Abstract
Mass drug administration with praziquantel is the mainstay of programs for the control of schistosomiasis morbidity. However, there is a growing recognition that treatment alone will not be sufficient for eventually effecting elimination and that additional measures will be required to interrupt transmission. In the absence of a safe and an effective vaccine for human schistosomiasis, the strategies to reduce infection levels will necessarily involve some interventions that affect the water-related stages of the schistosome life cycle: by reducing exposure to infectious water, by moderating availability of the intermediate snail host, or by decreasing contamination of water with egg-containing excreta. While much research on the importance of water on schistosomiasis has been performed, advances in these areas have perhaps languished with the ready availability of a cost-effective treatment. As some endemic areas near a shift to an elimination goal, a better understanding of water-based interventions that can be used alone or in concert with treatment will be needed. Reinvigoration of laboratory, field, and human behavioral aspects of this research now will ensure that the appropriate strategies are available by the time their implementation becomes necessary.
Keywords: Schistosomiasis, Control, Elimination, Water, Snail, Transmission.
Schistosomiasis differs from most waterborne diseases in that rather than through ingestion of contaminated water, people become infected when their skin comes into contact with fresh water bodies that contain the parasite. As a result, strategies to purify collected drinking water that may be effective against other waterborne pathogens do not necessarily prevent transmission of schistosome infection as the act of collecting water, along with bathing, washing, swimming, and certain occupations can expose individuals to infection. More than 230 million people in the world are infected with one of the three major species of schistosomes: Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium, and more than 700 million people are at risk for infection in developing countries in Africa, Asia, and South America.1,2 Infection occurs when individuals come into contact with water containing the life cycle form known as a cercaria (Figure 1). When cercariae encounter an appropriate host, they penetrate the skin, the tails detach, and the heads enter the body and transform into a larval stage known as a schistosomula. Over the next 4–6 weeks, the immature worms migrate through various organs in the body and mature into adults that live in the blood vessels surrounding the intestines (S. mansoni and S. japonicum) or bladder (S. haematobium). Male and female worms live together in copula and female worms produce between 300 and 3000 eggs per day, depending on the species. Many of the eggs become lodged in host tissue where they induce granulomatous reactions that lead to the pathology associated with schistosomiasis, but the life cycle is continued when eggs pass into the lumen of the intestine (S. mansoni and S. japonicum) or bladder (S. haematobium) and pass out of the definitive host in feces or urine, respectively. Eggs that pass out of the host must reach fresh water to hatch; those that do release miracidia, which are infectious for intermediate snail hosts. For each of the human schistosomes, the presence of a specific genus of snail is necessary for transmission to occur. Miracidia infect the snails and transform into mother sporocysts, which then produce daughter sporocysts that greatly amplify the number of parasites through asexual replication. Finally, the daughter sporocysts release the infectious cercariae that emerge from the snail usually between 4 and 6 weeks after it was infected with miracidia.
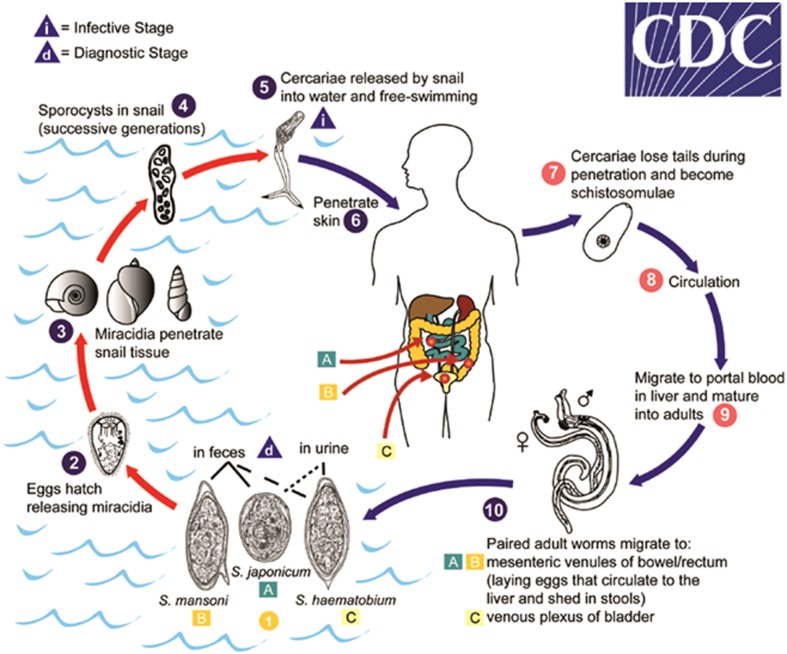
Figure 1.
Eggs are eliminated with feces or urine (1). Under optimal conditions, the eggs hatch and release miracidia (2), which swim and penetrate specific snail intermediate hosts (3). The stages in the snail include two generations of sporocysts (4) and the production of cercariae (5). Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host (6), and shed their forked tail, becoming schistosomulae (7). The schistosomulae migrate through several tissues and stages to their residence in the veins (8, 9). Adult worms in humans reside in the mesenteric venules in various locations, which are specific for each species (10). For instance, S. japonicum is more frequently found in the superior mesenteric veins draining the small intestine A, and S. mansoni occurs more often in the superior mesenteric veins draining the large intestine B. S. haematobium most often occurs in the venous plexus of bladder C, but it can also be found in the genital tract or rectal venules. The females deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S. mansoni and S. japonicum) and of the bladder and ureters (S. haematobium), and are eliminated with feces or urine, respectively (1) (source: http://www.cdc.gov/dpdx/schistosomiasis/index.html).
Although schistosomiasis only kills a relatively small proportion of the people who are infected, and then usually only after many years, the disease causes a great deal of disability and reduces the educational and work performance of infected individuals. It is thus known as a ‘disease of poverty’, both resulting from and contributing to lower socioeconomic conditions in the areas where it is endemic.3 In addition, infection with schistosomes can increase susceptibility to or severity of co-infecting pathogens.4 As a result, in recent years, schistosomiasis and other neglected tropical diseases (NTDs) have been targeted for control and elimination by the World Health Organization and governmental and non-governmental organizations interested in improving health and development in the endemic countries.1 Fortunately, schistosomiasis can be treated with a single dose of the drug praziquantel. Because treatment has few side effects, mass drug administration (MDA) is an appropriate approach for control. Recent donations by governmental and non-governmental organizations as well as industry have resulted in an extraordinary expansion of persons treated for schistosomiasis over the last decade.5 Some of these donations have also gone to supporting operational research in an attempt to identify the best MDA approaches to improve the health of affected populations.
Although MDA with praziquantel has clear benefits, people can rapidly become reinfected after treatment if they again enter water containing infectious cercariae. Furthermore, treatment often does not kill 100% of the worms, especially for individuals who have high-intensity infections. The size of the praziquantel tablets also makes it difficult to treat young children in MDA campaigns. Thus, there is a growing recognition that MDA alone is unlikely to reduce infection levels to the point where transmission is interrupted without additional control measures.6,7 With the recent World Health Assembly resolution calling for the elimination of schistosomiasis, the impetus for identifying schistosomiasis control measures in addition to treatment is intensified.8,9 Although a vaccine for schistosomiasis is a popular aspiration as an adjunct control approach and many investigators are working toward this goal, no vaccines for human schistosomiasis currently exist.10 Thus, the most practical available approach to augment the effects of treatment is through interventions that affect the aquatic stages of the schistosome life cycle.
Although earlier investigations of schistosomiasis control included research to reduce transmission, that work was considered less critical when a safe and an effective treatment became available.11 This is an appropriate time to revitalize this research and develop new water strategies or to better employ existing ones to reduce the global burden of schistosomiasis. Interventions could be pursued at three different points: (1) reducing human contact with water that contains infectious cercariae, (2) decreasing availability of the snail intermediate host, and (3) preventing snail infections by discontinuing contamination of fresh water by parasite eggs in human urine and stool.
Reduced Exposure to Infectious Water
Like most waterborne diseases, the most effective solution to control infection with schistosomes is ready access to ample supplies of clean water. However, water systems are very expensive both to install as well as to maintain and are seldom priorities in resource-constrained settings. Schistosomiasis control is even more complicated than diseases that can be controlled by filtration or chemical treatment of water for drinking or cooking as the volume of water needed for bathing and washing of dishes or clothes far exceeds what is necessary for consumption. Countries that have successfully eliminated schistosomiasis have either done so as a result of economic development (e.g. Japan, Puerto Rico), resulting in increased clean water access and fewer transmission sites, or because the number of transmission sites was already limited (e.g. Morocco). It is for this reason that concrete is sometimes jokingly referred to as the most effective control measure for schistosomiasis. As economic conditions improve, children also tend to have entertainment options other than playing in infectious waters, a rare situation in which playing video games may be a more healthy option than playing outside in endemic areas where water exposure can occur.
However, development can also have consequences that result in increased rather than decreased exposure. Irrigation practices have been associated with human schistosome infection for as long as 6000 years.12,13 A number of water resource development projects built for the purpose of providing hydroelectric power, improved irrigation, and piped water systems have also resulted in increased snail habitats and human water contact.2 At least two of these projects, the Aswan High Dam on the Nile and the Diama Dam on the Senegal River, are associated with uncharacteristic ‘outbreaks’ of schistosomiasis, with local prevalence levels rising from under 5% to over 70% in just a few years.14,15 The effect of the Diama Dam was compounded by also interrupting the life cycle of the freshwater prawn Macrobrachium vollenhovenii that is a natural predator for snails that serve as intermediate hosts for schistosomes.16 The reduction in prawns, combined with an increase in snail habitat in irrigation schemes, has been associated with the rapid rise in schistosomiasis prevalence following construction of the dam. The impact of these and other water resource projects on increased local schistosomiasis caused great concern in advance of the construction of the Three Gorges Dam on the Yangtze River and its effect on China’s progress toward reducing the prevalence of S. japonicum infections.17,18 Fortunately, to date, there has been no apparent effect of that dam on increasing schistosomiasis transmission.19 This has been attributed to the vigorous chemotherapy efforts in the surrounding area, confirming the recommendation to couple schistosome control efforts with water resource projects in areas endemic for schistosomiasis.
Health education, especially through schools, and establishment of alternative locations for washing or swimming are additional ways to decrease contact with infectious water.20–23 Here again, careful thought that includes consideration of sociologic factors is important in the design of any intervention as effecting changes in behaviors is much more challenging than simply performing MDA or installing new facilities. For example, during the Rockefeller Foundation-supported schistosomiasis control studies in Saint Lucia, researchers found that despite provision of household water supplies, women still preferred to do their laundry at the river because it provided the opportunity for social interactions.24 Subsequent development of communal wash stations that used clean water garnered greater acceptance. Artificial swimming pools were also not accepted as the mud from the feet of children rapidly clouded the water and residents considered them unclean.24 Despite the failures of these initiatives, water supplied at individual households was readily accepted for domestic purposes and bathing.25 It also had the collateral benefits of clean water for prevention of other waterborne diseases. Unfortunately, for many areas endemic for schistosomiasis, even getting clean drinking water is a challenge, much less having alternatives to contaminated water for washing, bathing, and swimming. In addition, certain occupations such as car washing, sand harvesting, canal cleaning, and fishing expose workers to a higher risk of infections.26–29
Control of Snail Intermediate Host
The three major species of schistosomes that infect humans, S. mansoni, S. haematobium, and S. japonicum, are transmitted by specific genera of snails, Biomphalaria spp., Oncomelania spp., and Bulinus spp., respectively. A second water-based approach to controlling schistosomiasis is to interrupt the life cycle by interventions that impact the intermediate host. This has most commonly been done through the use of chemical molluscicides such as niclosamide.30–32 The drawback of this approach, however, is that the chemicals that kill snails are nonspecific and are toxic for other aquatic animals such as fish, which often make up the protein source for persons in communities where schistosomiasis is endemic. Fish toxicity and yellowing of treated water by niclosamide decrease the acceptability of mollusciciding in communities.33 In addition, the chemicals are expensive and can be rapidly washed down streams following rains or diluted to nontoxic concentrations in larger water bodies, which necessitates frequent reapplication. Furthermore, a relatively high degree of training is needed for personnel who disburse molluscicides as efficacy is influenced by other environmental conditions such as water hardness and temperature. Despite these drawbacks, the combination of mollusciciding and praziquantel treatment is more effective for reducing prevalence of schistosomiasis in humans than is treatment alone and it is currently being employed in operational research for elimination of schistosome infections in some areas.8,32
Indigenous plant extracts are an attractive alternative to chemicals for killing snails. Costs are held down by local availability and the extracts are less toxic to other forms of aquatic life.11,34–36 Endod, the soapberry plant Phytolacca dodecandra, has received the most attention but as yet has not been effectively employed for schistosomiasis control. As with other health interventions that require community involvement, success or failure is highly dependent on participation rates, which in turn are affected by perceived importance of the intervention, the ability to observe impact or personal benefits, and the degree of input the population has in designing the intervention.37
Biological control is another approach to reducing snail populations and impacting transmission of schistosomiasis. As mentioned above, interruption of the predator prawn life cycle in Senegal may have contributed to the schistosomiasis epidemic that occurred following construction of the Diama Dam.16 M. vollenhovenii is a voracious consumer of snails, leading to new initiatives to reintroduce the prawns upstream of the dam.38 A proposed collateral benefit of producing the prawns for snail control is their potential for food production for either local or exported consumption. Similarly, following introduction of the crayfish Procambarus clarkii into water bodies in Kenya for aquaculture, investigators observed that water bodies contained either crayfish or snails, able to transmit schistosomiasis, but not both.39 Laboratory and field enclosure studies confirmed that P. clarkii readily consumes Bulinus and Biomphalaria snails, as well as their egg masses. Successful introduction of crayfish into man-made ponds near schools lead to significant decreases in snail populations and subsequent reductions in schistosome infections among children in high transmission areas.40 Unlike the prawns in Senegal, however, P. clarkii are not native to Africa and the potential impact of exotic species on the overall ecosystem is a critical consideration that may limit their introduction into additional areas.
Certain species of fish are also predators for the snails that transmit schistosomiasis. Like the example of prawns in Senegal, the cichlid populations in Lake Malawi are molluscivores that preferentially feed on Bulinus spp. compared to snails with thicker shells.41 The increased transmission of S. haematobium in Lake Malawi beginning in the mid-1980s coincided with decreases in the cichlid populations due to overfishing.42 As larger fish populations dwindled, residents used nets with smaller openings to obtain food. Eventually, the remaining fish were too small to eat snails and the Bulinus populations flourished, followed by increased transmission of schistosomiasis with an inverse correlation between transmission and density of molluscivorous fish.43,44 To counter this effect, a ban on fishing in shallow waters has been proposed but compliance among local populations remains a challenge. Laboratory and field research with fish indigenous to Brazil, Zimbabwe, and Ethiopia suggest that additional species could be stocked into local waters for snail control.45–47
Other attempts to alter snail populations include environmental alterations such as removal of vegetation on which they feed, lining canals with cement, or draining water bodies where they live.48,49 Vegetation removal has had some success for reducing snail populations but has the adverse effect of increasing risk of infection to workers who may not have tools or protective clothing. Similarly, attempts to directly remove snails, including financial incentives for numbers collected has been employed in China but could increase infection risk to persons doing the work.50 The efficacy of this method is also questionable.51 Even in areas of high schistosomiasis transmission, the percentage of infected snails is usually low; suggesting that it only takes a few positive snails for the life cycle to be maintained. Thus, the prospect of finding and removing a sufficient numbers of intermediate snail hosts in order to interrupt transmission is unlikely. Environmental modifications are more successful but are very expensive and impractical for resource-constrained areas.49
Introduction of other snails that are inappropriate hosts for human schistosomiasis and either compete with or predate on intermediate host snails has been used with some success. Introduction of ampullarid (e.g. Marisa cornuarietis) or thiarid (e.g. Melanoidees tuberculata) snails into several endemic areas in the Caribbean region has successfully replaced populations of Biomphalaria spp. snails, resulting in the reduction or interruption of transmission of S. mansoni.52 M. cornuarietis also rapidly replaced Biomphalaria and other pulmonate snails following its introduction into a man-made water body in northern Tanzania.53 Competitor snails function by consuming food sources and egg clusters of schistosome-transmitting snails and have higher rates of reproduction.54–56 As a result, the fiscal costs of using competitor snails are trivial in comparison to using molluscicides that need frequent reapplication.57 There are also certain trematode parasites that are non-infectious for humans, which infect intermediate host snails and render them nonfertile or prevent infection by schistosome miracidia.52,58 However, like the use of predator species, great consideration must be employed as success in one ecological setting may not be reproducible in a different endemic area. More importantly, the history of introducing non-indigenous species is replete with unintended consequences and is therefore highly discouraged, if not illegal in most countries. Introduction of competitor species of snails or parasites could cause greater harm to agriculture or other animals in the ecosystem. As an alternative, the identification of strains of schistosome intermediate host snails that are resistant to miracidial infection and the associated defense factors shows promise.59,60 By mollusciciding and replacing infection-susceptible snails with an infection-resistant strain of the same species, it may be possible to accentuate the benefits of competitor snail approaches while minimizing the consequences.
Prevention of Contamination of Fresh Water by Stool and Urine
The third area of water-associated intervention that reduces risks of infection is preventing fecal and urine contamination of water bodies. If stool (S. mansoni, S. japonicum) or urine (S. haematobium) from infected individuals can be prevented from getting into fresh water, schistosome eggs do not hatch and there are no miracidia to infect snails. However, like snail control, unless these efforts are highly effective, the life cycle can be maintained by the considerable amplification that takes place in the snail host. The situation is further complicated for S. japonicum infections that are zoonotic, with infections of bovines making a substantial contribution to the continuation of transmission in China.
Like piped water, traditional sanitation systems require extensive infrastructure with installation and maintenance costs that are beyond the means of most areas where schistosomiasis is endemic. In the absence of infrastructure, water, sanitation, and hygiene (WASH) have been promoted as a means to reduce excreta from reaching fresh water. The water component usually consists of point of use treatment, sanitation involves construction of latrines, and hygiene involves health education and behavioral modification approaches such as community-led total sanitation that discourages open defecation through social pressure. However, the emphasis of WASH programs has traditionally been more one of the human rights rather than infection control and in studies where the impact on infectious diseases is considered, the focus has been more on diarrheal diseases rather than schistosomiasis or other NTDs.61 In other words, there has been very little operational research to successfully identify what sanitation practices are effective for reducing the transmission of schistosomiasis. In a randomized control trial in Kenya that was designed to evaluate the effect of WASH on soil-transmitted helminth infections, there was no effect of the WASH intervention on reinfection with S. mansoni after treatment.62 Failure of WASH interventions to lower the risk of S. haematobium infection was similarly observed in Zanzibar.6 Simply providing latrines may not be sufficient, even if they are consistently used, in areas where toilet paper is scarce as hygienic bathing after defecation may be the source of the vast majority of miracidia released into transmission sites rather than stool being washed into the water body.63 For urogenital schistosomiasis, preventing urination into the water is challenging as it can occur unobserved and the sense of urgency to urinate may be increased by the combination of bladder irritation from infection and being in the water.8 The interplay between schistosomiasis and sanitation is on its face quite obvious but there is a clear need for additional investigation in this area, especially with respect to human behavior.
By contrast, prevention of zoonotic S. japonicum contamination of fresh water by bovines used for agricultural purposes has received much more attention. Preventing their access to snail-infected grasslands and replacing them with mechanized farm equipment is part of an integrated control strategy being used in China with promising results.64–66 Additionally, a vaccine for schistosomiasis is being tested in bovines with the goal of blocking transmission through reducing egg output.67 Regular treatment of bovines has a similar benefit but as in humans requires an ongoing program.
Conclusions and Future Directions
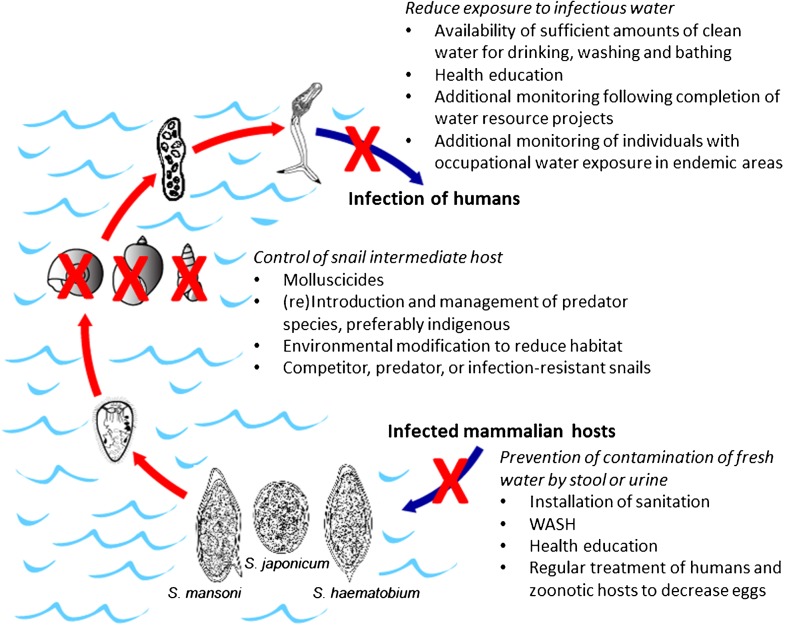
Chemotherapy with praziquantel is, and will remain, the backbone of control programs designed to prevent morbidity and decrease both prevalence and intensity of schistosomiasis. It is the most cost-effective approach and will continue to benefit populations in endemic areas as treatment becomes more widespread and operational research provides answers for better drug delivery. However, without additional control measures, it is highly unlikely that treatment will be able to break the transmission cycle of schistosomiasis, thus necessitating ongoing surveillance and continued chemotherapy with few prospects of ever reaching an endpoint. Current formulations of praziquantel are also inappropriate for mass treatment of very young children. Unless and until a safe, affordable and highly effective human vaccine for schistosomiasis can be developed, any adjunct to chemotherapy to reduce infection will involve the water stages of the life cycle; thus, further research in this area is both critical and timely. Even before reaching the elimination phase, effective water-based interventions that reduce transmission may be operational adjuncts to treatment for improved morbidity control. A summary of the strategies discussed above are shown in Figure 2.
Figure 2.
Putative waster-based interventions for schistosomiasis control. Measures could be employed individually or in concert but success of any intervention will be dependent on its acceptability to the endemic population and should be developed with local culture and customs in mind.
Beyond extension and refinement of the topics mentioned in this review, needs for investigations and tool development include methods to determine the force of transmission, i.e. how ‘infectious’ a water contact site may be. Such a method would not only help identify sites that pose the greatest risk of infection for focused remediation but would also be a more immediate and direct measure than subsequent human infection for determining whether a given water-associated intervention was effective. A number of attempts have been made to measure the number of infectious cercariae in a volume of water but none are as yet field applicable. Sentinel mice have been used with some success but results are not available for many weeks and the animal care costs associated with this method prevent the frequency and number of sampling sites that are needed for operational utility. Snail collections and shedding, along with PCR that can also detect prepatent infections is promising but still dependent on properly standardized snail collection methods for site comparison. Use of sentinel snails for identifying egg contamination of water bodies has shown promise for transmission mapping.68
Another area of needed research is determining what prevalence levels of human and/or snail infection merit changes in control measures. This knowledge would inform programs when an intervention that is too expensive for regular use (e.g. molluscicide) may be a worthwhile investment to initiate a control program when infection levels are very high or break the transmission cycle as a program moves into the elimination phase.31 Similarly, little research has evaluated timed treatments or other interventions in areas where transmission is seasonal. Scheduling MDA immediately after a transmission season or mollusciciding at the beginning of a transmission season may be a more cost-effective use of resources. Finally, once transmission has apparently ceased, it is not known how long additional surveillance is needed to verify that elimination has been achieved.
In any control program, including MDA, the participation of the affected population is necessary for success. Misunderstanding about an intervention or failure to see how it is of benefit to an individual leads to low participation while agreement with the goals and methods increases involvement.21,22,37,48,69 Thus, a critical but frequently overlooked aspect of control and elimination programs is community engagement and health education prior to initiation of the intervention. Because there are three major species of schistosomiasis with different transmission features, and sometimes S. mansoni and S. haematobium may overlap, it is not possible to design a ‘one size fits all’ plan for elimination, especially when water usage is involved. Even communities that share most features of the transmission dynamics may choose different responses to the same challenge. Continued consideration of the interplay between infection, environment, and human behavior with respect to water will be necessary for achieving the global control and elimination goals for schistosomiasis.
Disclaimer Statements
The opinions and conclusions in this report are those of the author and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Funding None.
Conflicts of interest I have no conflicts of interest.
Ethics approval Not applicable.
References
- 1.Montresor A, Gabrielli AF, Chitsulo L, Ichimori K, Mariotti S, Engels D, et al. Preventive chemotherapy and the fight against neglected tropical diseases. Expert Rev Anti Infect Ther. 2012;10:237–42. doi: 10.1586/eri.11.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383((9936)):2253–64. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020 Geneva: World Health Organization; 2012 [Google Scholar]
- 6.Knopp S, Stothard JR, Rollinson D, Mohammed KA, Khamis IS, Marti H, et al. From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. Acta Trop. 2013;128:412–22. doi: 10.1016/j.actatropica.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Njenga SM, Mutungi FM, Wamae CN, Mwanje MT, Njiru KK, Bockarie MJ. Once a year school-based deworming with praziquantel and albendazole combination may not be adequate for control of urogenital schistosomiasis and hookworm infection in Matuga District, Kwale County, Kenya. Parasit Vectors. 2014;7:74. doi: 10.1186/1756-3305-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopp S, Mohammed KA, Ali SM, Khamis IS, Ame SM, Albonico M, et al. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC Public Health. 2012;12:930. doi: 10.1186/1471-2458-12-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–40. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Mo AX, Agosti JM, Walson JL, Hall BF, Gordon L. Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg. 2014;90:54–60. doi: 10.4269/ajtmh.13-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chimbari MJ. Enhancing schistosomiasis control strategy for Zimbabwe: building on past experiences. J Parasitol Res. 2012;2012:353768. doi: 10.1155/2012/353768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibbs AC, Secor WE, Van Gerven D, Armelagos G. Irrigation and infection: the immunoepidemiology of schistosomiasis in ancient Nubia. Am J Phys Anthropol. 2011;145:290–8. doi: 10.1002/ajpa.21493. [DOI] [PubMed] [Google Scholar]
- 13.Anastasiou E, Lorentz KO, Stein GJ, Mitchell PD. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect Dis. 2014;14:553–4. doi: 10.1016/S1473-3099(14)70794-7. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab MF, Strickland GT, El-Sahly A, El-Kady N, Zakaria S, Ahmed L.Changing pattern of schistosomiasis in Egypt 1935–79. Lancet. 19792(8136):242–4. [DOI] [PubMed] [Google Scholar]
- 15.Stelma FF, Talla I, Polman K, Niang M, Sturrock RF, Deelder AM, et al. Epidemiology of Schistosoma mansoni infection in a recently exposed community in northern Senegal. Am J Trop Med Hyg. 1993;49:701–6. doi: 10.4269/ajtmh.1993.49.701. [DOI] [PubMed] [Google Scholar]
- 16.Sokolow SH, Lafferty KD, Kuris AM. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop. 2014;132:64–74. doi: 10.1016/j.actatropica.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J, Gu XG, Xu YL, Ge JH, Yang XX, He CH, et al. Relationship between the transmission of schistosomiasis japonica and the construction of the Three Gorge Reservoir. Acta Trop. 2002;82:147–56. doi: 10.1016/s0001-706x(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhu HM, Xiang S, Yang K, Wu XH, Zhou XN. Three Gorges Dam and its impact on the potential transmission of schistosomiasis in regions along the Yangtze River. Ecohealth. 2008;5:137–48. doi: 10.1007/s10393-008-0168-y. [DOI] [PubMed] [Google Scholar]
- 19.Gray DJ, Thrift AP, Williams GM, Zheng F, Li YS, Guo J, et al. Five-year longitudinal assessment of the downstream impact on schistosomiasis transmission following closure of the Three Gorges Dam. PLoS Negl Trop Dis. 2012;6:e1588. doi: 10.1371/journal.pntd.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou LY, Deng Y, Steinmann P, Yang K. The effects of health education on schistosomiasis japonica prevalence and relevant knowledge in the People's Republic of China: a systematic review and meta-analysis. Parasitol Int. 2013;62:150–6. doi: 10.1016/j.parint.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Mwanga JR, Lwambo NJ. Pre- and post-intervention perceptions and water contact behaviour related to schistosomiasis in north-western Tanzania. Acta Trop. 2013;128:391–8. doi: 10.1016/j.actatropica.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, et al. Community knowledge, attitudes and practices on schistosomiasis in western Kenya-The SCORE Project. Am J Trop Med Hyg. 2014;90:646–52. doi: 10.4269/ajtmh.13-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosinski KC, Adjei MN, Bosompem KM, Crocker JJ, Durant JL, Osabutey D, et al. Effective control of Schistosoma haematobium infection in a Ghanaian community following installation of a water recreation area. PLoS Negl Trop Dis. 2012;6:e1709. doi: 10.1371/journal.pntd.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan P, Bartholomew RK, Unrau GO, Upatham ES, Grist E, Christie JD. Further observations from St Lucia on control of Schistosoma mansoni transmission by provision of domestic water supplies. Bull World Health Organ. 1978;56:965–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan P, Unrau GO, Bartholomew RK, Cook JA, Grist E. Value of individual household water supplies in the maintenance phase of a schistosomiasis control programme in Saint-Lucia, after chemotherapy. Bull World Health Organ. 1982;60:583–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Karanja DMS, Hightower AW, Colley DG, Mwinzi PNM, Galil K, Andove J, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and the effect of HIV-1 coinfection on susceptibility to schistosomiasis: A longitudinal study. Lancet. 2002;360:592–6. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- 27.Black CL, Mwinzi PNM, Muok EMO, Abudho B, Fitzsimmons CM, Dunne DW, et al. Influence of exposure history on the immunology and development of resistance to human schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2010;4:e637. doi: 10.1371/journal.pntd.0000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tameim O, Abdu KM, el Gaddal AA, Jobin WR. Protection of Sudanese irrigation workers from schistosome infections by a shift to earlier working hours. J Trop Med Hyg. 1985;88:125–30. [PubMed] [Google Scholar]
- 29.Peng WX, Tao B, Clements A, Jiang QL, Zhang ZJ, Zhou YB, et al. Identifying high-risk areas of schistosomiasis and associated risk factors in the Poyang Lake region, China. Parasitology. 2010;137:1099–107. doi: 10.1017/S003118200999206X. [DOI] [PubMed] [Google Scholar]
- 30.Chu KY. Trials of ecological and chemical measures for the control of Schistosoma haematobium transmission in a Volta Lake village. Bull World Health Organ. 1978;56:313–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Yang GJ, Sun LP, Hong QB, Zhu HR, Yang K, Gao Q, et al. Optimizing molluscicide treatment strategies in different control stages of schistosomiasis in the People's Republic of China. Parasit Vectors. 2012;5:260. doi: 10.1186/1756-3305-5-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kariuki HC, Madsen H, Ouma JH, Butterworth AE, Dunne DW, Booth M, et al. Long term study on the effect of mollusciciding with niclosamide in stream habitats on the transmission of schistosomiasis mansoni after community-based chemotherapy in Makueni District, Kenya. Parasit Vectors. 2013;6:107. doi: 10.1186/1756-3305-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takougang I, Meli J, Wabo Poné J, Angwafo F., 3rd Community acceptability of the use of low-dose niclosamide (Bayluscide), as a molluscicide in the control of human schistosomiasis in Sahelian Cameroon. Ann Trop Med Parasitol. 2007;101:479–86. doi: 10.1179/136485907X193833. [DOI] [PubMed] [Google Scholar]
- 34.Mølgaard P, Chihaka A, Lemmich E, Furu P, Windberg C, Ingerslev F, et al. Biodegradability of the molluscicidal saponins of Phytolacca dodecandra. Regul Toxicol Pharmacol. 2000;32:248–55. doi: 10.1006/rtph.2000.1390. [DOI] [PubMed] [Google Scholar]
- 35.Abebe F, Erko B, Gemetchu T, Gundersen SG. Control of Biomphalaria pfeifferi population and schistosomiasis transmission in Ethiopia using the soap berry endod (Phytolacca dodecandra), with special emphasis on application methods. Trans R Soc Trop Med Hyg. 2005;99:787–94. doi: 10.1016/j.trstmh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Rapado LN, Pinheiro Ade S, Lopes PO, Fokoue HH, Scotti MT, Marques JV, et al. Schistosomiasis control using piplartine against Biomphalaria glabrata at different developmental stages. PLoS Negl Trop Dis. 2013;7:e2251. doi: 10.1371/journal.pntd.0002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ndekha A, Hansen EH, Mølgaard P, Woelk G, Furu P. Community participation as an interactive learning process: experiences from a schistosomiasis control project in Zimbabwe. Acta Trop. 2003;85:325–38. doi: 10.1016/s0001-706x(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 38.Jullien M.Using Prawns to Battle A Killer Disease in Senegal. BBC News, 0000Available at http://www.bbc.com/news/world-africa-21080224 [Google Scholar]
- 39.Hofkin BV, Mkoji GM, Koech DK, Loker ES. Control of schistosome-transmitting snails in Kenya by the North American crayfish Procambarus clarkii. Am J Trop Med Hyg. 1991;45:339–44. doi: 10.4269/ajtmh.1991.45.339. [DOI] [PubMed] [Google Scholar]
- 40.Mkoji GM, Hofkin BV, Kuris AM, Stewart-Oaten A, Mungai BN, Kihara JH, et al. Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. Am J Trop Med Hyg. 1999;61:751–9. doi: 10.4269/ajtmh.1999.61.751. [DOI] [PubMed] [Google Scholar]
- 41.Evers BN, Madsen H, McKaye KM, Stauffer JR., Jr The schistosome intermediate host, Bulinus nyassanus, is a 'preferred' food for the cichlid fish, Trematocranus placodon, at Cape Maclear, Lake Malawi. Ann Trop Med Parasitol. 2006;100:75–85. doi: 10.1179/136485906X78553. [DOI] [PubMed] [Google Scholar]
- 42.Stauffer JR, Jr, Arnegard ME, Cetron M, Sullivan JJ, Chitsulo LA, Turner GF, et al. Controlling vectors and hosts of parasitic diseases using fishes. BioScience. 1997;47:41–9. [Google Scholar]
- 43.Stauffer JR, Jr, Madsen H, McKaye K, Konings A, Bloch P, Ferreri CP, et al. Schistosomiasis in Lake Malawi: relationship of fish and intermediate host density to prevalence of human infection. Ecohealth. 2006;3:22–7. [Google Scholar]
- 44.Madsen H, Stauffer JR. Density of Trematocranus placodon (Pisces: Cichlidae): a predictor of density of the schistosome intermediate host, Bulinus nyassanus (Gastropoda: Planorbidae), in Lake Mala∧wi. Ecohealth. 2011;8:177–89. doi: 10.1007/s10393-011-0737-3. [DOI] [PubMed] [Google Scholar]
- 45.Weinzettl M, Jurberg P. Biological control of Biomphalaria tenagophila (Mollusca, Planorbidae), a schistosomiasis vector, using the fish Geophagus brasiliensis (Pisces, Cichlidae) in the laboratory or in a seminatural environment. Mem Inst Oswaldo Cruz. 1990;85:35–8. doi: 10.1590/s0074-02761990000100005. [DOI] [PubMed] [Google Scholar]
- 46.Chimbari MJ, Madsen H, Ndamba J. Laboratory experiments on snail predation by Sargochromis codringtoni, a candidate for biological control of the snails that transmit schistosomiasis. Ann Trop Med Parasitol. 1997;91:95–102. doi: 10.1080/00034983.1997.11813116. [DOI] [PubMed] [Google Scholar]
- 47.Gashaw F, Erko B, Teklehaymanot T, Habtesellasie R. Assessment of the potential of competitor snails and African catfish (Clarias gariepinus) as biocontrol agents against snail hosts transmitting schistosomiasis. Trans R Soc Trop Med Hyg. 2008;102:774–9. doi: 10.1016/j.trstmh.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 48.Boelee E, Laamrani H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop Med Int Health. 2004;9:997–1004. doi: 10.1111/j.1365-3156.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohmae H, Iwanaga Y, Nara T, Matsuda H, Yasuraoka K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol Int. 2003;52:409–17. doi: 10.1016/s1383-5769(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 50.Sleigh A, Li X, Jackson S, Huang K. Eradication of schistosomiasis in Guangxi, China. Part 1: setting, strategies, operations, and outcomes, 1953–92. Bull World Health Organ. 1998;76:361–72. [PMC free article] [PubMed] [Google Scholar]
- 51.Fan KW. Schistosomiasis control and snail elimination in China. Am J Public Health. 2012;102:2231–2. doi: 10.2105/AJPH.2012.300809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pointier JP, Jourdane J. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Trop. 2000;77:53–60. doi: 10.1016/s0001-706x(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 53.Nguma JF, McCollough FS, Masha E. Elimination of Biomphalaria pfeifferi, Bulinus tropicus and Lymnaea natalensis by the ampullarid snail, Marisa cornuarietis, in a man-made dam in northern Tanzania. Acta Trop. 1982;39:85–90. [PubMed] [Google Scholar]
- 54.Negrón-Aponte H, Jobin WR. Schistosomiasis control in Puerto Rico: twenty-five years of operational experience. Am J Trop Med Hyg. 1979;28:515–25. doi: 10.4269/ajtmh.1979.28.515. [DOI] [PubMed] [Google Scholar]
- 55.Pointier JP, Guyard A, Mosser A. Biological control of Biomphalaria glabrata and B. straminea by the competitor snail Thiara tuberculata in a transmission site of schistosomiasis in Martinique, French West Indies. Ann Trop Med Parasitol. 1989;83:263–9. [PubMed] [Google Scholar]
- 56.Chingwena G, Mukaratirwa S, Kristensen TK, Chimberi M. Susceptibility of freshwater snails to the amphistome Calicophoron microbothrium and the influence of the species on susceptibility of Bulinus tropicus to Schistosoma haematobium and Schistosoma mattheei infections. J Parasitol. 2002;88:880–3. doi: 10.1645/0022-3395(2002)088[0880:SOFSTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Jobin WR, Brown RA, Vélez SP, Ferguson FF. Biological control of Biomphalaria glabrata in major reservoirs of Puerto Rico. Am J Trop Med Hyg. 1977;26:1018–24. doi: 10.4269/ajtmh.1977.26.1018. [DOI] [PubMed] [Google Scholar]
- 58.Tang CT, LU MK, Guo Y, Wang YN, Peng JY, Wu WB, et al. Development of larval Schistosoma japonicum blocked in Oncomelania hupensis by pre-infection with larval Exorchis sp. J Parasitol. 2009;95:1321–5. doi: 10.1645/GE-2055.1. [DOI] [PubMed] [Google Scholar]
- 59.Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl Trop Dis. 2012;6:e1591. doi: 10.1371/journal.pntd.0001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Nassery SM, Abou-El-Naga IF, Allam SR, Shaat EA, Mady RF. Genetic variation between Biomphalaria alexandrina snails susceptible and resistant to Schistosoma mansoni infection. Biomed Res Int. 2013;2013:160320. doi: 10.1155/2013/160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, Amnie AG, et al. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis. 2013;7:e2439. doi: 10.1371/journal.pntd.0002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freeman MC, Clasen T, Brooker SJ, Akoko DO, Rheingans R. The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth reinfection: a cluster-randomized trial. Am J Trop Med Hyg. 2013;89:875–83. doi: 10.4269/ajtmh.13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sow S, Polman K, Vereecken K, Vercruysse J, Gryseels B, de Vlas SJ. The role of hygienic bathing after defecation in the transmission of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 2008;102:542–7. doi: 10.1016/j.trstmh.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 64.Hong QB, Yang K, Huang YX, Sun LP, Yang GJ, Gao Y, et al. Effectiveness of a comprehensive schistosomiasis japonica control program in Jiangsu province, China, from 2005 to 2008. Acta Trop. 2011;120((Suppl 1)):S151–7. doi: 10.1016/j.actatropica.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Hong XC, Xu XJ, Chen X, Li YS, Yu CH, Yuan Y, et al. Assessing the effect of an integrated control strategy for schistosomiasis japonica emphasizing bovines in a marshland area of Hubei Province, China: a cluster randomized trial. PLoS Negl Trop Dis. 2013;7:e2122. doi: 10.1371/journal.pntd.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YY, Liu JB, Huang XB, Cai SX, Su ZM, Zhong R, et al. New integrated strategy emphasizing infection source control to curb Schistosomiasis japonica in a marshland area of Hubei Province, China: findings from an eight-year longitudinal survey. PLoS One. 2014;9:e89779. doi: 10.1371/journal.pone.0089779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da'dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, et al. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–25. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allan F, Dunn AM, Emery AM, Stothard JR, Johnston DA, Kane RA, et al. Use of sentinel snails for the detection of Schistosoma haematobium transmission on Zanzibar and observations on transmission patterns. Acta Trop. 2013;128:234–40. doi: 10.1016/j.actatropica.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Omedo MO, Matey EJ, Awiti A, Ogutu M, Alaii J, Karanja DMS, et al. Community health workers' experiences and perspectives on mass drug administration for schistosomiasis control in western Kenya: the SCORE Project. Am J Trop Med Hyg. 2012;87:1065–72. doi: 10.4269/ajtmh.2012.12-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]