Abstract
The prevalence of obesity is growing at an alarming rate, placing many at risk for developing diabetes, hypertension, sleep apnea, or a combination of disorders known as “metabolic syndrome”. The evidence to date suggests that metabolic syndrome results from an imbalance in the mechanisms that link diet, physical activity, glucose-insulin control, and autonomic cardiovascular control. There is also growing recognition that sleep-disordered breathing and other forms of sleep disruption can contribute significantly to autonomic dysfunction and insulin resistance. Chronic sleep deprivation resulting from sleep-disordered breathing or behavioral causes can lead to excessive daytime sleepiness and lethargy, which in turn contribute to increasing obesity. Analysis of this complex dynamic system using a model-based approach can facilitate the delineation of the causal pathways that lead to the emergence of the metabolic syndrome. In this paper, we provide an overview of the main physiological mechanisms associated with obesity and sleep-disordered breathing that are believed to result in metabolic and autonomic dysfunction, and review the models and modeling approaches that are relevant in characterizing the interplay among the multiple factors that underlie the development of the metabolic syndrome.
Index Terms: Autonomic function, computational model, glucose-insulin regulation, obesity, sleep apnea
I. Introduction
The term “metabolic syndrome” has been used to characterize the clustering of symptoms that include obesity, insulin resistance, hypertension and dyslipidemia. The first standardized definition of metabolic syndrome (MS) was provided by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III report in 2001 [2]. According to the criteria established in the report, MS is diagnosed when three or more of the following measures are found in a subject: 1) elevated waist circumference (≥ 102 cm in men, ≥ 88 cm in women); 2) elevated triglycerides (≥ 150 mg/dL, or 1.7 mmol/L) or on drug treatment for elevated triglycerides; 3) reduced high-density lipoprotein cholesterol, HDL-C (< 40 mg/dL or 1.03 mmol/L in men, <50 mg/dL or 1.3 mmol/L in women) or on drug treatment for reduced HDL-C; 4) elevated blood pressure, systolic blood pressure or ≥85 mmHg diastolic blood pressure, or on antihypertensive drug treatment in a patient with a history of hypertension; 5) elevated fasting glucose (≥ 110 mg/dL) or on drug treatment for elevated glucose. The NCEP definition was subsequently updated [3] to use a cutoff of fasting glucose ≥ 110 mg/dl, consistent with the current American Diabetes Association definition of impaired fasting glucose [4].
Using the NCEP criteria, the National Health and Nutrition Examination survey (NHANES) conducted between 2003 and 2006 found that approximately 34% of all US adults have MS [5]. There was a strong association with age in these data: for subjects 20–39, 40–59 and >60 years, the prevalence rates were approximately 18%, 39% and 53%, respectively. MS was slightly more prevalent in males compared to females, except in the >60 group, where the trend was reversed. Prevalence of MS increased also with body mass index (BMI) in both sexes. Obese males were 32 times more likely to have MS compared to their under- and normal weight counterparts. Indeed, the prevalence of MS over the past few decades has increased in tandem with the prevalence rate of obesity [6]. This is a phenomenon that has been observed to occur not only in the US and other western countries, but also in developing countries, such as India and Mexico, where growing affluence, urbanization and the corresponding changes in lifestyle and diet are pushing prevalence rates progressively higher [7]. Since the components of MS, either individually or in combination, constitute risk factors for cardiovascular disease [8]–[10] and Type 2 diabetes [11], the implications for future healthcare costs and management appear challenging.
The evidence to date suggests that metabolic syndrome results from an imbalance in the mechanisms that link diet, physical activity, glucose-insulin control, and autonomic cardiovascular control with one another. There is also growing recognition that the added factors of sleep-disordered breathing (SDB) and other forms of sleep disruption can contribute significantly to autonomic imbalance and insulin resistance [12]–[14]. Moreover, chronic sleep deprivation resulting from SDB or behavioral causes can lead to excessive daytime sleepiness and lethargy, which in turn can contribute to increasing obesity [15]. The complex nature of this multifactorial system makes it difficult to establish the causal links that connect one factor to another. Apart from some preliminary efforts, no comprehensive model of MS exists to date. But it is precisely because of the complexity of this problem that a closed-loop systems approach, that takes into account multiple feedforward and feedback loops, should be useful in allowing us to better understand the underlying pathophysiology of autonomic and metabolic dysfunction in MS [16].
II. Measures of Metabolic, Automatic and Sleep Impairment
The physiological systems that regulate glucose metabolism, autonomic cardiovascular control, respiratory control and the sleep-wake cycle are in themselves complex and each constitutes the basis for an entire medical specialty. Thus, it is useful at the outset to define the terminology and lay out the basic concepts connected with each of these systems. Since much of these are closely tied to the methodology with which the key indicators of function are measured or assessed, we will briefly review the main techniques used for assessment of the parameters in question.
A. Measuring Metabolic Dysfunction
Insulin resistance refers to the condition in which some or all body tissues are unable to metabolize glucose at normal rates for the same amount of insulin delivered by the bloodstream. This is generally the starting point for the development of Type 2 diabetes. In response to insulin resistance, the pancreas exerts a compensatory response by producing more insulin. As insulin resistance worsens, for instance with progressive obesity, the beta cells of the pancreas eventually become dysfunctional. As such, glycemic control becomes impaired, leading to a condition known as “impaired glucose tolerance” (also referred to as “prediabetes”). With progressive deterioration of the beta cells, the body becomes hyperglycemic even under fasting conditions, at which point, overt Type 2 diabetes emerges [17].
Different methods have been proposed for measurement of the degree of insulin resistance of an individual. The hyperinsulinemic euglycemic clamp [18] has been usually regarded as the gold standard for quantifying the degree of insulin resistance. In this procedure, plasma glucose concentration is infused and held constant at the basal arterial plasma glucose concentration level, by periodically adjusting the glucose infusion, based on the negative feedback principle. In other words, if the actual glucose concentration is higher than the desired set point, the infusion is decreased, and vice-versa. Since this test maintains the basal glucose level after insulin administration, it avoids the physiological responses to hypoglycemia and thus provides a reliable estimate of tissue sensitivity to insulin.
Another commonly used surrogate measure for insulin sensitivity is the Homeostasis Model Assessment of Insulin Resistance, or HOMA-IR index. Since this score requires the use of only fasting plasma glucose and insulin, it is a more simple and inexpensive alternative to the determination of insulin resistance. The HOMA method derives an estimate of insulin sensitivity from the mathematical modeling of fasting plasma glucose and insulin concentrations [19]. A model of insulin-glucose interactions is used to determine an array of fasting plasma insulin and glucose concentrations that would be expected for varying degrees of -cell deficiency and insulin resistance. From this array, the insulin resistance and deficient -cell function which might have been expected to give the fasting plasma glucose and insulin concentrations observed in a patient can be estimated. High HOMA scores denote high insulin resistance. Since the HOMA score estimates the spontaneous homeostatic characteristics by inferring what degree of insulin sensitivity is compatible with the homeostatic characteristics of the metabolic system in each individual, it is less accurate than the clamp method for assessing insulin sensitivity. Even so, Bonora and colleagues [20] have shown that the HOMA score can account for 65% of the variability in insulin sensitivity assessed by the glucose clamp technique (p < 0.0001). Because of the need for only fasting blood assays, the HOMA index has been used frequently in large-scale or epidemiological studies. The authors do mention, however, that comparing HOMA scores obtained from different studies cannot be done unless the insulin assay is standardized across studies.
The oral glucose tolerance test is a dynamic test that stimulates both glucose disposal and insulin secretion. After overnight fasting, the subject ingests 75 g of anhydrous glucose over a period of 5 minutes; subsequently, blood is sampled 2 hours after the glucose bolus and plasma glucose is measured. The subject is said to have “impaired glucose tolerance” if the 2-hour glucose level exceeds 140 mg/dL but remains below 200 mg/dL [21]. Although the oral glucose tolerance test is a relatively simple test that activates the insulin-glucose homeostatic process, measuring glucose tolerance is not synonymous to measuring insulin resistance, since the response includes endogenous insulin secretion [22].
Relative to other tests that are commonly applied in the clinical setting, the frequently-sampled intravenous glucose tolerance test yields a more complete characterization of the dynamics of glucose metabolism in the presence of insulin, providing more accurate estimates of insulin resistance and also information about beta cell function. This involves, after collection of 2 baseline blood samples following overnight fasting, the intravenous infusion of a bolus of glucose (300 mg/kg of body weight) over a period of 2 minutes, followed by the timed collection of 6 sequential blood samples within 19 minutes of glucose administration. Then, at 20 minutes, insulin (0.02 units per kilogram body weight) is administered, followed by the subsequent collection of 11 timed blood samples over 3 hours following glucose administration. The 19 blood samples are assayed for plasma glucose and insulin concentrations and the dynamics of insulin-glucose interaction are analyzed using the Bergman minimal model [23], [24]. The model consists of two coupled differential equations: one characterizes the insulin-dependent and insulin-independent dynamics of glucose uptake by the body tissues; the second equation models the kinetics of insulin transport from plasma into the interstitial space where it exerts its physiologic actions on glucose disposal. From the model, one can estimate through nonlinear least squares fitting of the plasma glucose concentration profile, the insulin sensitivity (SI, which is inversely related to insulin resistance) and the glucose effectiveness (the effect of glucose on its own disposal independent of insulin action). From the endogenous response of insulin to the intravenous bolus of glucose (following external glucose administration and before intravenous administration of exogenous insulin), the acute response of insulin to glucose (AIRg) is deduced – which provides an indication of pancreatic beta cell function. The product of SI and AIRg is known as the “disposition index” (DI). If SI were to be reduced but AIRg were to compensate perfectly for the decrease in insulin sensitivity (or equivalently, the increase in insulin resistance), then DI would remain constant. Decreases in DI would indicate a reduced ability for the pancreatic beta cells to compensate for an increase in insulin resistance.
B. Measuring Autonomic Dysfunction
There is a substantial amount of evidence that points to abnormal autonomic control as a major contributor to the hypertension component of MS. Animal studies have demonstrated that overfeeding can lead to sympathetic overactivity [25]. Baseline sympathetic activity in humans has been shown to be increased in obesity [26]. Adiposity, hyperinsulinemia and elevated sympathetic drive all predispose strongly to hypertension [27]. Thus, apart from directly measuring blood pressure, many studies of MS have employed more direct methods of measuring autonomic function. The most direct assessment of sympathetic activity involves measurement of muscle sympathetic nerve activity using peroneal microneurography, but this method has been limited to the research setting since it requires considerable technical expertise and is highly susceptible to artifactual noise introduced by limb movements [28]. Moreover, microneurography gives only a regionally-confined assessment of sympathetic tone, which can be quantitatively different in the heart and various parts of the vasculature [29]. Radiotracer dilution methodology has been used to determine norepinephrine spillover in various organ systems as a measure of regional sympathetic activity [30], but these kinds of measurements are expensive, intrusive and technically challenging. Plasma or urinary catecholamine concentrations provide an integrated measure of sympathetic outflow over a period of many hours, and as such, are limited in sensitivity and temporal resolution [29]. Furthermore, plasma catecholamine levels can be confounded by the variability in the subject’s psychological state just prior to or as a consequence of the blood sampling process [31]. Autonomic stress tests are not commonly applied as they have been shown to be quite insensitive and to depend critically on subject cooperation [32].
Spectral analysis of heart rate variability (HRV) has been used extensively as a noninvasive and nonintrusive means of measuring cardiac autonomic function [33], which makes it an attractive and low-cost method for detecting autonomic dysfunction in MS. However, there are important limitations that are often overlooked. For instance, power in the high-frequency band (0.15 to 0.4 Hz) is highly sensitive to differences or changes in ventilatory pattern [34]. This caveat is particularly important when spectral analysis of HRV is performed in subjects under various conditions with irregular or periodic forms of ventilation [35]. The low-frequency power or the ratio of low-frequency to high-frequency power (LHR) is frequently cited as measures of sympathetic nervous system activity, but it is now fairly well established that these indices contain a substantial parasympathetic contribution [36]. Saul et al. [37] found no significant correlation between low-frequency power of HRV or the LHR and baseline peroneal sympathetic nerve activity. Indeed, one frequently overlooked premise in this technique is that HRV assesses fluctuations in heart rate, which in turn reflect fluctuations in autonomic activity but not necessarily autonomic tone [38].
The limitations inherent in using heart rate variability or blood pressure variability to infer autonomic function can be circumvented to some extent by focusing not on the oscillations themselves but on how they are correlated with each other [39]. The simplest example is the relationship between spontaneous fluctuations in blood pressure and those of heart rate – i.e., the determination of “spontaneous” baroreflex sensitivity (BRS). Spontaneous BRS can be estimated in the time domain using the “sequence” technique or in the frequency domain using the “spectral” method [40]. A further refinement of this approach involves the explicit incorporation of the very significant effects of respiration on heart rate and blood pressure, and consideration of feedback effects in a closed-loop model configuration [41]–[43]. Determining BRS may be a means of early detection of MS since it has been shown in a study on diabetic patients that impaired baroreflex function can precede the occurrence of daytime hypertension or overt autonomic dysfunction [44]. BRS has been shown to be impaired in elderly subjects with MS and negatively correlated with insulin resistance, as measured by HOMA-IR [45]. In another study on healthy non-diabetic subjects with no history of cardiovascular disease, BRS was also found to be negatively correlated with HOMA-IR [46]. Beske et al. [47] found BRS to be negatively correlated with increasing levels of abdominal visceral fat.
C. Measuring the Effects of SDB and Sleep Disruption
The measurements recorded in a standard clinical polysomnographic study include as the electroencephalogram, electrooculogram, electromyogram, electrocardiogram, respiratory airflow, respiratory effort (changes in ribcage and abdominal circumference), snoring, body position, and oxy-hemoglobin saturation. With these measurements, the sleep parameters that are generally taken to reflect severity of SDB include [48]: (a) apnea-hypopnea index (AHI), which is defined as the average number of apneas (greater than 90% decrease in airflow from baseline value) and hypopneas (between 50% and 90% decrease in airflow from baseline value) per hour of sleep; (b) desaturation index, a measure of the degree of exposure to intermittent hypoxia in SDB, defined as the number of events per hour of sleep in which oxyhemoglobin saturation is reduced by 3% or more from baseline; (c) arousal index, the average number of arousals per hour, which may be a superior marker of sleep fragmentation and better explain daytime sleepiness when compared with the apnea-hypopnea index; and (d) SpO2_nadir, which is the lowest value of arterial oxyhemoglobin saturation for detecting hypoxia, measured via pulse oximetry.
III. Obesity, Impaired Glucose Metabolism and Autonomic Dysfunction
Obesity results from a breakdown in the energy balance that relates ingestive behavior to energy storage in adipose tissue and energy expenditure. Although the influences that shape this regulatory system are multifactorial and complex, many studies have shown that the sympathetic nervous system plays an important role by regulating adipose tissue storage [49], as well as modulating energy expenditure through diet-induced thermogenesis [50]. As well, the ventromedial and lateral hypothalamus modulate hunger and the feeling of satiety through both branches of the autonomic nervous system [51]. Animal models have demonstrated that reduced sympathetic activity can play a causative role in the development of obesity [50], [51]. Thus, an early hypothesis was that obesity should be accompanied by low sympathetic activity, since the latter would reduce thermogenesis and thus lead to weight gain [52]. However, subsequent studies have shown the opposite in humans: that baseline sympathetic activity is increased in obesity [26], [53].
Although the question has not been totally resolved, the general consensus to date is that obesity is associated with sympathetic overactivity, and that this elevated sympathetic tone represents the body’s compensatory response aimed at achieving weight stabilization [54], [55]. Food intake triggers the release of insulin which acts to regulate glucose metabolism. However, in obesity, excessive feeding can lead to chronic hyperinsulinemia, which predisposes to insulin resistance. Since insulin stimulates sympathetic activity, it has been suggested that hyperinsulinemia, which is more prevalent in obese than non-obese humans, may be largely responsible for sympathetic overactivity associated with obesity [25], [27]. Thus, the current evidence suggests that diet, physical activity, glucose-insulin control, and the insulin-mediated regulation of sympathetic activity are tied together in a delicate balance that, if disrupted, can lead to obesity and obesity-related disorders. Such obesity-related disorders include Type 2 diabetes, hypertension, and the combination of autonomic and metabolic dysfunction that is now recognized as MS. Since the prevalence of SDB in obese adults is between 2 and 3 times as high as that in the general population [56], and approximately half of all SDB patients are overweight or obese [57], it is likely that SDB is also important as another risk factor in the development of MS.
IV. Effects of Sleep-Disordered Breathing and Sleep Disruption on Autonomic Control
Over the past decade or so, epidemiological studies, such as the multi-center Sleep Heart Health Study, have provided strong evidence suggesting that SDB constitutes an independent risk factor for the development of systemic hypertension, coronary artery disease, heart failure and stroke [58]–[60]. Three major factors accompany each cycle of SDB: large changes in intrathoracic pressure when efforts to breathe continue during upper airway obstruction, progressive hypercapnia and hypoxia, and finally, transient arousal from sleep, which restores upper airway patency and allows re-ventilation of the lungs. Several recent studies involving animal models have provided useful insight into the relative importance of these individual factors in producing the chronic abnormalities of cardiovascular control associated with SDB [61]. Using an elegant canine model of obstructive apnea, Brooks et al. [62] were able to produce nocturnal and daytime hypertension by exposing the animals to artificially-induced periodic airway obstructions for several weeks. On the other hand, sustained exposure to periodic acoustically-induced arousals without prior upper airway obstruction led only to nocturnal hypertension with no carry-over effect in the daytime. In a rat model, sustained hypertension developed after a few weeks of exposure to intermittent hypoxia without any accompanying upper airway obstruction [63]. In a porcine model, the cardiovascular effects of simulated central apneas were found to be greater than those resulting from obstructive apneas of similar durations [64]. These studies, taken together, suggest that although SDB is characterized by large intrathoracic pressure changes and arousal-induced sleep fragmentation, intermittent hypoxic stimulation appears to be the dominant factor that produces the chronic alterations in cardiovascular control.
There are a number of mechanisms through which intermittent hypoxia, produced by SDB, can lead to hypertension and other forms of cardiovascular disease, but abnormal autonomic control appears to play a major role. Studies utilizing peroneal microneurography or testing of plasma catecholamines have shown that sympathetic tone is abnormally high in subjects with SDB in both sleep and wakefulness [65], [66]. Treatment with continuous positive airway pressure (CPAP) partially reverses these effects [67]–[70]. CPAP therapy has been shown also to improve vagal control of heart rate, the degree of improvement varying directly with compliance level [71]. To determine whether exposure to intermittent hypoxia leads to changes in autonomic control, several prospective studies in normal humans have been carried out. Xie et al. [72] found that exposing healthy young subjects to intermittent asphyxia over a period of 20 min led to sympathetic activation that continued even after the stimulus was removed. In another study [73], prolonged sympathetic activation was produced after 20 min exposure to intermittent hypoxic apnea. The results were similar regardless of whether these exposures occurred against a background of hypercapnia or isocapnia, confirming that the primary mediator for the increase in sympathetic activity was the intermittent hypoxia. In two other studies [74], [75], healthy young subjects exposed to repetitive hypoxic apneas for a total duration of 30 mins displayed, in the post-recovery period, a small and short-lasting increase in mean arterial blood pressure, along with a more sustained and substantial increase in muscle sympathetic nerve activity. The mechanism relating cyclic intermittent hypoxia to sustained sympathetic activation, that outlasts the hypoxic stimulus, may be associated with augmentation of peripheral chemoreflex sensitivity and direct effects on sites of central sympathetic regulation [76]. Baroreflex impairment in patients with SDB has also been proposed as a possible explanation of altered autonomic function [77]–[79].
V. Effects of Sleep-Disordered Breathing and Sleep Deprivation on Metabolic Control
Recognition of an association between SDB and various manifestations of abnormal glucose metabolism, ranging from increased fasting insulin to overt Type 2 diabetes, emerged with early studies on this topic in the 1990s. A number of excellent reviews provide comprehensive coverage of these early studies as well as more recent ones [80]–[82]. The most compelling evidence of the association between SDB and abnormal glucose metabolism has come from 3 relatively recent studies. Ip and colleagues [83] studied a total of 270 subjects who had undergone polysomnography as a part of a community study or who were referred to the sleep laboratory for suspected sleep apnea. Using fasting blood glucose and insulin levels to assess insulin resistance, they found severity of SDB (assessed in terms of AHI and minimum arterial oxyhemoglobin saturation) to be significantly correlated to insulin resistance, independent of obesity (measured using BMI, waist circumference and waist/hip ratio). Punjabi et al. [84] performed oral glucose tolerance tests on 150 middle-aged overweight males who had undergone polysomnography, and found increasing AHI to be associated with impaired glucose tolerance as well as insulin resistance, calculated from fasting blood insulin and glucose levels, even after discounting for obesity in these subjects. In a subsequent study, analysis of data obtained from 2,656 community-dwelling participants of the multi-center Sleep Heart Health Study demonstrated that severity of SDB, as assessed using AHI and average oxyhemoglobin saturation during sleep, was associated with impaired glucose tolerance, independent of other confounding variables such as age, gender, weight, and body fat distribution [85]. More recently, two studies, employing the intravenous glucose tolerance test and the Bergman minimal model for subsequent analysis, found insulin sensitivity to be inversely correlated with SDB severity, represented in terms of either AHI or oxyhemoglobin desaturation [86], [87]. On the other hand, there have been some studies that have failed to find a significant association between SDB and insulin resistance; in these cases, obesity appeared to be the dominant factor affecting insulin resistance [88], [89].
Since cross-sectional studies, such as those cited above, only demonstrate association, several recent longitudinal studies have been conducted to test the hypothesis of a causal link between SDB and glucose intolerance by determining whether CPAP treatment can at least partially reverse the abnormalities in metabolic function. Harsch and colleagues [90], in a study with 40 adult non-diabetic patients with insulin responsiveness measured by a hyperinsulinemic euglycemic clamp technique, found that CPAP treatment (used on average for 5.2 hours per night) improved insulin sensitivity in as early as 2 days after beginning of treatment, with the beneficial effects remaining stable after 3 months of treatment. However, insulin sensitivity in patients with BMI greater than 30 kg/m appeared to be least affected by the treatment, suggesting that in obese individuals, SDB may not be as important a determinant of insulin resistance. Babu and colleagues [91] studied the effects of CPAP treatment of SDB on glycemic control in a group of 25 obese adult patients with type II diabetes. Using a 72-hour continuous glucose monitoring system to measure interstitial glucose levels, they found that for patients who used CPAP for more than 4 h/day there was a correlation between days of CPAP use and reduction in hemoglobin A1C level, a measure of long-term serum (4 weeks to 3 months) glucose regulation. Another study [92] reported a correlation between adherence to CPAP treatment (more than 4 h/night for 6 months) and reduction in hemoglobin A1C levels in adult non-diabetic SDB patients, but found no effect of CPAP on markers of insulin resistance. The authors also found a negative correlation between average sleep oxyhemoglobin saturation and fasting insulin levels. In the study conducted by Coughlin et al. [93], 34 obese males with confirmed diagnosis of SDB participated in a randomized placebo-controlled blinded crossover protocol in which each subject was first assigned to 6 weeks of either CPAP therapy or sham CPAP (pressures were set to 1 cm H2O or less); subsequently, the same subject was crossed over to the other treatment for another 6 weeks. Fasting insulin and fasting glucose were measured at the end of each 6-week treatment limb. The difference between the values at the end of each treatment period was taken to reflect the effectiveness of CPAP on improving glycemic control. However, it was determined that there was no significant change in insulin resistance following CPAP treatment for 6 weeks. Thus, at this time, no definitive conclusion can be drawn regarding the effectiveness of CPAP in improving metabolic function in people with SDB.
A likely mechanism linking SDB to insulin resistance in humans is increased lipolysis and fatty acid availability resulting from the sustained sympathetic activation produced by intermittent hypoxia [94]. Intermittent hypoxia from SDB can also lead to pro-inflammatory states and elevated cytokine levels [95], [96]. The desaturation-reoxygenation effect of intermittent hypoxia can also lead to oxidative stress and consequently impaired glucose metabolism [97]. Sleep deprivation may also play a role in the association of SDB and insulin resistance. There may be an indirect pathway between lack of sleep and the metabolic syndrome through its relation with obesity, the latter being a known risk factor for the metabolic syndrome. Gangwisch and colleagues [98] studied longitudinal data of the 1982–1984, 1987, and 1992 NHANES I follow-up studies and cross-sectional analysis of the 1982–1984 study in adults, and found a nearly inverse linear relationship between weight and sleep time. Sleep deprivation may also be directly implicated as a risk factor for metabolic syndrome. Healthy young adult subjects sleep deprived for six consecutive days have been found to show reduced glucose tolerance and a blunted insulin response to glucose, along with an increase in sympathetic activity [99]. Shigeta and colleagues [100] have shown that sustained sleep debt is associated with obesity and an increase in insulin resistance. In a recently published study [101], 812 community participants were evaluated via questionnaires for sleep disturbances related to insomnia or SDB and tested for metabolic abnormalities at baseline and subsequently after 3 years. The study found that sleep symptoms, such as difficulty falling asleep and loud snoring, predicted the development of MS in this pool of subjects.
VI. Modeling the Development of Metabolic Syndrome
A. Key Questions and Hypotheses
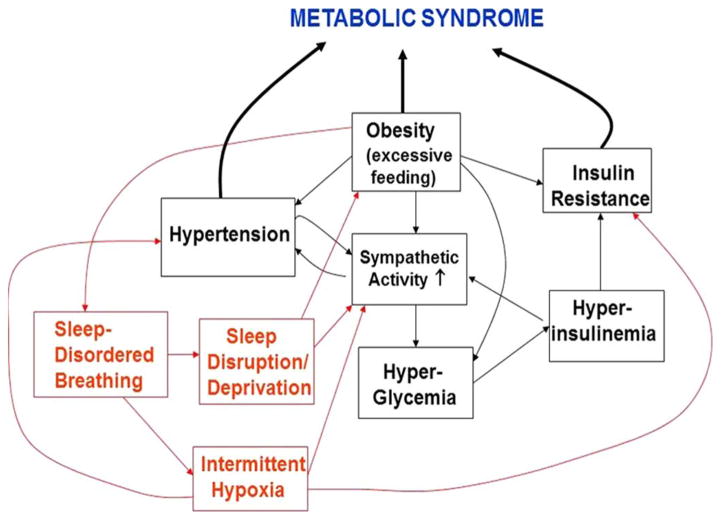
While the cumulative evidence has shown that obesity, and in particular, visceral obesity, plays a key role in the pathogenesis of cardiovascular disease and Type 2 diabetes, the intermediate steps in the longitudinal process that lead eventually to one or both of these outcomes remain unclear. The available evidence also suggests that SDB can independently contribute to the development of cardiovascular disease and diabetes. In fact, the term “Syndrome Z” has been used to describe the clustering of symptoms that include hypertension, obesity, insulin resistance, hyperlipidemia and SDB [102]. But what are the major steps in this progression and what mechanisms are involved? Fig. 1 displays a simplified schematic that suggests the major possibilities that may individually or in combination constitute pathways to MS:
Fig. 1.
Schematic representation of the plausible causal pathways through which obesity, insulin resistance, autonomic activity and sleep-disordered breathing can interact to produce the metabolic syndrome.
Overfeeding stimulates insulin-mediated glucose metabolism in the cells of the ventral medial hypothalamus, which in turn reduces neural traffic along an inhibitory pathway to the brain-stem sympathetic centers that subsequently increase central sympathetic outflow [103]. Obesity also leads to the dysregulation of a number of endocrine, neural, inflammatory and cell-intrinsic mechanisms that interact to produce insulin resistance [104]. As a result of the insulin resistance, the pancreas compensates by secreting more insulin, thus causing hyperinsulinemia and further sympathetic overactivity. Sustained sympathetic overactivity leads through a variety of mechanisms to hypertension [105].
Obesity leads to a reduction in arterial compliance, which in turn leads to reduced BRS [106]. The depressed baroreflex gain leads to sympathetic overactivity which produces hyperglycemia and subsequent hyperinsulinemia, thus increasing insulin resistance. Sympathetic overactivity also leads subsequently to hypertension.
Chronic exposure to intermittent hypoxia and repetitive sympathetic surges that accompany arousals in the obese patient with SDB leads to increased sympathetic activity and eventually hypertension. Sympathetic overactivity leads to increased catecholamine production, which stimulates glycogenolysis and lipolysis, thus promoting insulin resistance.
Intermittent hypoxia could directly impact glucose metabolism through a leptin-related mechanism and thus increase insulin resistance [107]. Then, the hyperinsulinemia that follows produces sympathetic overactivity, which predisposes to the development of hypertension.
B. Mathematical Models of Metabolic and Autonomic Dysregulation
The hypotheses listed in the previous section are by no means exhaustive, but they do represent, albeit rather simplistically, the main potential pathways through which insulin resistance and hypertension could develop in obese individuals, without or with the added complication of SDB. To test these hypotheses and to determine whether the postulated pathways could act in parallel and which may be the more dominant ones, an approach based on computational modeling provides the most systematic way to proceed. More specifically, an approach that applies both “minimal” and “structured” modeling in parallel would likely yield the greatest dividend. “Minimal” modeling is important in allowing us to estimate all key parameters from the observable data from a given experiment – since, under practical circumstances, not all physiological variables can be measured at the same time. On the other hand, minimal modeling does not generally provide insights into the details of the underlying physiology. In contrast, “comprehensive” or “structured” models allow our knowledge of the underlying physiology to be systematized and encapsulated concisely into an efficient library of mathematical “rules”. The presence of model parameters that are isomorphic to key physiological entities greatly simplifies the problem of interpretation vis-a-vis some forms of minimal models that are expressed as nonparametric functions. However, because of the large number of parameters, estimation of all these parameters from experimental data is generally not practicable or feasible. On the other hand, simulations performed with the structured model can provide insight into which physiological parameters are more important in the mediation of the process under study, and this insight can be used in the development of the corresponding minimal models that can be employed to refine the parameter estimates.
Since the introduction of the simple dynamic model by Bolie [108] in 1961, a broad variety of mathematical models of the regulation of glucose and insulin have appeared in the literature [109]–[119]. Some of these fall into the “structured model” category, involving a multi-parameter characterization of glucose, insulin and glucagon dynamics and their interactions such that most model compartments can be identified with the physiological entities that they were designed to represent [109]–[113]. At the same time, minimal models have been employed ubiquitously to derive useful indices that quantify insulin resistance, pancreatic beta cell function and other physiological parameters related to metabolic control and insulin-glucose regulation [114]–[119]; a recent comprehensive review is given by Cobelli and colleagues [120]. Most of these models were designed to simulate the body’s responses to various types of glucose or insulin challenges, and different parameter values were employed to represent “normal” versus “diabetic” or “prediabetic” responses. More challenging has been the quest to develop computational models that can simulate the progression from normal to prediabetic state to overt Type 2 diabetes. Using data collected from a longitudinal study of Type 2 diabetes, Bagust and Beal [121] developed an empirical model characterizing the loss of beta-cell function over time. Their analysis was consistent with a two-phase process in beta-cell function deterioration: an initial long phase with very slow beta-function decay followed, after a critical point in the disease progression process, by a much faster phase of metabolic dysregulation. Topp et al. [122] augmented a minimal model of insulin-glucose regulation with a much slower dynamical component representing the dependence of beta-cell mass on glucose. Their model predicted a steady state operating point with relatively stable beta-cell mass under conditions of mild hyperglycemia. However, it also predicted that more severe levels of hyperglycemia would lead to reduction of beta-cell mass, thus creating a positive feedback effect, which drives the model towards diabetes. The predictions of the Topp model are thus consistent with the two-phase progression process suggested by Bagust and Beal. However, all these models of diabetes do not explicitly take into account the dynamics associated with the regulation of body weight, which is the focus of a number of existing models of energy homeostasis [123]. For instance, Hall’s model [124] characterizes in exquisite detail how changes in the intake of carbohydrates, fat and protein can lead to changes in body weight and body composition over time scales of days or longer. On the other hand, this model and others that focus on the development of obesity do not explicitly include considerations of glucose-insulin regulation.
Computational models of cardiovascular control also have had a long history that may be traced back to the pioneering works of Guyton [125] and Grodins [126] in the late 1960’s and early 1970’s. However, it was only in the past decade and a half that detailed and physiologically validated representations of the heart and autonomic neural control were added to produce more realistic dynamic simulations of the key cardiovascular reflexes [127]–[133]. As well, some of these models incorporated respiratory mechanics and pulmonary gas exchange in order to simulate the cardiovascular responses to Valsalva maneuvers [132] or inhalation of hypoxic or hypercapnic gas mixtures [128], [129]. More recently, the model of Cheng et al. [133] integrated sub-models of respiratory, cardiovascular and sleep regulation to characterize the dynamic interactions among these systems during SDB. To date, the Guyton model [134], [135] remains the dominant and accepted model in guiding our understanding of the development of hypertension. However, this model focuses only on the cardiovascular and renal systems, and therefore, it is limited in its application to MS.
C. A Structured Model of Autonomic-Metabolic Interaction
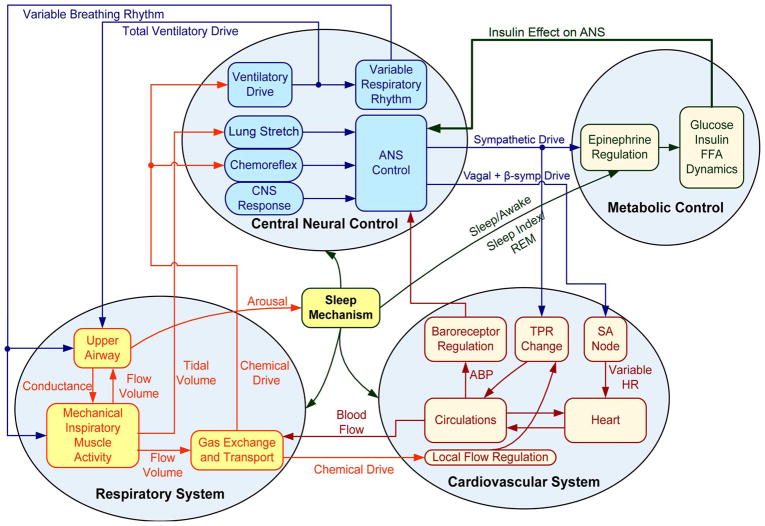
As implied in the previous section, while a large number of models of glucose-insulin, cardiovascular and respiratory control have been developed over the past several decades, none have been used to explore the interactions between autonomic and metabolic dysfunction, such as what might be expected to occur in MS. We have made some preliminary efforts in the development of a structured model of autonomic-metabolic interactions [136] by extending the existing sleep-cardiorespiratory control model of Cheng et al. [133] to incorporate a metabolic sub-model. The metabolic portion of the new model is a hybrid that includes features from both the Bergman minimal model [118] and the model by Roy and Parker [119] that includes the regulation of free fatty acids (FFA). Glucose and FFA metabolism in this “extended minimal metabolic model” are also assumed to be influenced by plasma epinephrine levels using the formulation employed by Kim et al. [137] in which their model was designed to explore the effects of exercise on whole-body metabolism. Inputs from the dietary intake of glucose and external interventions, such as insulin injections, have also been incorporated into the model. The primary connection between the sleep-cardiorespiratory portion of the model and the extended metabolic portion is the efferent sympathetic output produced by the former. Changes in sympathetic output from the cardiorespiratory portion of the model, as well as changes in sleep-wake state, lead to changes in epinephrine production, which in turn affects the metabolism of glucose, insulin and FFA. Sympathetic activity sends epinephrine to the heart, muscle and pancreas compartments. The sum of all the metabolic fluxes that result from these impacts works as internal inputs for the glucose and FFA compartments. Inputs from the dietary intake of glucose and external interventions, such as insulin injections, along with changes in metabolism produced by changes in sleep-wake state, are also incorporated into the model. An important feature is the incorporation of “feedback” from the metabolic component to the autonomic portion of the model. This “feedback” comes in the form of changes in insulin level, which lead to changes in sympathetic tone. A schematic illustration of this large scale simulation model is displayed in Fig. 2.
Fig. 2.
Block diagram of the integrative model of autonomic and metabolic interactions by Cheng and Khoo [136]. Figure as originally published in Cheng L and Khoo MCK (2012) “Modeling the autonomic and metabolic effects of obstructive sleep apnea: a simulation study”. Front. Physio. 2:111. doi: 10.3389/fphys. 2011.00111.
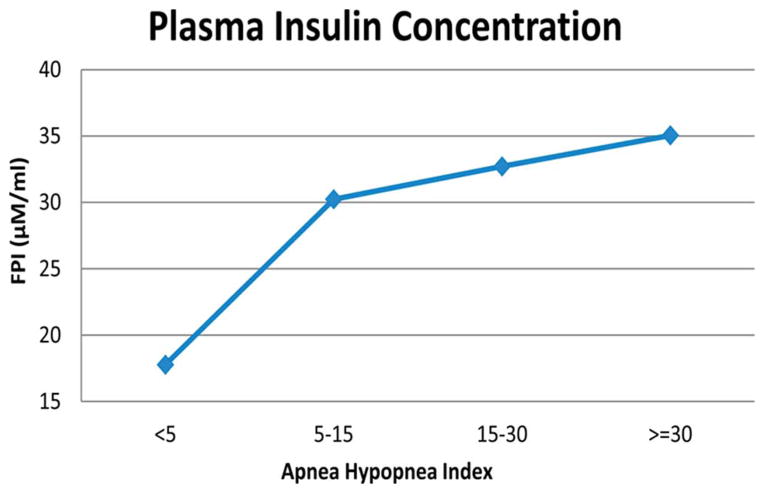
The model allows the comparison of daytime blood pressure, generalized sympathetic tone (represented by epinephrine level), and blood glucose, FFA and insulin levels between the conditions simulating a normal subject and the conditions simulating an individual with SDB. The results of model simulations that have been run for several days indicate higher levels of blood pressure, epinephrine, FFA and insulin, along with slightly elevated plasma glucose levels. Essentially, SDB produces sympathetic overactivity, elevating epinephrine levels which stimulate glycogenolysis and gluconeogenesis, increasing blood glucose. This, along with the elevated epinephrine level, stimulates the production of insulin, which helps to attenuate the rise in blood glucose. The system ends up in a hyperinsulinemic state. Although the parameters of the metabolic sub-model that collectively represent insulin sensitivity remain unchanged in the model, whole-body insulin resistance is effectively increased. The model predicts that increased severity of SDB, as reflected in an increase in AHI, leads to higher levels of fasting plasma insulin (Fig. 3). However, the time course of “disease progression”, as currently predicted by the model in terms of the development of elevated epinephrine, insulin and FFA levels, is substantially more rapid than one might expect based on clinical observation. This is largely related to the fact that the model parameters in both the autonomic and metabolic subsystems remain unchanged, even though the model variables (e.g., mean blood pressures, insulin levels and FFA levels) are altered by the presence or absence of SDB. As well, the current model structure allows for only one of many pathways through which metabolic dysfunction can develop.
Fig. 3.
Model-predicted fasting insulin levels are positively correlated to severity of sleep-disordered breathing, as represented by the apnea-hypopnea index.
D. Future Directions
The model presented in the last section should be viewed as merely a starting point of the quest to obtain a better understanding of how the key physiological subsystems that represent the autonomic, metabolic and sleep regulation may interact dynamically to yield “symptoms” that are consistent with MS. However, with the basic structure of the model in place, future efforts can be launched to incorporate other avenues of interaction among the different subsystems of the model. For instance, the present capabilities of the model are currently limited to addressing the question of whether SDB can independently lead to expected changes in autonomic and metabolic function. What additional features are required to be added to the model in order for us to simulate the development of insulin resistance and/or hypertension in an individual as a consequence of progressive obesity? It is clear that some, if not many, of the model parameters would change slowly with time as the “disease” progresses. But what are the time courses of these parameter changes?
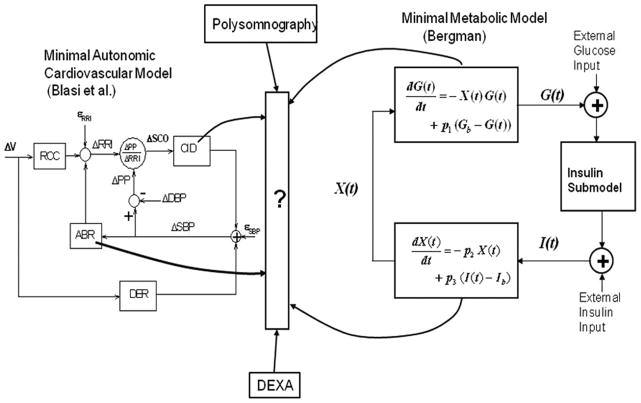
To address this question, the ideal solution would be to conduct large-scale prospective longitudinal studies in which the participating subjects are each tested for autonomic cardiovascular function, metabolic function and severity of SDB (if present). This approach is not likely to be feasible due to cost considerations and the intrusiveness of the experimental protocols involved. Thus, a more practical approach might be to conduct cross-sectional studies in which multiple measurements reflecting adiposity, autonomic activity, metabolic function and SDB status are made in each individual, but the individuals are sampled from a large population pool with a broad range of clinical characteristics, including normal to low values of insulin resistance, normal to high BMI values, and normal to severe SDB. The Bergman minimal model [118] would be used to estimate the key metabolic parameters (SI, AIRg, Sg) and the minimal model of cardiorespiratory control of Blasi et al. [138] would be employed to estimate autonomic parameters such as baroreflex gain. This would allow functional relationships between the metabolic and autonomic parameters to be determined for subjects with different levels of adiposity (which is best measured using dual-energy Xray absorptiometry or DEXA) and severity in SDB (as determined from polysomnography). A schematic representation of this multimodal approach is displayed in Fig. 4. The empirically derived functional relationships that link obesity, autonomic and metabolic function, and SDB severity would need to be interpreted and “translated” into the physiological context so that the information can be employed for the construction of a disease progression model of MS, similar to what was done by Bagust and Beal [121] to characterize loss of beta-cell function over time. This approach is the basis of some of the ongoing work in this research direction in our laboratory; some preliminary findings have been reported in the form of a conference report [139].
Fig. 4.
Schematic representation of the “multimodal” approach in which minimal models of glucose-insulin regulation (e.g., Bergman [118]) and cardiovascular autonomic control (e.g., Blasi et al. [138]) can be employed along with measures derived from polysomnography and dual-energy Xray absorptiometry to establish functional relationships that link autonomic and metabolic function with obesity and severity of sleep-disordered breathing.
Depending on the kinds of nonlinearities that reside in the model structure, gradual changes in the parameter values with time may not always translate to a slow progressive deterioration in metabolic or autonomic function. For instance, in the model of Topp et al. [122], the slow dynamical mechanism representing the effect of glucose on beta-cell mass produces rapid deterioration in metabolic function only after a threshold level of hyperglycemia has been exceeded. Another example is the simplified model of MS introduced by Kitano and associates [16]. Here, they focused on the long-term control of blood glucose via the regulatory feedback loops that involve tumor necrosis factor (TNF)- and adiponectin. As overfeeding continues, increased adipocyte size leads to increased TNF-secretion inhibiting adiponectin, the net result of which is reduced insulin-mediated peripheral glucose utilization and increased glucose production by the liver. Thus, hyperglycemia and hyperinsulinemia occur. Increased TNF- enhances lipolysis in the adipocytes leading to increased plasma FFAs, which in turn increases TNF- from skeletal muscle and the liver. This can transform what was originally a stable negative feedback system into one with an unstable operating point dominated by positive feedback.
An important piece of the puzzle that is missing from the handful of existing quantitative MS models is the link between diet and sympathetic nervous system activity, an observation established by Landsberg and colleagues in a series of elegant experimental studies since the late 1970’s [140]. Insulin-mediated glucose metabolism in the neurons of the ventromedial hypothalamus is increased with overfeeding; this leads to a reduction in the inhibitory influence of these neurons on the brainstem sympathetic centers, resulting in an increase in central sympathetic outflow. Thus, in this case, hyperinsulinemia is the primary driving force for the elevated sympathetic activity, rather than the other way around. Obesity also leads to an increase in leptin levels which are meant to reduce overfeeding by suppressing appetite; however, this occurs at a price, since elevated leptin levels lead to increased sympathetic activity [141]. In our view, incorporating these pathways is crucial for the development of an all-encompassing model of MS.
In summary, while there is great contemporary interest in modeling the mechanisms underlying MS at the molecular level [142], we contend that adopting a “systems” approach to understanding the development and progression of this syndrome is likely to yield the highest practical dividends.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health under Grant EB001978, Grant HL-090451, and Grant HL-105210.
Biographies

Michael C. K. Khoo (M’86-SM’10-F’12) received the B.Sc.(Eng.) degree in mechanical engineering from Imperial College of Science and Technology, University of London, in 1976, and the M.S. and Ph.D. degrees in bioengineering from Harvard University, Cambridge, MA, in 1977 and 1981, respectively.
From 1981 to 1983, he was a Research Associate at the V.A. Hospital, West Roxbury, MA, and the Brigham and Women’s Hospital, Boston, MA. Since September 1983, he has been on the faculty of the University of Southern California (USC), Los Angeles, where he is currently Professor of Biomedical Engineering and Pediatrics. From 2003 to 2010, he was Chair of the Department of Biomedical Engineering and Co-Director of Education and Outreach in the Biomimetic Microelectronic Systems Engineering Research Center at USC. His current research interests include cardiorespiratory regulation in sleep apnea and metabolic syndrome, physiological modeling, and biomedical signal processing. Dr. Khoo is a Fellow of the American Institute of Medical and Biological Engineering, the IEEE, and the Biomedical Engineering Society. He is a member of the American Physiological Society, Sleep Research Society, and the American Heart Association. He is also on the editorial board of Respiratory Physiology and Neurobiology, and the IEEE Press Book Series on Biomedical Engineering. He is the author of the biomedical engineering textbook: Physiological Control Systems: Analysis, Simulation and Estimation (Wiley-IEEE Press, 2000), as well as two reference volumes on physiological modeling.

Flavia M. G. S. Oliveira (M’99) received the B.Sc. degree in electrical engineering from the University of Brasilia, Brazil, in 1996, the M.Sc. degree in electrical engineering from the University of Campinas, Brazil, in 1998, and the M.Sc. and Ph.D. degrees in biomedical engineering from the University of Southern California (USC) in 2009 and 2011, respectively.
She is currently a member of the faculty (Professor Adjunto) in the Department of Electrical Engineering, University of Brasilia. She received an undergraduate scholarship (PIBIC) from the National Council for Scientific and Technological Development (CNPq) of the Brazilian Ministry of Education (1993–1995), graduate scholarships from CNPq (1996–1998), the USC Viterbi School of Engineering Graduate Fellowship Award (2007–2009), and a Fulbright/CAPES Graduate Scholarship (2007–2011). Her research interests include physiological modeling, the investigation of metabolic and cardiovascular complications of sleep disorders, and physiological control systems identification using orthonormal basis functions.

Limei Cheng (S’06–M’11) received the M. Sc. in electrical engineering from University of California, Los Angeles in 2002 and the Ph.D. degree in Biomedical Engineering from University of Southern California (USC), Los Angeles, in 2009, respectively.
She was a Research Associate at the Biomedical Simulation Resource in the Department of Biomedical Engineering at USC during 2009 and 2012, where she was involved in developing novel modeling and computational methodologies for the experimental study of biological systems and understanding, diagnosing and treating human diseases. She is currently a Member Research Scientist in the Department of Clinical Decision Support Solutions in Philips Research, North America, Briarcliff Manor, NY. Her research interests include computational modeling and simulation of human physiology, clinical decision support for emergency care and intensive care, and noninvasive physiological monitoring.
Contributor Information
Michael C. K. Khoo, Email: khoo@bmsr.usc.edu, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA.
Flavia M. G. S. Oliveira, Email: flavia@ene-unb.br, Department of Electrical Engineering, University of Brasilia, Brazil.
Limei Cheng, Email: limei.cheng@philips.com, Department of Clinical Decision Support Solutions, Philips Research, North America.
References
- 1.Young GO. Synthetic structure of industrial plastics (Book style with paper title and editor) In: Peters J, editor. Plastics. 2. Vol. 3. New York: McGraw-Hill; 1964. pp. 15–64. [Google Scholar]
- 2.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 5.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Nat Health Statist Reports. 2009;13:1–7. [PubMed] [Google Scholar]
- 6.Fontaine KR, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Procopiou M, Philippe J. The metabolic syndrome and type 2 diabetes: Epidemiological figures and country specificities. Cerebrovasc Dis. 2005;20(Suppl 1):2–8. doi: 10.1159/000088231. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 9.Isomaa B, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 10.Lakka HM, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;25:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 11.Henry RR. Type 2 diabetes care: The role of insulin-sensitizing agents and practical implications for cardiovascular disease prevention. Am J Med. 1998;105(1A):20S–26S. doi: 10.1016/s0002-9343(98)00207-1. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR, Mawdsle L, Mugarza JA, Calverley PMA, Wilding JPH. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Tasali E, Ip MSM. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 14.Levy P, Tamisier R, Minville C, Launois S, Pepin J-L. Sleep apnoea syndrome in 2011: Current concepts and future directions. Eur Respir Rev. 2011;20:134–146. doi: 10.1183/09059180.00003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasali E, Van Cauter E. Sleep-disordered breathing and the current epidemic of obesity: Consequence or contributing factor? Am J Respir Crit Care Med. 2002;165:562–563. doi: 10.1164/ajrccm.165.5.2201001b. [DOI] [PubMed] [Google Scholar]
- 16.Kitano H, Oda K, Kimura T, Matsuoka Y, Csete M, Doyle J, Muramatsu M. Metabolic syndrome and robustness tradeoffs. Diabetes. 2004;53(Suppl 3):S6–S15. doi: 10.2337/diabetes.53.suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- 17.Chavez BE, Henry RR. Type 2 diabetes: Insulin resistance, beta cell dysfunction, and other metabolic and hormonal abnormalities. In: Fonseca VA, editor. Clinical Diabetes – Translating Research into Practice. New York: Elsevier; 2006. pp. 21–34. [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol: Endocrinol Metab Gastrointest Physiol. 1979;6:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Bonora, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 22.Muniyappa R, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol. 2008;297:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 23.Bergman RN, et al. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN, et al. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension. 1991;17:669–677. doi: 10.1161/01.hyp.17.5.669. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 27.Ward KD, Sparrow D, Landsberg L, Young JB, Vokonas PS, Weiss ST. Influence of insulin, sympathetic nervous system activity, and obesity on blood pressure: The normative aging study. J Hypertens. 1996;14:301–308. doi: 10.1097/00004872-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Daffonchio A, Di Rienzo M, Ferrari AU, Grassi G. Methods to quantify sympathetic cardiovascular influences. Eur Heart J. 1998;19(Suppl F):F7–F13. [PubMed] [Google Scholar]
- 30.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DL, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake and angiotension neuromodulation. Hypertens. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 31.Dimsdale JE, Moss J. Short-term catecholamine to psychologic stress. Psychosom Med. 1980;42:493–497. doi: 10.1097/00006842-198009000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Veale D, Pepin JL, Levy PA. Autonomic stress tests in obstructive sleep apnea syndrome and snoring. Sleep. 1992;15:505–513. [PubMed] [Google Scholar]
- 33.Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 34.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human RR interval power spectra is largely ignored. J Appl Physiol. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 35.Khoo MCK, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22:443–451. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- 36.Eckberg DL. Sympathovagal balance: A critical appraisal. Circulation. 1997;96:3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 37.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol. 1990;258:H713–H721. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- 38.Malik M, Camm AJ. Components of heart rate variability–What they really mean and what we really measure. Am J Cardiol. 1993;72:821–822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 39.Parati G, Mancia G, Di Rienzo M. Cardiovascular variability is an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–678. doi: 10.1152/japplphysiol.00446.2006. [DOI] [PubMed] [Google Scholar]
- 40.Parati G, Di Rienzo MM, Mancia G. How to measure baroreflex sensitivity: From the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- 41.Baselli G, Cerutti S, Civardi S, Malliani A, Pagani M. Cardiovascular variability signals: Towards the identification of a closed-loop model of the neural control mechanisms. IEEE Trans Biomed Eng. 1988 Dec;35(12):1033–1045. doi: 10.1109/10.8688. [DOI] [PubMed] [Google Scholar]
- 42.Mullen TJ, Appel ML, Mukkamala R, Mathias JM, Cohen RJ. System identification of closed-loop cardiovascular control: Effects of posture and autonomic blockade. Am J Physiol. 1997;272:H448–H461. doi: 10.1152/ajpheart.1997.272.1.H448. [DOI] [PubMed] [Google Scholar]
- 43.Belozeroff V, Berry RB, Sassoon CSH, Khoo MCK. Effects of CPAP therapy on cardiovascular variability in obstructive sleep apnea: A closed-loop analysis. Am J Physiol. 2002;282:H110–H121. doi: 10.1152/ajpheart.2002.282.1.H110. [DOI] [PubMed] [Google Scholar]
- 44.Weston PJ, James MA, Panerai RB, McNally PG, Potter HF, Thurston H. Evidence of defective cardiovascular autoregulation in insulin-dependent diabetic patients without clinical autonomic dysfunction. Diabetes Res Clin Pract. 1998;42:141–148. doi: 10.1016/s0168-8227(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 45.Lindgren K, Hagelin E, Hansen N, Lind L. Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J Hypertens. 2006;24:143–150. doi: 10.1097/01.hjh.0000198024.91976.c2. [DOI] [PubMed] [Google Scholar]
- 46.Lucini DD, Cusumano GG, Bellia AA, Kozakova MM, DiFede GG, Lauro RR, Pagani MM. Is reduced baroreflex gain a component of the metabolic syndrome? Insights from the LINOSA study. J Hypertens. 2006;24:361–370. doi: 10.1097/01.hjh.0000202817.02836.9c. [DOI] [PubMed] [Google Scholar]
- 47.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: Implications for the metabolic syndrome. Am J Physiol. 2002;282:H630–H635. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 48.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 49.Penicau L, Cousin B, Leloup C, Lorsignol A, Casteilla L. The autonomic nervous system, adipose tissue plasticity, and energy balance. Nutrition. 2000;16:903–908. doi: 10.1016/s0899-9007(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 50.Landsberg L, Young JB. The role of the sympathoadrenal system in modulating energy expenditure. Clin Endocrinol Metab. 1984;13:475–499. doi: 10.1016/s0300-595x(84)80034-1. [DOI] [PubMed] [Google Scholar]
- 51.Bray GA. Integration of energy intake and expenditure in animals and man: The autonomic and adrenal hypothesis. Clin Endocrinol Metab. 1984;13:521–546. doi: 10.1016/s0300-595x(84)80036-5. [DOI] [PubMed] [Google Scholar]
- 52.Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Eng J Med. 1988;318:1077–1083. doi: 10.1056/NEJM198804283181701. [DOI] [PubMed] [Google Scholar]
- 53.Eikelis N, Schlich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 54.Rumantir MS, et al. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 55.Landsberg L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006 doi: 10.1007/s10571-006-9010-7. [DOI] [PubMed] [Google Scholar]
- 56.Hiestand DM, et al. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the National Sleep Foundation sleep in America 2005 poll. Chest. 2006;130:780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 57.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: Association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–540. [PubMed] [Google Scholar]
- 58.Nieto FJ, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a community-based study. Sleep heart health study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 59.Peppard PE, Young T, Palta M, Skatrud JB. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 60.Shahar EE, et al. Sleep disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2000;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 61.Schaub CG, Schneider H, O’Donnell CP. Mechanisms of acute and chronic blood pressure elevation in animal models of obstructive sleep apnea. In: Bradley TD, Floras JS, editors. Sleep Apnea: Implications in Cardiovascular and Cerebrovascular Disease. New York: Marcel Dekker; 2000. pp. 159–179. [Google Scholar]
- 62.Brooks D, Horner RL, Kozar LF, Render-Teixera CLB, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: Evidence from a canine model. J Clin Invest. 1997;99:106–119. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fletcher EC, Lesske J, Behm R, Miller CC, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 64.Chen L, Scharf SM. Comparative hemodynamic effects of periodic obstructive and simulated central apneas in sedated pigs. J Appl Physiol. 1997;83:485–494. doi: 10.1152/jappl.1997.83.2.485. [DOI] [PubMed] [Google Scholar]
- 65.Carlson JT, Hedner J, Elam JM, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 66.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waravdekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–1338. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 68.Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: Cardiovascular implications. Eur Respir J. 1995;8:222–229. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 69.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 70.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- 71.Khoo MCK, Belozeroff V, Berry RB, Sassoon CSH. Cardiac autonomic control in obstructive sleep apnea: Effects of long-term CPAP therapy. Am J Respir Crit Care Med. 2001;164:807–812. doi: 10.1164/ajrccm.164.5.2010124. [DOI] [PubMed] [Google Scholar]
- 72.Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent hypoxia in humans. J Appl Physiol. 2000;89:1333–1338. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- 73.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 74.Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Leuenberger UA, Hogeman CS, Quraishi S, Linton-Frazier L, Gray KS. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans. J Appl Physiol. 2007;103:835–842. doi: 10.1152/japplphysiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- 76.Weiss JW, Liu Y, Huang J. Physiological basis for a causal relationship of obstructive sleep apnoea to hypertension. Exp Physiol. 2007;92:21–26. doi: 10.1113/expphysiol.2006.035733. [DOI] [PubMed] [Google Scholar]
- 77.Cortelli P, Parchi P, Sforza E, Contin M, Pierangeli G, Barletta G, Lugaresi E. Cardiovascular autonomic dysfunction in normotensive awake subjects with obstructive sleep apnoea syndrome. Clin Auton Res. 1994;4:57–62. doi: 10.1007/BF01828839. [DOI] [PubMed] [Google Scholar]
- 78.Lai CJ, et al. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- 79.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstrucive sleep apnea. Hypertension. 1998;32:1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 80.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 81.Tasali E, Ip MSM. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 82.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and Type 2 diabetes: Interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 83.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 84.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 85.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The sleep heart health study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 86.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lesser DJ, Tran WH, Khoo MCK, Keens TG, Ortega R, Goran MI, Mittelman SD, Davidson Ward SL. Sleep fragmentation and intermittent hypoxemia associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr Res. 2012;72:293–298. doi: 10.1038/pr.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stoohs R, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 89.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Harsch IA, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 91.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 92.Steiropoulos P, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with obstructive sleep apnea hypopnea syndrome: Does adherence to CPAP treatment improve glycemic control? Sleep Med. 2009;10:887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 94.Kjeldsen SE, Rostrup M, Moan A, Mundal HH, Gjesdal K, Eide IK. The sympathetic nervous system may modulate the metabolic cardiovascular syndrome in essential hypertension. J Cardiovasc Pharmacol. 1992;20(Suppl 8) [PubMed] [Google Scholar]
- 95.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J Clin Endocrin Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 96.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Rudich A. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998 Oct;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 98.Gangwisch JE. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 99.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 100.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001;24:608. doi: 10.2337/diacare.24.3.608. [DOI] [PubMed] [Google Scholar]
- 101.Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. “Syndrome Z”: The interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998;53:S25–S28. [PMC free article] [PubMed] [Google Scholar]
- 102.Troxel WM, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633–1640. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Landsberg L. Insulin-mediated sympathetic stimulation: Role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 104.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007;21:1443–55. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 105.Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9:113S–120S. doi: 10.1016/0895-7061(96)00287-7. [DOI] [PubMed] [Google Scholar]
- 106.Weston PJ. Insulin resistance and hypertension: Is impaired arterial baroreceptor sensitivity the missing link? Clin Sci. 2000;98:125–126. doi: 10.1042/cs0980125. [DOI] [PubMed] [Google Scholar]
- 107.Polotsky VY, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bolie VW. Coefficients of normal blood glucose regulation. J Appl Physiol. 1961;16:783–788. doi: 10.1152/jappl.1961.16.5.783. [DOI] [PubMed] [Google Scholar]
- 109.Ackerman E, Gatewood LC, Rosevar JW, Molnar GD. Model studies of blood-glucose regulation. Bull Math Biophys. 1965;27:21–38. doi: 10.1007/BF02477259. [DOI] [PubMed] [Google Scholar]
- 110.Srinivasan R, Kadish A, Shridar R. A mathematical model for the control mechanisms of free fatty acid-glucose metabolism in normal humans. Comput Biomed Res. 1970;3:146–166. doi: 10.1016/0010-4809(70)90021-2. [DOI] [PubMed] [Google Scholar]
- 111.Cobelli C, Federspil G, Pacini G, Salvan A, Scandellari C. An integrated mathematical model of the dynamics of blood glucose and its hormonal control. Math Biosci. 1982;58:27–60. [Google Scholar]
- 112.Summers RL, Montani JP, Woodward LH, Coleman TG, Hall JE. Theoretical analysis of the mechanisms of chronic hyperinsulinemia. Comput Biol Med. 1997;27:249–256. doi: 10.1016/s0010-4825(97)83147-2. [DOI] [PubMed] [Google Scholar]
- 113.Sturis J, Polonsky KS, Mosekilde E, Van Cauter E. Computer model for mechanisms underlying ultradian oscillations of insulin and glucose. Am J Physiol. 1991;260:E801–E809. doi: 10.1152/ajpendo.1991.260.5.E801. [DOI] [PubMed] [Google Scholar]
- 114.Insel PA, Liljenquist JE, Tobin JD, Sherwin RS, Watkins P, Andres R, Berman M. Insulin control of glucose metabolism in man: A new kinetic analysis. J Clin Invest. 1975;55:1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cobelli C, Toffolo G, Ferrannini E. A model of glucose kinetics and their control by insulin, compartmental and noncompartmental approaches. Math Biosci. 1984;72:291–315. [Google Scholar]
- 116.Sherwin RS, Kramer KJ, Tobin TD, Insel PA, Liljenquist JE, Berman M, Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974;53:1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dalla Man CC, Caumo AA, Cobelli C. The oral glucose minimal model: Estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002 May;49(5):419–429. doi: 10.1109/10.995680. [DOI] [PubMed] [Google Scholar]
- 118.Bergman RN. Minimal model: Perspective from 2005. Horm Res. 2005;64(Suppl 3):8–15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- 119.Roy A, Parker RS. Dynamic modeling of free fatty acid, glucose, and insulin: An extended “minimal model”. Diabetes Technol Ther. 2006;8:617–626. doi: 10.1089/dia.2006.8.617. [DOI] [PubMed] [Google Scholar]
- 120.Cobelli C, Man CD, Sparacino G, Magni L, De Nicolao G, Kovatchev BP. Diabetes: Models, signals, and control. IEEE Rev Biomed Eng. 2009;2:54–96. doi: 10.1109/RBME.2009.2036073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bagust A, Beale S. Deteriotaing beta-cell function in type 2 diabetes: A long term model. QJ Med. 2003;96:281–288. doi: 10.1093/qjmed/hcg040. [DOI] [PubMed] [Google Scholar]
- 122.Topp B, Promislow K, DeVries G, Finegood DT. A model of beta cell mass, insulin and glucose kinetics: Pathways to diabetes. J Theor Biol. 2000;206:605–619. doi: 10.1006/jtbi.2000.2150. [DOI] [PubMed] [Google Scholar]
- 123.Pattaranit R, van den Berg A. Mathematical models of energy homeostasis. J Roy Soc Interface. 2008;5:1119–1135. doi: 10.1098/rsif.2008.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298:E449–E466. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guyton AC, Coleman TG, Granger HJ. Circulation: Overall regulation. Annu Rev Physiol. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 126.Grodins FS. Integrative cardiovascular physiology: A mathematical synthesis of cardiac and blood vessel hemodynamics. Q Rev Biol. 1959;34:93–116. doi: 10.1086/402631. [DOI] [PubMed] [Google Scholar]
- 127.Ursino M. Interaction between carotid baroregulation and the pulsating heart: A mathematical model. Am J Physiol Heart Circ Physiol. 1998;275:H1733–H1747. doi: 10.1152/ajpheart.1998.275.5.H1733. [DOI] [PubMed] [Google Scholar]
- 128.Ursino M, Magosso E. Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model. Am J Physiol Heart Circ Physiol. 2000;279:H149–H165. doi: 10.1152/ajpheart.2000.279.1.H149. [DOI] [PubMed] [Google Scholar]
- 129.Magosso E, Ursino M. A mathematical model of CO2 effect on cardiovascular regulation. Am J Physiol Heart Circ Physiol. 2001;281:H2036–H2052. doi: 10.1152/ajpheart.2001.281.5.H2036. [DOI] [PubMed] [Google Scholar]
- 130.Olansen JB, Clark JW, Khoury D, Ghorbel F, Bidani A. A closed loop model of the canine cardiovascular system that includes ventricular interaction. Comp Biomed Res. 2000;33:260–295. doi: 10.1006/cbmr.2000.1543. [DOI] [PubMed] [Google Scholar]
- 131.Lu K, Clark JW, Ghorbel FH, Ware DL, Bidani A. A human cardiopulmonary system model applied to the analysis of the Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2001;281:H2661–H2679. doi: 10.1152/ajpheart.2001.281.6.H2661. [DOI] [PubMed] [Google Scholar]
- 132.Liang FY, Liu H. Simulation of hemodynamic responses to the Valsalva maneuver: An integrative computational model of the cardiovascular system and the autonomic nervous system. J Physiol Sci. 2006;56:45–65. doi: 10.2170/physiolsci.rp001305. [DOI] [PubMed] [Google Scholar]
- 133.Cheng L, Ivanova O, Fan H, Khoo MCK. An integrative model of respiratory and cardio vascular control in sleep-disordered breathing. Respir Physiol Neurobiol. 2010;174:4–28. doi: 10.1016/j.resp.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guyton AC, Coleman TG, Cowley AW, Manning RD, Norman RA, Ferguson JD. A systems analysis approach to understanding long-range arterial blood pressure control and hypertension. Circ Res. 1974;35:159–176. [Google Scholar]
- 135.Guyton AC. Long-term arterial pressure control: An analysis from animal experiments and computer and graphic models. Am J Physiol Regul Integr Comp Physiol. 1990;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 136.Cheng L, Khoo MCK. Modeling the autonomic and metabolic effects of obstructive sleep apnea: A simulation study. Front Physiol. 2012;2(111):1–20. doi: 10.3389/fphys.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim J, Saidel GM, Cabrera ME. Multi-scale computational model of fuel homeostasis during exercise: Effect of hormonal control. Ann Biomed Eng. 2007;35:69–90. doi: 10.1007/s10439-006-9201-x. [DOI] [PubMed] [Google Scholar]
- 138.Blasi A, Jo J, Valladares E, Juarez R, Baydur A, Khoo MCK. Autonomic cardiovascular control following transient arousal from sleep: A time-varying closed-loop model. IEEE Trans Biomed Eng. 2006 Jan;53(1):74–82. doi: 10.1109/TBME.2005.859789. [DOI] [PubMed] [Google Scholar]
- 139.Oliveira FMS, Tran WH, Lesser DJ, Bhatia R, Ortega R, Mittelman SD, Keens TG, Davidson Ward SL, Khoo MCK. Autonomic and metabolic effects of OSA in childhood obesity. Proc IEEE Eng Med Biol Soc Conf. 2010;2010:6134–6137. doi: 10.1109/IEMBS.2010.5627789. [DOI] [PubMed] [Google Scholar]
- 140.Landsberg L. Insulin-mediated sympathetic stimulation: Role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 141.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: From epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]