).
Yogis and Controls Use Different Mental Strategies to Deal with Pain, Including Strategies Involving Increased Parasympathetic Function and Interoceptive Awareness
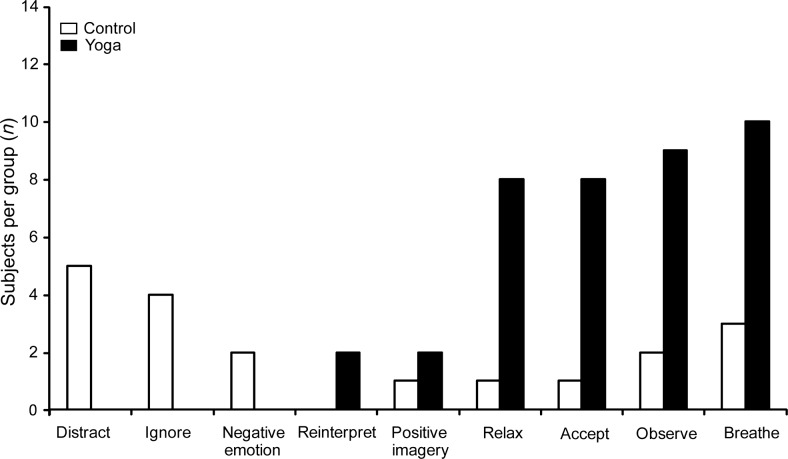
When interviewed about mental strategies used to tolerate pain during water immersion, yoga practitioners most commonly reported using strategies based on relaxation, acceptance, and nonjudgemental focusing on the pain (reminiscent of mindfulness). These strategies included using or focusing on breathing (10 yogis), focusing on the sensation—observing it without reacting (9 yogis), relaxing their mind or body (8 yogis), and accepting the painful sensation (8 yogis) (Fig. 5). In contrast, most of the controls tried to either distract themselves (5 controls) or ignore the pain (4 controls).
Figure 5.
Frequency histogram of categories of mental strategies used by yogis (filled bars) and controls (open bars) during the cold pain tolerance task. Yoga practitioners used strategies involving relaxation, acceptance, and increased interoceptive awareness, but only controls tried to actively distract themselves or to ignore the pain. Subjects made use of or focus on breathing (10 yogis/3 controls), focused on sensation—observed without reacting (9/2), accepted the sensation (8/1), relaxed the mind or the body or parts of the body (8/1), made use of positive imagery (2/1), reinterpreted the sensation (2/0), demonstrated negative emotions (0/2), ignored the pain (0/4), or distracted the self from the pain (0/5).
Discussion
These results show that experienced North American yoga practitioners tolerate more pain and have more brain GM in multiple cortical regions related to affective pain processing, pain regulation, and attention than do individually matched controls. Nevertheless, only the increased GM in the insular cortex correlated with the higher pain tolerance across groups. Further, the results of our exploratory whole-brain regression analysis indicate that the duration of yoga practice positively correlated with GM volume in the left insular cortex, suggesting that yoga practice contributed to the anatomical differences, rather than the yoga practitioners having fundamentally different brains before beginning to practice yoga. In addition to GM differences, the WM tract running along the posterior/anterior length of the left insular GM showed signs of higher integrity, indicating an increased intrainsular connectivity. We also observed that yoga practitioners, but not control subjects, used cognitive strategies involving parasympathetic activation and interoceptive awareness to tolerate pain. These kind of strategies constitute an integral part of yoga practice. Since other studies show a sequential processing of pain in the human insula, from nociceptive input in the posterior insula to autonomic integration in the midinsula to subjective feelings in the anterior insula (reviewed by Craig 2011), the increased parasympathetic activation and interoceptive awareness of yoga practitioners could have led to use-dependent hypertrophy and connectional strengthening in the insular cortex, thereby altering pain tolerance.
Based on the strategies used by yoga practitioners to tolerate pain, it appears that it is the yoga training itself that equips individuals with tools to tolerate more pain, and this increased pain tolerance could be mediated through autonomic activation of the insular cortex. The strategies used by yogis, including breathing techniques, focusing on sensations without reacting, relaxing the mind or body, and accepting pain, are all part of yoga training and are similar to mindfulness practice that has been shown to improve pain tolerance (Kingston et al. 2007). Yoga practitioners are encouraged to adopt an emotionally detached observation of the present moment and, accordingly, yoga has been shown to improve scores on the Freiburg Mindfulness Inventory (Brisbon and Lowery 2011). Consequently, and although not specifically instructed to do so, a majority of yogis in our study reported adopting this detached observer's perspective as a strategy to tolerate pain, a strategy that was used by only 2 controls. Unsurprisingly, the cultivation of this emotionally detached attitude toward events and sensations lead a majority of yoga practitioners from our study to expect that their practice would result in better control over pain in everyday life. Therefore, expectation of greater control over pain might have contributed to the observed behavioral findings, since expectation of pain relief has been found to be associated with increased pain tolerance in placebo studies, a finding that is mediated through activation of the descending opioid system (Benedetti et al. 1999). However, it is also worth mentioning that when testing for pain tolerance, subjects were not given any indication as to how long they were expected to leave their hand in the water. In other words, they could not know if they were doing better or worse than other people in the same situation. Only if they reached the 120-s predertermined limit were they told to remove their hand from the water. Control subjects, on the other hand, mainly tried to distract themselves or to ignore the pain. While distraction can alter pain sensation, it does not diminish the emotional (unpleasantness) aspect of the pain, which is the main determinant of tolerance (Rainville et al. 1992; Villemure et al. 2003; Villemure and Bushnell 2009). Further, there is little evidence that efforts to deliberately try to ignore something are successful (Wegner 1994).
Across groups, individual differences in cold pain tolerance were positively correlated with GM volume in areas encompassing the mid and posterior insula. Though our GM findings largely cluster in the more posterior parts of the insula, we find higher WM anisotropy in a tract leading from the posterior into anterior insula, which is consistent with findings of Craig et al. (2000) suggesting a posterior to mid-to-anterior pattern of integration of thermal information in the human brain. These correlations between anatomical findings and pain perception are consistent with data from functional MRI studies, showing that pain-related activity in the insular cortex correlates with individual differences in pain perception (Coghill et al. 1999; Baliki et al. 2009) and suggests that individual differences in insular anatomy could be one of the underlying causes of perceptual differences observed across individuals.
Although both the left and right insular cortices were larger in yoga practitioners than in controls, with the size correlating with pain tolerance, only the left insula showed a relationship with the duration of yoga practice and with WM integrity. This asymmetry is consistent with the homeostatic neuroanatomical model of emotion (Craig 2005) that proposes a left insular association with parasympathetic activity, and thus, the positive affect and affiliative emotions. As described above, yoga training encourages positive emotions and increased parasympathetic activity, which according to this model involve the left insula. Consistent with this idea are data showing that aspects of yoga practice, such as low breathing rate, reduce pain, and negative affect (Zautra et al. 2010). Furthermore, anatomical differences involving the insula, such as GM density, cortical thickness, and the extent of cortical gyrification, have been reported between meditators and nonmeditators (Lazar et al. 2005; Holzel et al. 2008; Luders et al. 2012). The increased GM could be due to many factors, including neural or glial cell genesis, increased cell size or spine density, or even changes in blood flow or interstitial fluid (May et al. 2007). The rapid changes in GM volume observed in some longitudinal studies (Draganski et al. 2004; Holzel et al. 2011) suggest that the involvement of a rapidly adjusting system, such as dendritic spine or synapse turnover (Trachtenberg et al. 2002), and the induction of long-term synaptic potentiation are associated with the enlargement of the dendritic spines (Bosch and Hayashi 2012).
Our finding of higher FA values in yoga practitioners in WM adjacent to the left posterior insula and connecting this region with the anterior portion of the insula, which was best described in terms of higher water diffusivity along the axons with concomitant lower diffusivity perpendicular to the axonal membrane, suggests the possibility of a enhanced intrainsular connectivity in yogis related to their yoga practice. Such enhanced connectivity could involve increased myelination, since longitudinal studies in animals combining DTI measures with histology show that WM FA can be modulated by changes in the myelination pattern (De Groof et al. 2008; Blumenfeld-Katzir et al. 2011). Higher WM FA have recently been linked to better performance across a wide range of cognitive (Floel et al. 2009; Tsang et al. 2009), emotional (Olson et al. 2009), and motor skills (Schmithorst and Wilke 2002; Bengtsson et al. 2005; Della-Maggiore et al. 2009; Tomassini et al. 2011), supporting the idea that increased connectivity is associated with increased training and enhanced performance. Longitudinal studies in humans are now beginning to provide direct evidence for such use-dependent plasticity, by correlating FA increase in the appropriate WM tracts with skill acquisition (Keller and Just 2009; Engvig et al. 2011). Meditation, another practice that encourages increased parasympathetic activity and interoceptive awareness, has also been associated with enhanced WM FA (Tang et al. 2010; Luders et al. 2011).
Taken together, we interpret insular GM and WM alterations in yoga practitioners to reflect use-dependent GM hypertrophy and increased intrainsular processing related to pain affect regulation. From the strategies used to tolerate pain reported by the yogis, we suggest that the observed insular adaptive changes related to successful pain affect regulation are mediated by increased parasympathetic activity and interoceptive processing.
This study bears certain limitations. We did not use standard questionnaires measuring factors such as mindfulness, anxiety, or suggestibility. These types of questionnaires could have added useful information and their use should be considered in future studies to further delineate what traits are associated with yoga-related pain modulation. Secondly, because of the cross-sectional nature of this study, our findings do not allow any definitive causal conclusion. The differences observed in the brain anatomy of yoga practitioners associated with greater pain tolerance could have been innate; on the other hand, regular and long-term yoga practice could have altered brain anatomy, as is suggested by the positive correlation between the duration of yoga practice and GM volume in the left insular cortex. It is also possible that the observed differences between yoga practitioners and control subjects are the result of an interaction between innate and acquired factors. Only longitudinal studies could disentangle with certainty these possibilities.
In summary, in a sample of experienced North American yoga practitioners, we found neuroanatomical enhancements of the insula that were related to higher cold pain tolerance. Yoga practitioners had higher intrainsular connectivity and they, but not the control subjects, dealt with pain using strategies involving insula-related interoceptive awareness, the use of which is promoted during yoga training. Based on these findings, we suggest that the insular cortex mediates the increase in pain tolerance associated with yoga as practiced and widely accessible in western societies.
Funding
This research was supported in part by the Intramural Research Program of the NIH and National Center for Complementary and Alternative Medicine.
Notes
We thank the team at the McConnell Brain Imaging Centre of the Montreal Neurological Institute for expert MRI data acquisition. We also wish to thank our colleagues who evaluated the strategies used by the participants to tolerate pain. We thank Petra Schweinhardt and David Seminowicz for valuable comments on the manuscript. Part of the psychophysical dataset was presented at the 2010 Canadian Pain Society Annual Conference held in Calgary, Alberta. Parts of the imaging dataset were presented at the Montreal 2010 IASP 13th World Congress on Pain and at the 2011 Society for 10 Neuroscience meeting in Washington, DC. Conflict of Interest: None declared.
References
- Ad-Dab'bagh Y, Lyttelton O, Muehlboeck JS, Lepage C, Einarson D, Mok K, Ivanov O, Vincent RD, Lerch J, Fombonne E, et al. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: Corbetta M, editor. Abstract presented at the 12th annual meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006. [DOI] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–887. doi: 10.1111/j.1464-410X.2008.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–3648. doi: 10.1056/NEJMoa0810084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1111/j.1442-2042.2010.02613.x. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb Cortex. 2009;19:1583–1596. doi: 10.1007/s10147-011-0267-6. [DOI] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6:e20678. doi: 10.1056/NEJMoa1011967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;131:1433–1444. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- Brisbon NM, Lowery GA. Mindfulness and levels of stress: a comparison of beginner and advanced hatha yoga practitioners. J Relig Health. 2011;50:931–941. doi: 10.1016/j.juro.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, Rapoport JL, Evans AC. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1002/cncr.11884. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1093/jnci/93.24.1864. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. doi: 10.1046/j.1464-410x.2000.00468.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1111/j.1464-410X.2010.09982.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1442-2042.2012.03193.x. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- De Groof G, Verhoye M, Van MV, Balthazart J, Van der Linden A. Seasonal rewiring of the songbird brain: an in vivo MRI study. Eur J Neurosci. 2008;28:2475–2485. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Scholz J, Johansen-Berg H, Paus T. The rate of visuomotor adaptation correlates with cerebellar white-matter microstructure. Hum Brain Mapp. 2009;30:4048–4053. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2011;33:2390–2406. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca's area predicts grammar learning success. Neuroimage. 2009;47:1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kingston J, Chadwick P, Meron D, Skinner TC. A pilot randomized control trial investigating the effect of mindfulness practice on pain tolerance, psychological well-being, and physiological activity. J Psychosom Res. 2007;62:297–300. doi: 10.1016/j.jpsychores.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lavey R, Sherman T, Mueser KT, Osborne DD, Currier M, Wolfe R. The effects of yoga on mood in psychiatric inpatients. Psychiatr Rehabil J. 2005;28:399–402. doi: 10.2975/28.2005.399.402. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57:1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front Hum Neurosci. 2012;6:34. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain. 2012;153:1467–1477. doi: 10.1016/j.pain.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992;9:265–277. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TE. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Bushnell MC. Pain imaging in health and disease–how far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable Bowel Syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D, Cook IA, Davydov DM, Ottaviani C, Leuchter AF, Abrams M. Yoga as a complementary treatment of depression: effects of traits and moods on treatment outcome. Evidence-based complementary and alternative medicine. eCAM. 2007;4:493–502. doi: 10.1093/ecam/nel114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsang JM, Dougherty RF, Deutsch GK, Wandell BA, Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proc Natl Acad Sci USA. 2009;106:22546–22551. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Mood and attention influence supra-spinal pain processing differently. J Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–108. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Wegner DM. Ironic processes of mental control. Psychol Rev. 1994;101:34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Woolery A, Myers H, Sternlieb B, Zeltzer L. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10:60–63. [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wren AA, Wright MA, Carson JW, Keefe FJ. Yoga for persistent pain: new findings and directions for an ancient practice. Pain. 2011;152:477–480. doi: 10.1016/j.pain.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Davis MC, Craig AD. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain. 2010;149:12–18. doi: 10.1016/j.pain.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]